The Role of Gravity in the Evolution of the Concentration Field in the Electrochemical Membrane Cell
Abstract
1. Introduction
2. Electrochemical Membrane Cell
3. Methodology for Measuring the Volume Flux
4. Results and Discussion
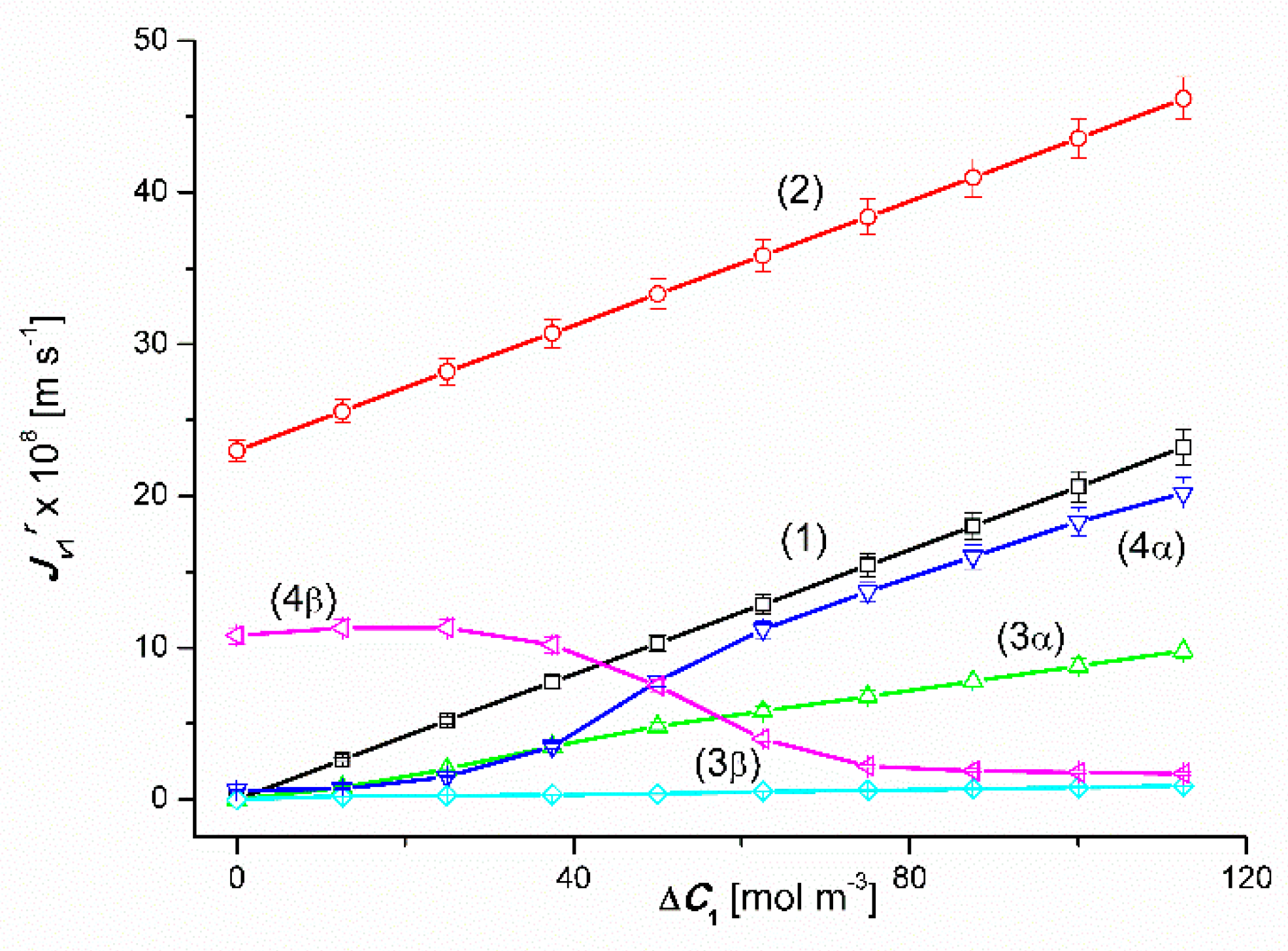
4.1. The Effect of Concentration Polarization
4.2. Convection Effect
4.3. The Effect of Asymmetry of the Volume Osmotic Flux
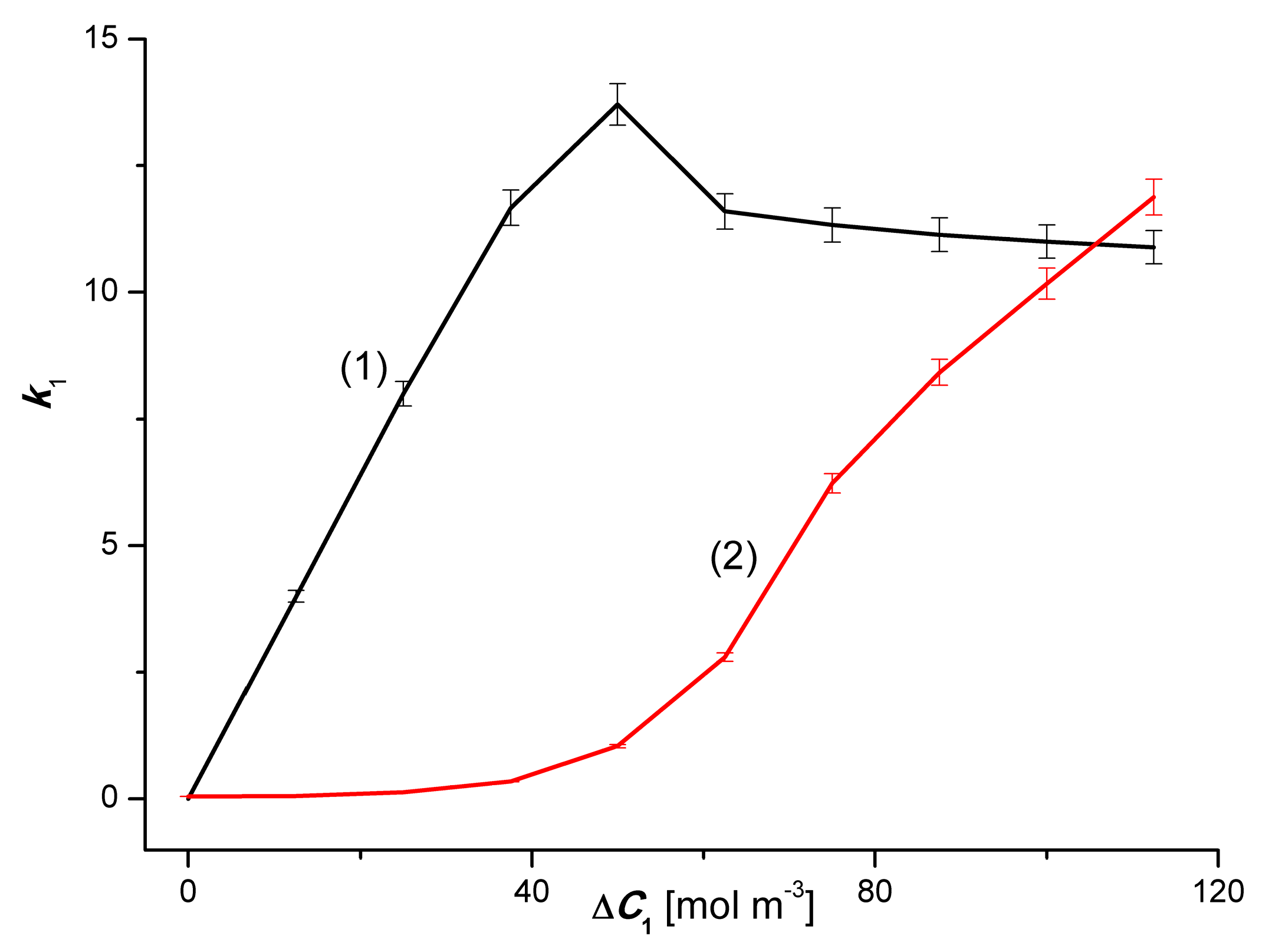
4.4. The Effect of Amplification the Volume Osmotic Flux
4.5. Evaluation of Osmotic Entropy Production
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lipton, B. The Biology of Belief: Unleashing the Power of Consciousness; Hay House: Carlsbad, CA, USA, 2018; ISBN 10: 1401923127. [Google Scholar]
- Baker, R. Membrane Technology and Application; John Wiley & Sons: New York, NY, USA, 2012; ISBN 978-0-470-74372-0. [Google Scholar]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Nguyen, T.P.N.; Jun, B.M.; Hwa Lee, J.; Kwon, Y.-M. Comparison of integrally asymmetric and thin film composite structures for a desirable fashion of forward osmosis membranes. J. Membr. Sci. 2015, 495, 457–470. [Google Scholar] [CrossRef]
- Nga Nguyen, T.P.; Byung-Moon, N.; Kwon, Y.N. The chlorination mechanism of integrally asymmetric cellulose triacetate (CTA)-based and thin film composite polyamide-based forward osmosis membrane. J. Membr. Sci. 2017, 523, 111–121. [Google Scholar] [CrossRef]
- Barry, P.H.; Diamond, J.M. Effects of unstirred layers on membrane phenomena. Physiol. Rev. 1984, 64, 763–872. [Google Scholar] [CrossRef]
- Dworecki, K.; Ślęzak, A.; Ornal-Wąsik, B.; Wąsik, S. Effect of hydrodynamic instabilities on solute transport in membrane system. J. Membr. Sci. 2005, 265, 94–100. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistet, P.; Pourcelly, G. Desalination at overlimitinng currents: state-of-theart and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Batko, K.M.; Ślęzak-Prochazka, I.; Ślęzak, A. Network hybrid form of the Kedem-Katchalsky equations for non-homogenous binary non-electrolyte solutions: Evaluation of Pij* Peusner’s tensor coefficients. Transp. Porous Med. 2015, 106, 1–20. [Google Scholar] [CrossRef]
- Ślęzak, A.; Ślęzak-Prochazka, I.; Grzegorczyn, S.; Jasik-Ślęzak, J. Evaluation of S-Entropy production in a single-membrane system in concentration polarization conditions. Trans. Porous Med. 2017, 116, 941–957. [Google Scholar] [CrossRef]
- Dermirel, Y. Nonequilibrium Thermodynamics: Transport and Rate Processes in Physical, Chemical and Biological Systems; Elsevier: Amsterdam, The Netherlands, 2007; pp. 275–540. ISBN 978-0-444-53079-0. [Google Scholar]
- Delmotte, M.; Chanu, J. Non-equilibrium thermodynamics and membrane potential measurement in biology. In Topics Bioelectrochemistry and Bioenergetics; Millazzo, G., Ed.; John Wiley Publish & Sons: Chichester, UK, 1979; pp. 307–359. [Google Scholar]
- Przestalski, S.; Kargol, M. Graviosmotic volume flow through membrane systems. Stud. Biophys. 1972, 34, 7–14. [Google Scholar]
- Kargol, M.; Dworecki, K.; Przestalski, S. Graviosmotic flow amplification effects in a series membrane system. Stud. Biophys. 1979, 76, 137–144. [Google Scholar]
- Kargol, M. The graviosmotic hypothesis of xylem transport of water in plants. Gen. Physiol. Biophys. 1992, 11, 469–487. [Google Scholar] [PubMed]
- Ślęzak, A.; Dworecki, K.; Anderson, J.A. Gravitational effects on transmembrane flux: the Rayleigh-Taylor convective instability. J. Membr. Sci. 1985, 23, 71–81. [Google Scholar] [CrossRef]
- Ślęzak, A. Irreversible thermodynamic model equations of the transport across a horizontally mounted membrane. Biophys. Chem. 1989, 34, 91–102. [Google Scholar] [CrossRef]
- Ślęzak, A.; Grzegorczyn, S.; Jasik-Ślęzak, J.; Michalska-Małecka, K. Natural convection as an asymmetrical factor of the transport through porous membrane. Transp. Porous Media 2010, 84, 685–698. [Google Scholar] [CrossRef]
- Batko, K.M.; Ślęzak-Prochazka, I.; Grzegorczyn, S.; Ślęzak, A. Membrane transport in concentration polarization conditions: network thermodynamics model equations. J. Porous. Media 2014, 17, 573–586. [Google Scholar] [CrossRef]
- Ślęzak, A.; Jasik-Ślęzak, J.; Wąsik, J.; Sieroń, A.; Pilis, W. Volume osmotic flows of non-homogeneous electrolyte solutions through horizontally mounted membrane. Gen. Physiol. Biophys. 2001, 21, 115–146. [Google Scholar]
- Katchalsky, A.; Curran, P.F. Nonequilibrium Thermodynamics in Biophysics; Harvard University Press: Cambridge, MA, USA, 1965; ISBN 9780674494121. [Google Scholar]
- Ślęzak, A.; Dworecki, K.; Ślęzak, I.H.; Wąsik, S. Permeability coefficient model equations of the complex: Membrane-concentration boundary layers for ternary nonelectrolyte solutions. J. Membr. Sci. 2005, 267, 50–57. [Google Scholar] [CrossRef]
- Dworecki, K.; Wąsik, S.; Ślęzak, A. Temporal and spatial structure of the concentration boundary layers In membrane system. Physica A 2003, 326, 360–369. [Google Scholar] [CrossRef]
- Ślęzak, A.; Jasik-Ślęzak, J.; Grzegorczyn, S.; Ślęzak-Prochazka, I. Nonlinear effects in osmotic volume flows of electrolyte solutions through double-membrane system. Transp. Porous Med. 2012, 92, 337–356. [Google Scholar] [CrossRef]
- Lebon, G.; Jou, D.; Casas-Vasquez, J. Understanding Non-Equilibrium Thermodynamics. Foundations, Applications, Frontiers; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Jasik-Ślęzak, J.; Olszówka, K.M.; Ślęzak, A. Estimation of thickness of concentration boundary layers by osmotic volume flux determination. Gen. Physiol. Biophys. 2011, 30, 186–195. [Google Scholar] [CrossRef]
- Ślęzak, A.; Dworecki, K.; Jasik-Ślęzak, J.; Wąsik, J. Method to determine the critical concentration Rayleigh number in isothermal passive membrane transport processes. Desalination 2004, 168, 397–412. [Google Scholar] [CrossRef]
- Klinkman, H.; Holtz, M.; Willgerodt, W.; Wilke, G.; Schoenfelder, D. “Nephrophan”— eine neue dialysemembran. Zeits. Urolog. Nephrol. 1969, 62, 285–294. [Google Scholar]
- Richter, T.; Keipert, S. In vito permeation studies comparing bovine nasal mucosa, porcine cornea and art.ificial membrane: Androdtenedione in microemulsions and their components. Europ. J. Pharmac. Biopharmac. 2004, 58, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Korus, I.; Rajca, M. Membranes and membrane processes in environmental protection. In Proceedings of the MEMPEP 2018, 12th Scientific Conference, Zakopane, Poland, 13–16 June 2018; Silesia Technical University Press: Gliwice, Poland, 2018; pp. 120–121. [Google Scholar]
- Gerbaud, V.; Shcherbakova, N.; Da Cunha, S. A nonequilibrium thermodynamics perspective on nature-inspired chemical engineering processes. Chem. Eng. Res. Des. 2020, 154, 316–330. [Google Scholar] [CrossRef]













© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batko, K.M.; Ślęzak, A.; Bajdur, W.M. The Role of Gravity in the Evolution of the Concentration Field in the Electrochemical Membrane Cell. Entropy 2020, 22, 680. https://doi.org/10.3390/e22060680
Batko KM, Ślęzak A, Bajdur WM. The Role of Gravity in the Evolution of the Concentration Field in the Electrochemical Membrane Cell. Entropy. 2020; 22(6):680. https://doi.org/10.3390/e22060680
Chicago/Turabian StyleBatko, Kornelia M., Andrzej Ślęzak, and Wioletta M. Bajdur. 2020. "The Role of Gravity in the Evolution of the Concentration Field in the Electrochemical Membrane Cell" Entropy 22, no. 6: 680. https://doi.org/10.3390/e22060680
APA StyleBatko, K. M., Ślęzak, A., & Bajdur, W. M. (2020). The Role of Gravity in the Evolution of the Concentration Field in the Electrochemical Membrane Cell. Entropy, 22(6), 680. https://doi.org/10.3390/e22060680






