Movable and Focus-Tunable Lens Based on Electrically Controllable Liquid: A Lattice Boltzmann Study
Abstract
:1. Introduction
2. Theoretical Method and Numerical Model
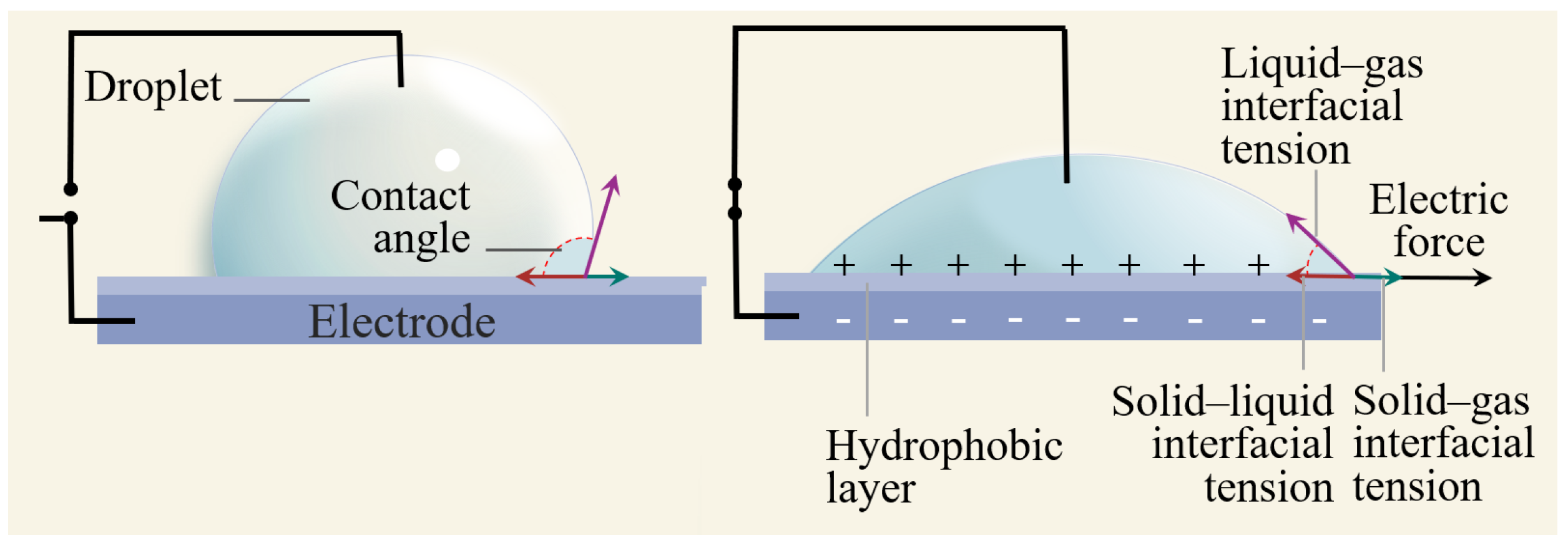
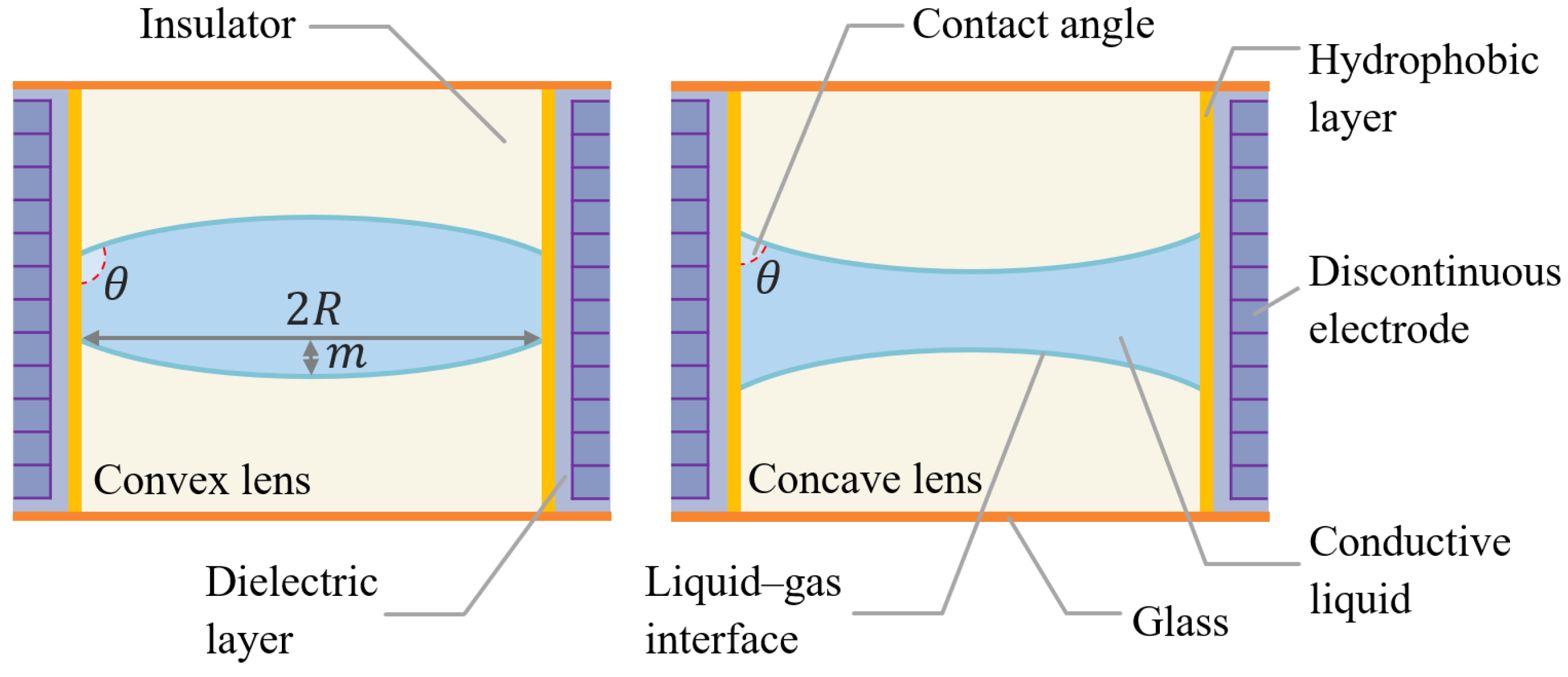
2.1. Schematic and Principle
2.2. LB-ED Numerical Method
2.2.1. The Multiple-Relaxation-Time LBM
2.2.2. Multiphase Model Based on Chemical-Potential
2.2.3. Electrodynamic Model
3. Model Verification
4. Results and Discussion
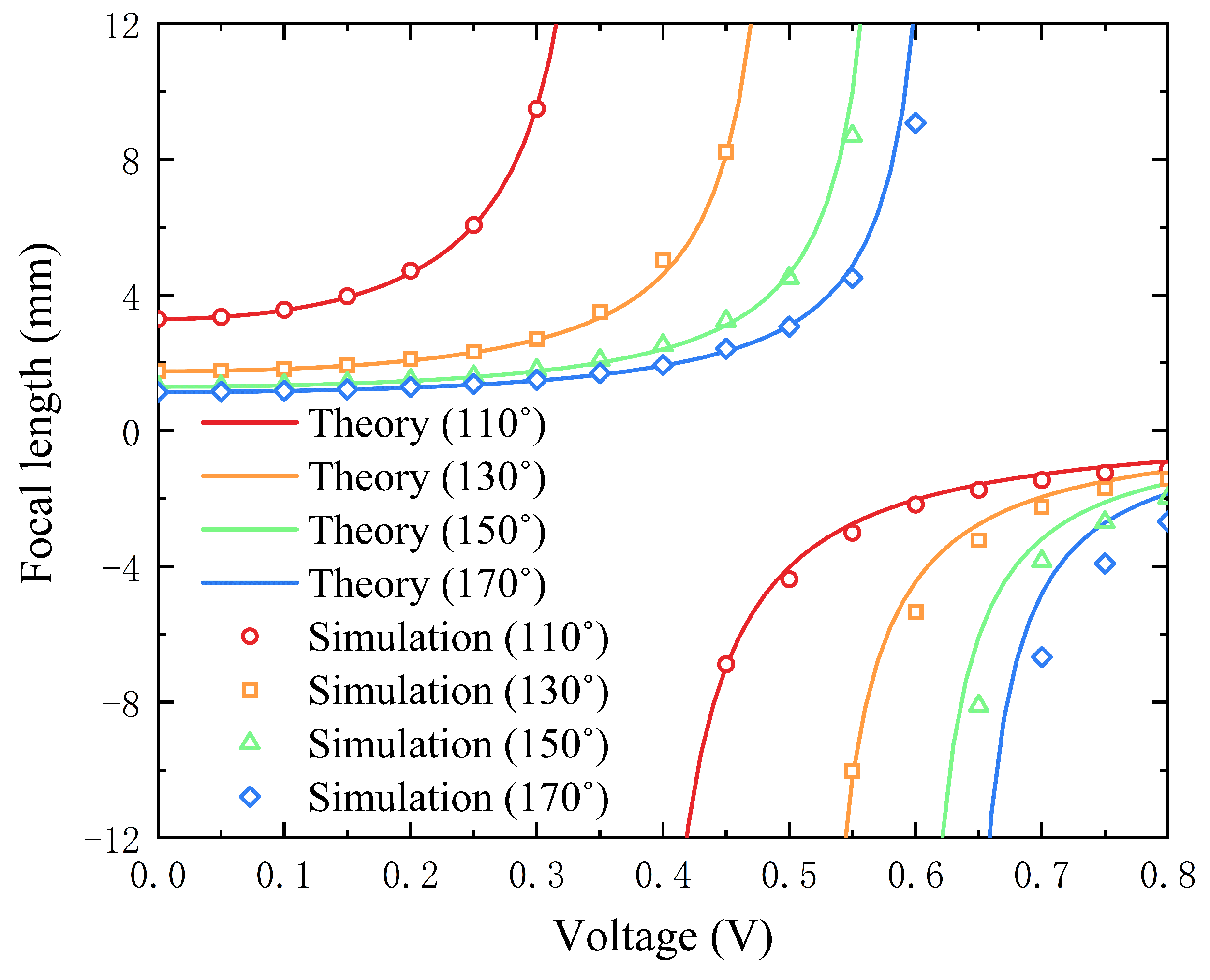
4.1. The Effect of Voltage on the Focal Length of the Lens
4.2. The Effect of Liquid Viscosity on the Performance of the Lens
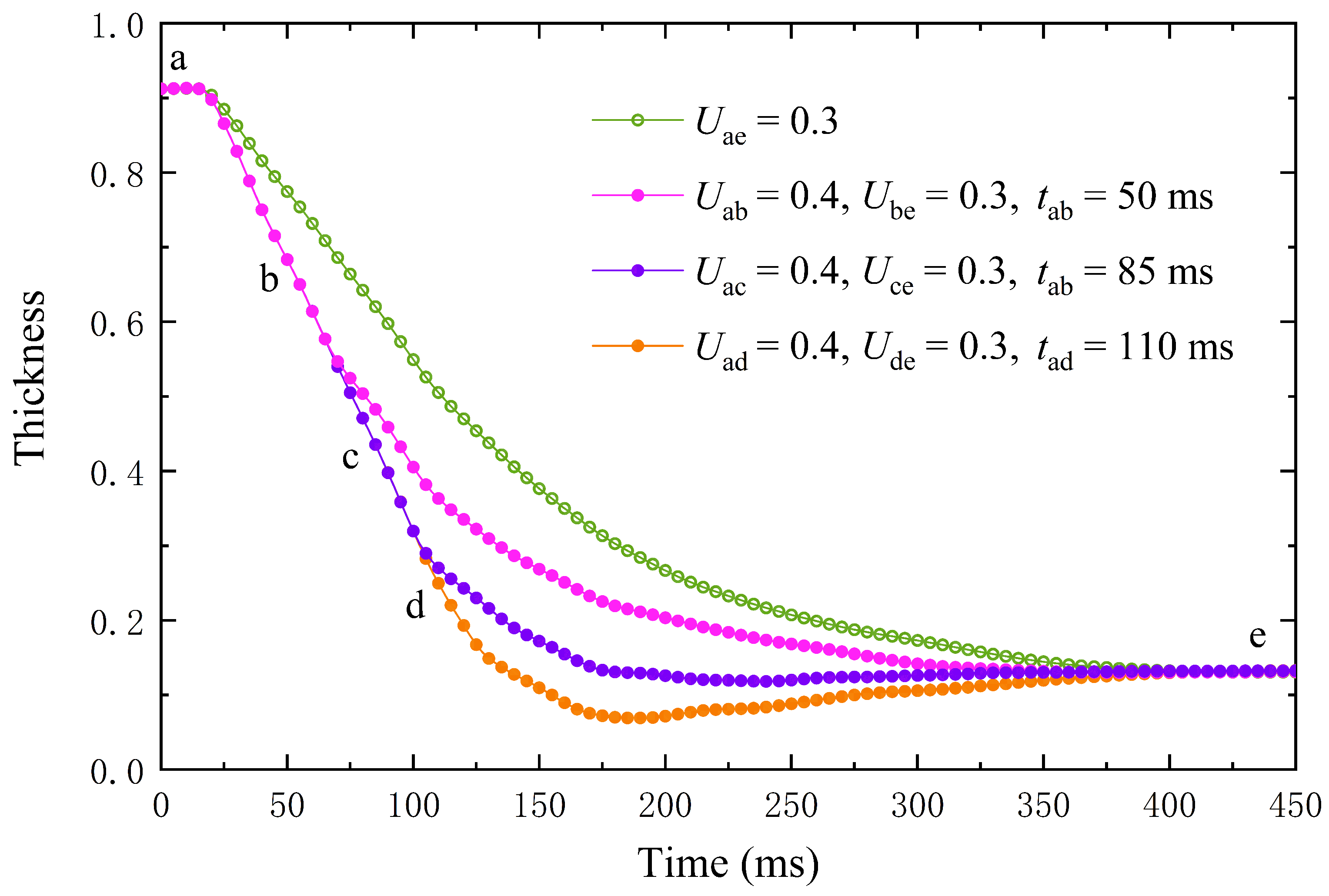
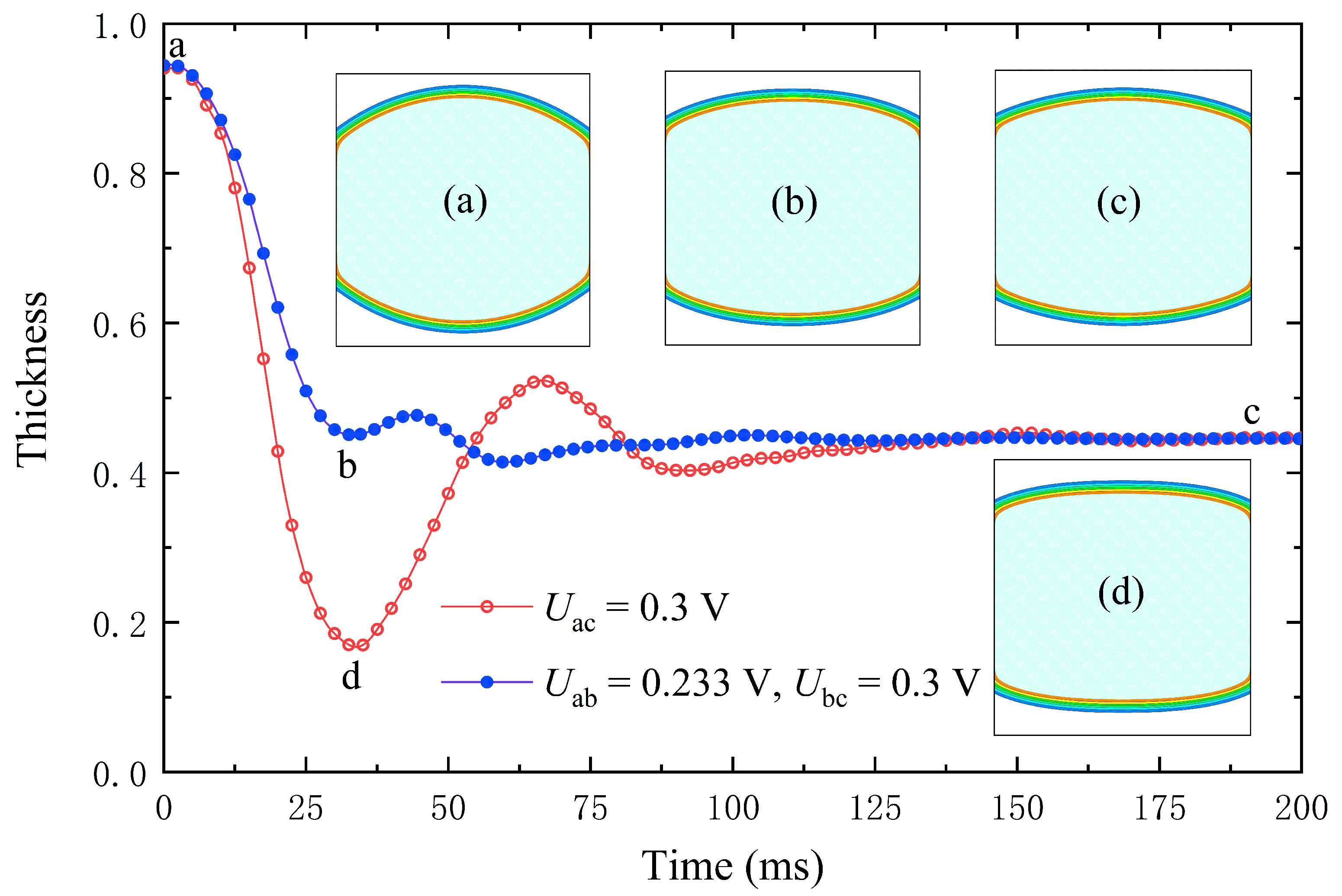
4.3. Accelerate Lens Zoom with an Overdrive Voltage
4.4. Acceleration Lens Zoom Is Driven by Undervoltage
4.5. Influence of Driving Voltage on the Moving Speed of the Lens
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kuiper, S.; Hendriks, B. Variable-focus liquid lens for miniature cameras. Appl. Phys. Lett. 2004, 85, 1128–1130. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Liu, Y.; Yuan, Y.; Lu, H.; Qiu, L. Variable-focus liquid lens based on electrically responsive fluid. Opt. Lett. 2022, 47, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Agarwal, A.K.; Beebe, D.J.; Jiang, H. Adaptive liquid microlenses activated by stimuli-responsive hydrogels. Nature 2006, 442, 551–554. [Google Scholar] [CrossRef] [PubMed]
- López, C.A.; Hirsa, A.H. Fast focusing using a pinned-contact oscillating liquid lens. Nat. Photonics 2008, 2, 610–613. [Google Scholar] [CrossRef]
- Miccio, L.; Memmolo, P.; Merola, F.; Netti, P.; Ferraro, P. Red blood cell as an adaptive optofluidic microlens. Nat. Commun. 2015, 6, 6502. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Guo, R.; Fei, C.; Li, D.; Chen, D.; Zheng, C.; Wu, R.; Feng, W.; Yang, Y. Liquid lens with adjustable focus for ultrasonic imaging. Appl. Acoust. 2021, 175, 107787. [Google Scholar] [CrossRef]
- Bosch, M.; Shcherbakov, M.R.; Won, K.; Lee, H.-S.; Kim, Y.; Shvets, G. Electrically actuated varifocal lens based on liquid-crystal-embedded dielectric metasurfaces. Nano Lett. 2021, 21, 3849–3856. [Google Scholar] [CrossRef]
- Xiong, J.; Hsiang, E.-L.; He, Z.; Zhan, T.; Wu, S.-T. Augmented reality and virtual reality displays: Emerging technologies and future perspectives. Light Sci. Appl. 2021, 10, 216. [Google Scholar] [CrossRef]
- Yin, K.; Hsiang, E.-L.; Zou, J.; Li, Y.; Yang, Z.; Yang, Q.; Lai, P.-C.; Lin, C.-L.; Wu, S.-T. Advanced liquid crystal devices for augmented reality and virtual reality displays: Principles and applications. Light Sci. Appl. 2022, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Fox, D.; Anderson, P.A.; Wu, B.; Wu, S.-T. Tunable-focus liquid lens controlled using a servo motor. Opt. Express 2006, 14, 8031–8036. [Google Scholar] [CrossRef]
- Ren, H.; Wu, S.T. Variable-focus liquid lens. Opt. Express 2007, 15, 5931–5936. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Zhou, Y.; Kuang, F.-L.; Li, L. Extended the depth of field and zoom microscope with varifocal lens. Sci. Rep. 2022, 12, 11015. [Google Scholar] [CrossRef]
- Ren, H.; Fan, Y.H.; Wu, S.-T. Liquid-crystal microlens arrays using patterned polymer networks. Opt. Lett. 2004, 29, 1608–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.C.; Chang, C.A.; Yeh, J.A. Variable focus dielectric liquid droplet lens. Opt. Express 2006, 14, 4101–4106. [Google Scholar] [CrossRef]
- Xu, S.; Ren, H.; Wu, S.-T. Dielectrophoretically tunable optofluidic devices. J. Phys. D Appl. Phys. 2013, 46, 483001. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.-H.; Wang, Q.-H.; Wu, S.-T. Displaceable and focus-tunable electrowetting optofluidic lens. Opt. Express 2018, 26, 25839–25848. [Google Scholar] [CrossRef]
- Li, L.; Xiao, L.; Wang, J.; Wang, Q. Movable electrowetting optofluidic lens for optical axial scanning in microscopy. Opto-Electron. Adv. 2019, 2, 180025-1–180025-5. [Google Scholar] [CrossRef]
- Liu, S.; Hua, H. Extended depth-of-field microscopic imaging with a variable focus microscope objective. Opt. Express 2011, 19, 353–362. [Google Scholar] [CrossRef]
- Sancataldo, G.; Scipioni, L.; Ravasenga, T.; Lanzanò, L.; Diaspro, A.; Barberis, A.; Duocastella, M. Three-dimensional multiple-particle tracking with nanometric precision over tunable axial ranges. Optica 2017, 4, 367–373. [Google Scholar] [CrossRef]
- Berge, B.; Peseux, J. Variable focal lens controlled by an external voltage: An application of electrowetting. Eur. Phys. E 2000, 3, 159–163. [Google Scholar] [CrossRef]
- Choi, D.-S.; Jeong, J.; Shin, E.-J.; Kim, S.-Y. Focus-tunable double convex lens based on non-ionic electroactive gel. Opt. Express 2017, 25, 20133–20141. [Google Scholar] [CrossRef]
- Song, X.; Zhang, H.; Li, D.; Jia, D.; Liu, T. Electrowetting lens with large aperture and focal length tunability. Sci. Rep. 2020, 10, 16318. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Zhao, L.; Qiu, W.; Ye, Y.; Shan, X. Chemical-potential multiphase lattice boltzmann method with superlarge density ratios. Phys. Rev. E 2020, 102, 013303. [Google Scholar] [CrossRef]
- Vallet, M.; Vallade, M.; Berge, B. Limiting phenomena for the spreading of water on polymer films by electrowetting. Eur. Phys. B-Condens. Matter Complex Syst. 1999, 11, 583–591. [Google Scholar] [CrossRef]
- Lippmann, G. Relations entre les phénomènes électriques et capillaires. Ann. Chim. Phys. 1875, 5, 494–549. [Google Scholar]
- Berge, B. Electrocapillarity and wetting of insulator films by water. Comptes Rendus Acad. Sci. Ser. II 1993, 317, 157–163. [Google Scholar]
- Vallet, M.; Berge, B.; Vovelle, L. Electrowetting of water and aqueous solutions on poly (ethylene terephthalate) insulating films. Polymer 1996, 37, 2465–2470. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ito, T.; Wakimoto, T.; Katoh, K. Numerical and theoretical analyses of the dynamics of droplets driven by electrowetting on dielectric in a hele-shaw cell. J. Fluid Mech. 2018, 839, 468–488. [Google Scholar] [CrossRef]
- Chen, S.; Doolen, G.D. Lattice boltzmann method for fluid flows. Annu. Rev. Fluid Mech. 1998, 30, 329–364. [Google Scholar] [CrossRef] [Green Version]
- Aidun, C.K.; Clausen, J.R. Lattice-boltzmann method for complex flows. Annu. Rev. Fluid Mech. 2010, 42, 439–472. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, P. A paradigm shift in liquid cooling by multitextured surface design. Innovation 2022, 3, 100222. [Google Scholar] [CrossRef]
- Lin, X.; Yang, F.; You, L.-X.; Wang, H.; Zhao, F. Liposoluble quinone promotes the reduction of hydrophobic mineral and extracellular electron transfer of shewanella oneidensis mr-1. Innovation 2021, 2, 100104. [Google Scholar] [CrossRef]
- Huang, C.; Shi, B.; Guo, Z.; Chai, Z. Multi-gpu based lattice boltzmann method for hemodynamic simulation in patient-specific cerebral aneurysm. Commun. Comput. Phys. 2015, 17, 960–974. [Google Scholar] [CrossRef]
- Lallemand, P.; Luo, L.-S. Theory of the lattice boltzmann method: Dispersion, dissipation, isotropy, galilean invariance, and stability. Phys. Rev. E 2000, 61, 6546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellar, P.J. An interpretation and derivation of the lattice boltzmann method using strang splitting. Comput. Math. Appl. 2013, 65, 129–141. [Google Scholar] [CrossRef]
- Luo, L.; Liao, W.; Chen, X.; Peng, Y.; Zhang, W. Numerics of the lattice boltzmann method: Effects of collision models on the lattice boltzmann simulations. Phys. Rev. E 2011, 83, 056710. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Zheng, C.; Shi, B. Discrete lattice effects on the forcing term in the lattice boltzmann method. Phys. Rev. E 2002, 65, 046308. [Google Scholar] [CrossRef]
- McCracken, M.E.; Abraham, J. Multiple-relaxation-time lattice-boltzmann model for multiphase flow. Phys. Rev. E 2005, 71, 036701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupershtokh, A.; Medvedev, D.; Karpov, D. On equations of state in a lattice boltzmann method. Comput. Math. Appl. 2009, 58, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.-H.; d’Humières, D.; Lallemand, P. Lattice bgk models for navier-stokes equation. EPL (Europhys. Lett.) 1992, 17, 479. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, C.; Tu, Y.; Wang, C.; Fang, H. Galilean invariant fluid–solid interfacial dynamics in lattice boltzmann simulations. J. Comput. Phys. 2014, 266, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Wen, B.; Qin, Z.; Zhang, C.; Fang, H. Thermodynamic-consistent lattice boltzmann model for nonideal fluids. Europhys. Lett. 2015, 112, 44002. [Google Scholar] [CrossRef]
- Wen, B.; Zhou, X.; He, B.; Zhang, C.; Fang, H. Chemical-potential-based lattice boltzmann method for nonideal fluids. Phys. Rev. E 2017, 95, 063305. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Qin, C.; Zhou, S.; Wen, B. Analysis of depinning behavior of drop on chemically heterogeneous surface. Phys. Rev. Fluids 2020, 5, 114003. [Google Scholar] [CrossRef]
- Wen, B.; Huang, B.; Qin, Z.; Wang, C.; Zhang, C. Contact angle measurement in lattice boltzmann method. Comput. Math. Appl. 2018, 76, 1686–1698. [Google Scholar] [CrossRef] [Green Version]
- Wen, B.; Zhang, C.; Fang, H. Hydrodynamic force evaluation by momentum exchange method in lattice boltzmann simulations. Entropy 2015, 17, 8240–8266. [Google Scholar] [CrossRef] [Green Version]
- Swift, M.R.; Osborn, W.R.; Yeomans, J.M. Lattice boltzmann simulation of nonideal fluids. Phys. Rev. Lett. 1995, 75, 830. [Google Scholar] [CrossRef] [Green Version]
- Rowlinson, J.S.; Widom, B. Molecular Theory of Capillarity; Clarendon: Oxford, UK, 1982. [Google Scholar]
- Yang, Z.; Liu, S.; Zhuo, C.; Zhong, C. Free-energy-based discrete unified gas kinetic scheme for van der waals fluid. Entropy 2022, 24, 1202. [Google Scholar] [CrossRef]
- Ghoderao, P.N.P.; Narayan, M.; Dalvi, V.H.; Byun, H.-S. Predictions of thermodynamic properties of pure fluids, refrigerants, and binary mixtures using modified peng-robinson equation of state. Korean J. Chem. Eng. 2022, 1–12. [Google Scholar] [CrossRef]
- He, B.; Qin, C.; Chen, W.; Wen, B. Numerical simulation of pulmonary airway reopening by the multiphase lattice boltzmann method. Comput. Math. Appl. 2022, 108, 196–205. [Google Scholar] [CrossRef]
- Butt, H.-J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gillespie, D.; Petsev, D.N.; van Swol, F. Electric double layers with surface charge regulation using density functional theory. Entropy 2020, 22, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.H.; Kang, I.S.; Lee, C.M. Electrostatic contribution to line tension in a wedge-shaped contact region. Langmuir 2003, 19, 9334–9342. [Google Scholar] [CrossRef]
- Flittner, R.; Přibyl, M. Computational fluid dynamics model of rhythmic motion of charged droplets between parallel electrodes. J. Fluid Mech. 2017, 822, 31–53. [Google Scholar] [CrossRef]
- Li, Z.-T.; Li, G.-J.; Huang, H.-B.; Lu, X.-Y. Lattice boltzmann study of electrohydrodynamic drop deformation with large density ratio. Int. J. Mod. Phys. C 2011, 22, 729–744. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Ghosh, A.; Pattader, P.S.G.; Bandyopadhyay, D. Electric field mediated von kármán vortices in stratified microflows: Transition from linear instabilities to coherent mixing. J. Fluid Mech. 2019, 865, 169–211. [Google Scholar] [CrossRef]
- Huang, H.; Krafczyk, M.; Lu, X. Forcing term in single-phase and shan-chen-type multiphase lattice boltzmann models. Phys. Rev. E 2011, 84, 046710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.K.; Moon, H.; Kim, C.-J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar]
- Jiang, Z.; Wang, D.; Zheng, Y.; Liu, C.; Wang, Q.-H. Continuous optical zoom microscopy imaging system based on liquid lenses. Opt. Express 2021, 29, 20322–20335. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-H.; Ren, H.; Xiao, L.; Wang, H.; Wu, S.-T. Liquid Crystal Microlens Arrays With Switchable Positive and Negative Focal Lengths. J. Disp. Technol. 2005, 1, 151–156. [Google Scholar] [CrossRef]
- Wu, S.-T. Nematic liquid crystal modulator with response time less than 100 μs at room temperature. Appl. Phys. Lett. 1990, 57, 986–988. [Google Scholar] [CrossRef]
- Pollack, M.G.; Fair, R.B.; Shenderov, A.D. Electrowetting-based actuation of liquid droplets for microfluidic applications. Appl. Phys. Lett. 2000, 77, 1725–1726. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Wei, S.; Qu, X.; Liu, F. Electrohydrodynamic analysis of electrowetting-on-dielectric (ewod)-induced transport of a microdroplet based on the lattice boltzmann method. AIP Adv. 2019, 9, 055021. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhuang, Z.; Qin, Z.; Wen, B. Movable and Focus-Tunable Lens Based on Electrically Controllable Liquid: A Lattice Boltzmann Study. Entropy 2022, 24, 1714. https://doi.org/10.3390/e24121714
Wang F, Zhuang Z, Qin Z, Wen B. Movable and Focus-Tunable Lens Based on Electrically Controllable Liquid: A Lattice Boltzmann Study. Entropy. 2022; 24(12):1714. https://doi.org/10.3390/e24121714
Chicago/Turabian StyleWang, Fei, Zijian Zhuang, Zhangrong Qin, and Binghai Wen. 2022. "Movable and Focus-Tunable Lens Based on Electrically Controllable Liquid: A Lattice Boltzmann Study" Entropy 24, no. 12: 1714. https://doi.org/10.3390/e24121714





