Breaking Barriers in Emerging Biomedical Applications
Abstract
1. Introduction
2. Emerging Biomedical Applications
2.1. Use Cases
2.1.1. In-Body Sensors
- Glucose Sensors (GS): These are widely used across the globe. They are used to measure the blood glucose concentration and help patients cope with diabetes mellitus. In recent years a new type of GS has been developed. Such sensors are typically implanted under the skin [4], featuring an interface circuit while they offer continuous measurement and monitoring of blood glucose in patients with diabetes. A GS will typically transmit at a rate below 1 kbps with expected latency under 150 ms. According to the World Health Organization, the number of people with diabetes rose from 108 million (approximately 2.4% of the entire population) in 1980 to 422 million (approximately 5.8% of the entire population) in 2014, while in 2019 diabetes was the ninth leading cause of death with an estimated 1.5 million deaths directly caused by diabetes [5]. It is worth noting that diabetes is found to be significantly more common in urban than rural areas [6,7,8]. This means that in urban areas, the network must be able to cope with a high density of users, causing significant traffic to the network.
- Pacemaker: Cardiovascular diseases are among the most common diseases across the world. Pacemakers have been invented to sense irregularities in heart beating and to send a signal to the heart that makes it beat at the correct pace. A pacemaker is a generally small, battery-operated device. The typical data rate expected to be transmitted is below 1 kbps for a 12-bit pacemaker with a 500 Hz sampling rate. The latency expected for a pacemaker should be less than 150 ms. According to [8], cardiovascular diseases are the leading cause of death globally, taking an estimated 17.9 million lives each year. According to [9], rural residents experience higher death rates compared with residents of urban areas. In many cases, the cause of this death could have been prevented [10]. A pacemaker along with an ECG can prove lifesaving, offering vital information about the state of the heart. As with the glucose sensor, it is expected that IoT networks in urban areas will be required to support this critical data in real-time, while in some cases priority will be given to people in critical conditions. At the same time, it is expected that this network will have to handle the traffic found in urban densely populated areas.
- Capsule Endoscopy (CE): This has been possible thanks to modern miniaturized electronics and Ultra-Wideband (UWB) communications that enable the investigation of the small bowel providing a non-invasive, well-tolerated means of accurately visualizing the distal duodenum, jejunum, and ileum [11]. CE is typically used when flexible endoscopy fails to identify a diagnosis. It consists of a capsule for imaging, a device for receiving and storing data, a computer, and software. The capsule itself consists of a light source, lens, battery, and a transmission device. A CE would typically require 1 Mbps of data rate with less than 150 ms of latency.
2.1.2. On-Body Sensors
- Electromyography (EMG): This measures the electrical activity of muscles by recording the electrical impulses that muscles produce. The nerve conduction test measures the speed at which impulses travel along a nerve during rest, slight contraction, and forceful contraction. A 12-channel EMG requires 1.536 Mbps of data rate at 8 kHz of sampling rate. The latency should be less than 250 ms. This implies that the communication channel carrying this sort of data will have to have enough bandwidth to meet these transmission requirements. Such application can be used to examine changes in muscle activity during acute and subacute phases of stroke recovery [12].
- ECG: As mentioned before, cardiovascular diseases are among the most common and life-threatening diseases across the world. People who suffer from cardiovascular diseases are usually fitted with an ECG, which measures changes in electrical signals on different areas of skin. Such signals are basically electrical and chemical signals used to enable communication between our nerve and muscle cells. Regular electrical signals also control our heartbeat. An ECG is mainly used for recording how often the heart beats (heart rate) and how regularly it beats (heart rhythm). In some cases, during the monitoring process, patients are encouraged to continue their daily routines in order to be able to capture possible irregularities of the heartbeat. A 12-channel ECG requires 72 kbps data rate to communicate recorded data at a sampling rate of 500 Hz and latency of less than 250 ms. As with the EMG, the communication channel required for carrying this sort of data need to have enough bandwidth to meet the transmission requirements.
- Temperature, Heart Rate (HR), Blood Pressure (BP), Blood Oxygen (BO): The main vital signs of the human body such as Temperature, HR, BP, and BO are associated with a single value that is not expected to change rapidly within a given time. Thus, the data rates required for such applications are expected to be below 10 kbps employing low power channels for the data transmission. Vital signs monitoring provides an easy way for the early detection of various diseases. The subtle variation of vital signs, such as the core body temperature, can be a significant indicator for older patients as it often indicates a more severe infection and is associated with increased rates of life-threatening consequences [13].
2.1.3. Intelligent Things in Smart Hospitals
- Automated Medicine Dispenser: Automated dispensing machines are used to securely store medication on patient care units. They also feature electronic tracking of the use of narcotics and other controlled drugs. These machines can save nursing time by eliminating a need for manual end-of-shift narcotic counts in the patient care units. Another clinical feature of automated dispensing machines is the capability to track and proactively monitor drug usage patterns. This is accomplished by setting up clinical indicators during the removal of specified drugs [14].
- Urine Monitoring System: According to [15], a urine monitoring device has been designed and implemented for monitoring postoperative urination. This device has been designed to reduce the burden of the nursing staff required to regularly monitor and empty the urine bags as well as to provide crucial information about the rate of flow of urine in real time that is vital information for postoperative urination. Authors have implemented this using WiFi, but this assumes adequate WiFi coverage across the hospital. Using Low Power Wide Area Network (LP-WAN) type of technology should alleviate this burden and enable the mass deployment of such low-cost devices in a hospitalized environment.
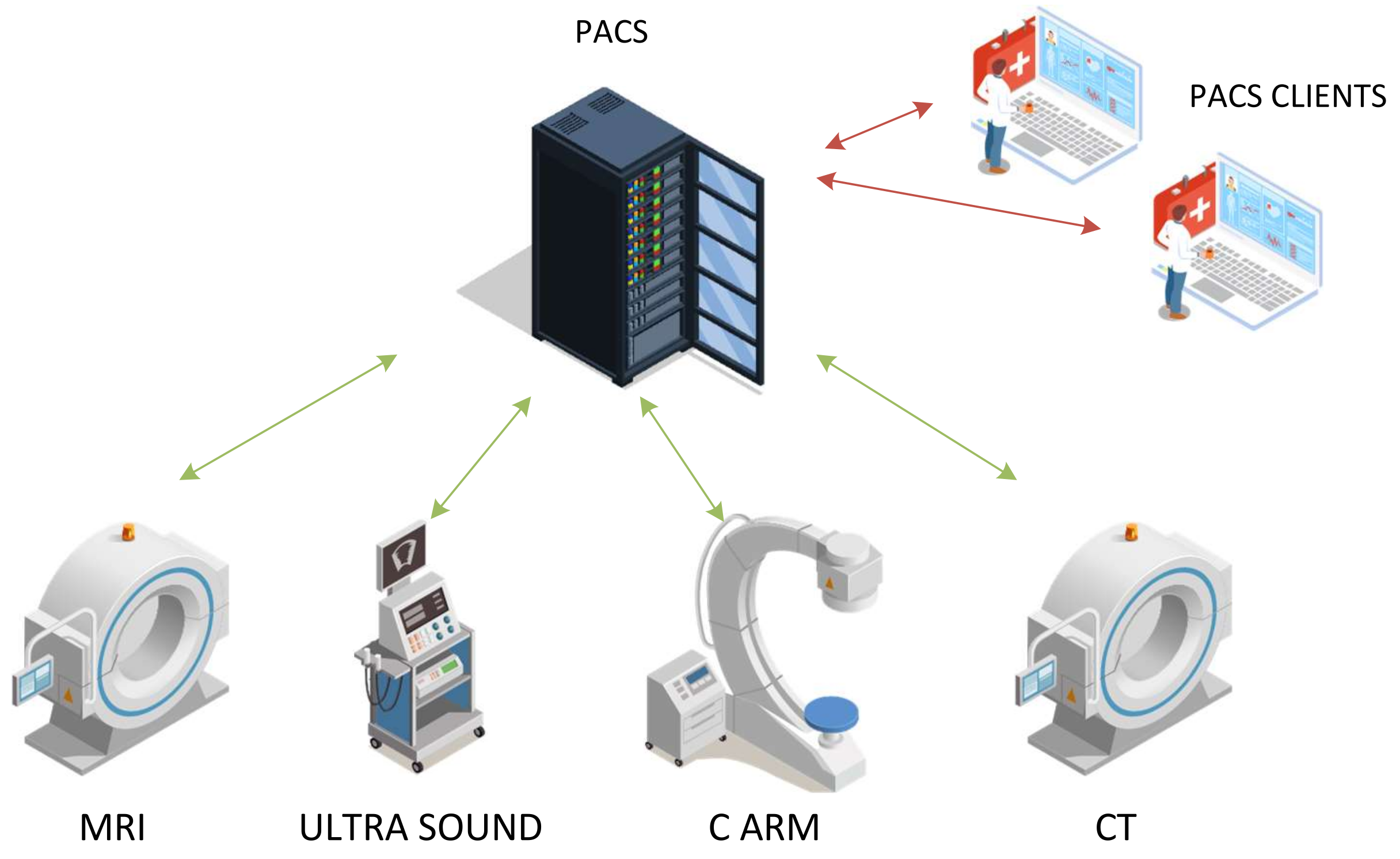
- Intensive Care Unit (ICU): An ICU is a true example where information technology and clinical informatics are used to acquire, process, and transform data into actionable information. Furthermore, it is required that this information disseminates effectively to improve patient care. Intensive care in ICUs involves highly complex decision making based on data [16]. In general, this is real-time data and the whole decision-making mechanism must provide decisions in real time. The basic approach of collecting and managing the data involves communicating data from many disparate sensors into a database/system (possibly located at the hospital). The system must be reliable, resilient, and responsive to rapid changes recorded by the sensors. For the ICU scenario, we consider that some of these devices can be connected using the IoT network available at the hospital, especially in emergency erected ICUs such as the one’s setup during the pandemic [17]. Devices (sensors/actuators) used in an ICU are automated medicine dispenser, the infusion monitoring system, ECG, vital signs, respiratory ventilator, syringe pump, infusion pump, etc.According to [18], to connect intelligent things using Narrow Band Internet of Things (NB-IoT) architecture, there are still numerous challenges to be addressed. Such challenges are the limited accuracy and reliability of data collection, which is a major challenge in the building of smart hospitals. Furthermore, security is a general challenge since the IoT network will have to handle sensitive/critical data. The encryption mechanism of terminal devices must employ an encryption algorithm and key management mechanism to strengthen authentication.
2.2. Communication Requirements and Network Limitations
3. Communication Standards in Medical Applications
- Low Rate Wireless Personal Area Network (LR-WPAN);
- Low Power Wide Area Network (LP-WAN).
3.1. LR-WPAN
3.2. LP-WAN
- NB-IoT is envisioned to support Wireless Sensor Networks (WSN) within legacy cellular networks. Over time, some of the older generations of cell networks have become outdated. NB-IoT is expected to further increase their life time and bring new business models with a simple software update of the network infrastructure. These networks have narrow bandwidth of only 180 kHz which can be assigned within LTE guard band. Although such narrow bandwidth supports lower data rates, at the same time they provide extended coverage and reduced power consumption [40].
- Long Term Evolution-Machine Type Communications (LTE CAT-M) [41] enables connectivity of IoT devices with reduced complexity of the device. This technology can be implemented on the already existing LTE base stations. Nevertheless, it supports longer communication range and longer battery lifetime. Besides being based on the currently available mobile networks, LTE CAT-M has enhanced security and privacy, which is especially important when dealing with patient’s health data. It uses 1.4 MHz bandwidth in contrast to regular LTE which used 20 MHz, and supports the data transfer speeds of up to 1 Mbps, which is particularly suitable for healthcare monitoring that need higher data rates.
- Extended Coverage GSM Internet of Things (EC-GSM-IoT) represents the standard that operates within GSM frequency bands which is based on eGPRS [42]. It is capable of providing extended range with higher energy efficiency for IoT applications. Since this technology is based on the legacy GSM networks, their lifetime can be effectively extended and bring new business opportunities to the mobile network operators. It provides maximum data throughput of 240 kbps while requiring 200 kHz of bandwidth. Similarly to NB-IoT technology, this is suitable for healthcare applications requiring lower data rates. The battery life of connected devices is expected to be up to 10 years.
- Long Range Wide Area Network (LoRaWAN) operates in sub-GHz frequency band and employs a proprietary spread spectrum modulation technique. It has been designed with the aim to support the mobile and fixed devices that are powered with batteries, where the energy efficiency is one of the most important aspects. In contrast to ZigBee, LoRaWAN is based on a star topology where different gateways communicate with the network nodes. It envisions three device types, based on the way they communicate with the network. More specifically, Class A enables bidirectional communication between network node and a gateway. For the uplink transmission, the data is randomly transmitted, whereas for the downlink, the receiver is turned on 1 and 2 s after the uplink transmission. Class B works in a similar manner where the receiving window is scheduled, while Class C enables bi-directional communication with low latency by allowing the receiving windows to be open at any time. LoRaWAN allows data rates up to 37.5 kbps [43] and transmission range of about 30 km, where these depend on the transmitter configuration, such as Tx power, signal bandwidth, spreading factor and propagation channel characteristics [44].
- SigFox represents a communication technology based on Shift Keying (DBPSK) and Gaussian Frequency Shift Keying (GFSK). It uses only 100 Hz out of total 192 kHz of total spectrum. Besides this, Sigfox message payload is limited to 12 bytes, with a limit of only 140 messages per day. In Europe, it operates on 868 MHz, whereas in North America a 902 MHz band is allocated for its use. The data received by the gateways are collected on the SigFox cloud servers and made available to the end users through an Application Programming Interface (API) or web-based interface. The limits of the number of messages per day and message size makes it suitable for non-critical scenarios with less frequent measurements [45].
- INGENU operates on ISM 2.4 GHz frequency band, which allows for wider usage since this band is less regulated worldwide. For the uplink communication, it employs Random Phase Multiple Access (RPMA) Direct Sequence Spread Spectrum (DSSS) as the transmission scheme. By using such an approach, multiple transmitters are able to share one time slot. Since each RPMA channel takes only 1 MHz of bandwidth, it is possible to have 40 uplink and downlink channels within 2.4 GHz spectrum. INGENU supports data rate of 19.5 kbps for downlink and 78 kbps for uplink. Transmission can reach up to 3 km in urban and up to 15 km in rural environments [46]. For security, it employs AES 256 bit encryption.
- WEIGHTLESS is a protocol that enables two-way communication by using PSK/GMSK and O-QPSK modulation with spread spectrum [47]. Similarly to other LP-WAN communication standards, it also uses sub-GHz frequency bands that do not require license. It supports adaptive bit rate from 625 bps to 100 kbps, while its channels are only 12.5 kHz narrow [48]. In order to optimize on the network capacity, WEIGHTLESS uses control of power in uplink and downlink directions. Finally, in order to enable secure transmission of data, it employs AES encryption with 128 and 256 bits.
4. Advanced Concepts in IoT for Biomedical Applications
5. Compression as the Past and Future of Medical Information
5.1. Piling the Cardiovascular Data
5.2. Medical Image Compression
5.3. Future Medical Practices and Paradigms
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAL | Ambient Assisted Living |
| ACR | American College of Radiology |
| AES | Advanced Encryption Standard |
| AI | Artificial Intelligence |
| API | Application Programming Interface |
| CE | Capsule Endoscopy |
| CT | Computed Tomography |
| GS | Glucose Sensors |
| BDA | Big Data Analytics |
| BLE | Bluetooth Low Energy |
| BP | Blood Pressure |
| BO | Blood Oxygen |
| CAR | Canadian Association of Radiologists |
| DAIC | Diagnostically Acceptable Irreversible Compression |
| DBPSK | Differential Binary Phase-Shift Keying |
| DICOM | Digital Imaging and Communication in Medicine |
| DSSS | Direct Sequence Spread Spectrum |
| EaaS | Entropy-as-a-Service |
| EC-GSM-IoT | Extended Coverage GSM Internet of Things |
| ECG | Electrocardiogram |
| EMG | Electromyography |
| ESI | Emergency Safety Index |
| ESR | European Society of Radiology |
| FDA | Food and Drug Administration |
| GFSK | Gaussian Frequency Shift Keying |
| GSM | Global System for Mobile communications |
| HR | Heart Rate |
| ICU | Intensive Care Unit |
| ISM | Industrial, Scientific and Medical |
| IoT | Internet of Things |
| LoRaWAN | Long Range Wider Area Network |
| LR-WPAN | Low Rate Wireless Personal Area Network |
| LP-WAN | Low Power Wide Area Network |
| LTE | Long Term Evolution |
| LTE CAT-N | Long Term Evolution Category N |
| LTE CAT-M | Long Term Evolution-Machine Type Communications |
| ML | Machine Learning |
| M2M | Machine to Machine |
| NB-IoT | Narrow Band Internet of Things |
| NFC | Near Field Communication |
| NB-IoT | Narrow Band Internet of Things |
| NEMA | National Electrical Manufacturers Association |
| PACS | Picture Archiving and Communication System |
| PHD | Personal Health Dashboard |
| PPG | Photoplethysmography |
| PPPM | Predictive, Preventing and Personalised Medicine |
| RCR | Royal College of Radiologists |
| RPMA | Random Phase Multiple Access |
| QoS | Quality of Service |
| SDN | Software Defined Networking |
| WBAN | Wireless Body Area Network |
| WPAN | Wireless Personal Area Network |
| WSN | Wireless Sensor Networks |
| MRI | Magnetic Resonance Imaging |
| URLLC | Ultra-Reliable Low-Latency Communications |
| US | Ultrasound |
| UWB | Ultra-Wideband |
References
- Queirós, A.; Dias, A.; Silva, A.G.; Rocha, N.P. Ambient Assisted Living and Health-Related Outcomes—A Systematic Literature Review. Informatics 2017, 4, 19. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Movassaghi, S.; Abolhasan, M.; Lipman, J.; Smith, D.; Jamalipour, A. Wireless Body Area Networks: A Survey. IEEE Commun. Surv. Tutor. 2014, 16, 1658–1686. [Google Scholar] [CrossRef]
- Zhang, J.X.; Hoshino, K. Chapter 8—Implantable and wearable sensors. In Molecular Sensors and Nanodevices, 2nd ed.; Micro and Nano Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 489–545. [Google Scholar] [CrossRef]
- Diabetes. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 19 December 2021).
- Bragg, F.; Holmes, M.V.; Iona, A.; Guo, Y.; Du, H.; Chen, Y.; Bian, Z.; Yang, L.; Herrington, W.; Bennett, D.; et al. Association Between Diabetes and Cause-Specific Mortality in Rural and Urban Areas of China. JAMA 2017, 317, 280–289. [Google Scholar] [CrossRef]
- Shera, A.; Jawad, F.; Maqsood, A. Prevalence of diabetes in Pakistan. Diabetes Res. Clin. Pract. 2007, 76, 219–222. [Google Scholar] [CrossRef] [PubMed]
- The Burden of Diabetes in Rural America. 2016. Available online: https://www.ruralhealthresearch.org/projects/100002380 (accessed on 12 December 2021).
- Cross, S.H.; Mehra, M.R.; Bhatt, D.L.; Nasir, K.; O’Donnell, C.J.; Califf, R.M.; Warraich, H.J. Rural-Urban Differences in Cardiovascular Mortality in the US, 1999–2017. JAMA 2020, 323, 1852–1854. [Google Scholar] [CrossRef]
- Garcia, M.C.; Rossen, L.M.; Bastian, B.; Faul, M.; Dowling, N.F.; Thomas, C.C.; Schieb, L.; Hong, Y.; Yoon, P.W.; Iademarco, M.F.; et al. Potentially Excess Deaths from the Five Leading Causes of Death in Metropolitan and Nonmetropolitan Counties—United States, 2010–2017. MMWR Surveill. Summ. 2019, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.F.; Sidhu, R.; McAlindon, M.E. Capsule endoscopy: Current practice and future directions. World J. Gastroenterol. 2014, 20, 7752–7759. [Google Scholar] [CrossRef]
- Feldner, H.A.; Papazian, C.; Peters, K.M.; Creutzfeldt, C.J.; Steele, K.M. Clinical Use of Surface Electromyography to Track Acute Upper Extremity Muscle Recovery after Stroke: A Descriptive Case Study of a Single Patient. Appl. Syst. Innov. 2021, 4, 32. [Google Scholar] [CrossRef]
- Chester, J.G.; Rudolph, J.L. Vital Signs in Older Patients: Age-Related Changes. J. Am. Med Dir. Assoc. 2011, 12, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Leung, B.; Hamilton, D.; Hope, J. Do Automated Dispensing Machines Improve Patient Safety? Can. J. Hosp. Pharm. 2009, 62, 516–517. [Google Scholar] [CrossRef] [PubMed]
- Katzis, K.; Despotou, G.; Jones, R.W. UMOD: A Device for Monitoring Postoperative Urination. Data Inform. Technol. Inspir. Improv. Healthc. 2018, 251, 67–70. [Google Scholar] [CrossRef]
- De Georgia, M.A.; Kaffashi, F.; Jacono, F.J.; Loparo, K.A. Information Technology in Critical Care: Review of Monitoring and Data Acquisition Systems for Patient Care and Research. Sci. World J. 2015, 2015, 727694. [Google Scholar] [CrossRef]
- Candel, F.J.; Canora, J.; Zapatero, A.; Barba, R.; González Del Castillo, J.; García-Casasola, G.; San-Román, J.; Gil-Prieto, R.; Barreiro, P.; Fragiel, M.; et al. Temporary hospitals in times of the COVID pandemic. An example and a practical view. Rev. Esp. Quimioter. 2021, 34, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Wen, B.; Xun, Y.; Liu, J. Connecting Intelligent Things in Smart Hospitals Using NB-IoT. IEEE Internet Things J. 2018, 5, 1550–1560. [Google Scholar] [CrossRef]
- Global IoT Market to Grow to 24.1 Billion Devices in 2030, Generating 1.5 Trillion USD Annual Revenue. Transforma Insights. 2020. Available online: https://www.prnewswire.com/news-releases/global-iot-market-will-grow-to-24-1-billion-devices-in-2030–generating-1-5-trillion-annual-revenue-301061873.html (accessed on 12 December 2021).
- Soós, G.; Ficzere, D.; Varga, P. The Pursuit of NB-IoT Transmission Rate Limitations by Real-life Network Measurements. In Proceedings of the 2020 43rd International Conference on Telecommunications and Signal Processing (TSP), Milan, Italy, 7–9 July 2020; pp. 430–434. [Google Scholar] [CrossRef]
- Reverberi, L.; Oswald, D. Breaking (and Fixing) a Widely Used Continuous Glucose Monitoring System. In 11th USENIX Workshop on Offensive Technologies (WOOT 17); USENIX Association: Vancouver, BC, Canada, 2017. [Google Scholar]
- Nikita, K.S. Biosensor Communication Technology and Standards. In Handbook of Biomedical Telemetry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 330–367. [Google Scholar] [CrossRef]
- DS1624 Digital Thermometer and Memory: Maxim Integrated. 2021. Available online: https://www.maximintegrated.com/en/products/sensors/DS1624.html (accessed on 12 December 2021).
- MAX30100 Pulse Oximeter and Heart-Rate Sensor IC for Wearable Health. 2021. Available online: https://www.maximintegrated.com/en/products/sensors/MAX30100.html (accessed on 12 December 2021).
- MAX32664 Ultra-Low Power Biometric Sensor Hub: Maxim Integrated. 2021. Available online: https://www.maximintegrated.com/en/products/interface/signal-integrity/MAX32664.html (accessed on 12 December 2021).
- Dittgen, A. Logements et taille des ménages dans la dynamique des populations locales. L’exemple de Paris. Population 2005, 60, 307–347. [Google Scholar] [CrossRef]
- Baker, S.B.; Xiang, W.; Atkinson, I. Internet of Things for Smart Healthcare: Technologies, Challenges, and Opportunities. IEEE Access 2017, 5, 26521–26544. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Song, H.; Cao, X. Ubiquitous WSN for Healthcare: Recent Advances and Future Prospects. IEEE Internet Things J. 2014, 1, 311–318. [Google Scholar] [CrossRef]
- Alam, M.M.; Malik, H.; Khan, M.I.; Pardy, T.; Kuusik, A.; Le Moullec, Y. A Survey on the Roles of Communication Technologies in IoT-Based Personalized Healthcare Applications. IEEE Access 2018, 6, 36611–36631. [Google Scholar] [CrossRef]
- Darroudi, S.M.; Gomez, C.; Crowcroft, J. Bluetooth Low Energy Mesh Networks: A Standards Perspective. IEEE Commun. Mag. 2020, 58, 95–101. [Google Scholar] [CrossRef]
- Elsts, A.; Fafoutis, X.; Woznowski, P.; Tonkin, E.; Oikonomou, G.; Piechocki, R.; Craddock, I. Enabling Healthcare in Smart Homes: The SPHERE IoT Network Infrastructure. IEEE Commun. Mag. 2018, 56, 164–170. [Google Scholar] [CrossRef]
- Ugrenovic, D.; Gardasevic, G. CoAP Protocol for Web-based Monitoring in IoT Healthcare Applications. In Proceedings of the 2015 23rd Telecommunications Forum Telfor (TELFOR), Belgrade, Serbia, 24–26 November 2015; pp. 79–82. [Google Scholar]
- Jara, A.J.; Lopez, P.; Fernandez, D.; Zamora, M.A.; Ubeda, B.; Skarmeta, A.F. Communication Protocol for Enabling Continuous Monitoring of Elderly People through Near Field Communications. Interact. Comput. 2014, 26, 145–168. [Google Scholar] [CrossRef]
- Monge, J.; Postolache, O.; Plopa, O.; Trandabat, A.; Schreiner, O.; Schreiner, T. Glucose Detection in Sweat using Biosensors. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Hasan, R.R.; Rahman, M.A.; Sinha, S.; Uddin, M.; Niloy, T.S.R. In Body Antenna for Monitoring Pacemaker. In Proceedings of the 2019 International Conference on Automation, Computational and Technology Management (ICACTM), London, UK, 24–26 April 2019; pp. 99–102. [Google Scholar] [CrossRef]
- Takizawa, K.; Aoyagi, T.; Hamaguchi, K.; Kohno, R. Performance Evaluation of Wireless Communications through Capsule Endoscope. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 6897–6900. [Google Scholar] [CrossRef]
- Gardašević, G.; Katzis, K.; Bajić, D.; Berbakov, L. Emerging Wireless Sensor Networks and Internet of Things Technologies—Foundations of Smart Healthcare. Sensors 2020, 20, 3619. [Google Scholar] [CrossRef]
- Green, R.; Asili, M.; Topsakal, E. Wireless Smartphone Communication for Medical Telemetry Systems. In Proceedings of the 2013 US National Committee of URSI National Radio Science Meeting (USNC-URSI NRSM), Boulder, CO, USA, 9–12 January 2013; p. 1. [Google Scholar] [CrossRef]
- Cao, T.; Tao, L.; Liu, D.; Wang, Q.; Sun, J. Design and Realization of Blood Oxygen and Heart Rate Sensor Nodes in Wireless Body Area Network. In Proceedings of the 2020 IEEE International Conference on Artificial Intelligence and Computer Applications (ICAICA), Dalian, China, 27–29 June 2020; pp. 469–473. [Google Scholar] [CrossRef]
- Migabo, E.M.; Djouani, K.D.; Kurien, A.M. The Narrowband Internet of Things (NB-IoT) Resources Management Performance State of Art, Challenges, and Opportunities. IEEE Access 2020, 8, 97658–97675. [Google Scholar] [CrossRef]
- Sultan, R.; Refaey, A.; Hamouda, W. Resource Allocation in CAT-M and LTE-A Coexistence: A Joint Contention Bandwidth Optimization Scheme. In Proceedings of the 2020 IEEE Canadian Conference on Electrical and Computer Engineering (CCECE), London, ON, Canada, 30 August–2 September 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Elsaadany, M.; Ali, A.; Hamouda, W. Cellular LTE-A Technologies for the Future Internet-of-Things: Physical Layer Features and Challenges. IEEE Commun. Surv. Tutor. 2017, 19, 2544–2572. [Google Scholar] [CrossRef]
- Lavric, A.; Popa, V. Performance Evaluation of LoRaWAN Communication Scalability in Large-Scale Wireless Sensor Networks. Wirel. Commun. Mob. Comput. 2018, 2018, 6730719. [Google Scholar] [CrossRef]
- Haxhibeqiri, J.; De Poorter, E.; Moerman, I.; Hoebeke, J. A Survey of LoRaWAN for IoT: From Technology to Application. Sensors 2018, 18, 3995. [Google Scholar] [CrossRef] [PubMed]
- Olatinwo, D.D.; Abu-Mahfouz, A.; Hancke, G. A Survey on LPWAN Technologies in WBAN for Remote Health-Care Monitoring. Sensors 2019, 19, 5268. [Google Scholar] [CrossRef]
- Goursaud, C.; Gorce, J.M. Dedicated Networks for IoT: PHY/MAC State of the Art and Challenges. EAI Endorsed Trans. Internet Things 2015. [Google Scholar] [CrossRef]
- WEIGHTLESS Website. Available online: http://www.weightless.org/ (accessed on 12 December 2021).
- Raza, U.; Kulkarni, P.; Sooriyabandara, M. Low Power Wide Area Networks: An Overview. IEEE Commun. Surv. Tutor. 2017, 19, 855–873. [Google Scholar] [CrossRef]
- Habibzadeh, H.; Dinesh, K.; Rajabi Shishvan, O.; Boggio-Dandry, A.; Sharma, G.; Soyata, T. A Survey of Healthcare Internet of Things (HIoT): A Clinical Perspective. IEEE Internet Things J. 2020, 7, 53–71. [Google Scholar] [CrossRef]
- Ranchal, R.; Bastide, P.; Wang, X.; Gkoulalas-Divanis, A.; Mehra, M.; Bakthavachalam, S.; Lei, H.; Mohindra, A. Disrupting Healthcare Silos: Addressing Data Volume, Velocity and Variety With a Cloud-Native Healthcare Data Ingestion Service. IEEE J. Biomed. Health Inform. 2020, 24, 3182–3188. [Google Scholar] [CrossRef]
- Goecks, J.e.a. How Machine Learning Will Transform Biomedicine. Cell 2020, 181, 92–101. [Google Scholar] [CrossRef]
- Bharadwaj, H.K.; Agarwal, A.; Chamola, V.; Lakkaniga, N.R.; Hassija, V.; Guizani, M.; Sikdar, B. A Review on the Role of Machine Learning in Enabling IoT Based Healthcare Applications. IEEE Access 2021, 9, 38859–38890. [Google Scholar] [CrossRef]
- Abdulkareem, K.H.; Mohammed, M.A.; Gunasekaran, S.S.; Al-Mhiqani, M.N.; Mutlag, A.A.; Mostafa, S.A.; Ali, N.S.; Ibrahim, D.A. A Review of Fog Computing and Machine Learning: Concepts, Applications, Challenges, and Open Issues. IEEE Access 2019, 7, 153123–153140. [Google Scholar] [CrossRef]
- Alarsan, F.; Younes, M. Analysis and Classification of Heart Diseases using Heartbeat Features and Machine Learning Algorithms. J. Big Data 2019, 6, 81. [Google Scholar] [CrossRef]
- Alfaras, M.; Soriano, M.C.; Ortín, S. A Fast Machine Learning Model for ECG-based Heartbeat Classification and Arrhythmia Detection. Front. Phys. 2019, 7, 103. [Google Scholar] [CrossRef]
- Moody, G.; Mark, R. The Impact of the MIT-BIH Arrhythmia Database. IEEE Eng. Med. Biol. Mag. 2001, 20, 45–50. [Google Scholar] [CrossRef]
- Zipes, D.P.; Camm, A.J.; Borggrefe, M.; Buxton, A.E.; Chaitman, B.; Fromer, M.; Gregoratos, G.; Klein, G.; Moss, A.J.; Myerburg, R.J.; et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—Executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur. Heart J. 2006, 27, 2099–2140. [Google Scholar] [CrossRef]
- Pincus, S.M. Approximate Entropy as a Measure of System Complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis using Approximate Entropy and Sample Entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Bajić, D.; Skoric, T.; Milutinovic-Smiljanic, S.; Japundzic-Zigon, N. Cardiovascular Dependency Structures in Different Ambient Conditions: An Entropy Study. Entropy 2019, 21, 1103. [Google Scholar] [CrossRef]
- Bajić, D.; Mišić, N.; Škorić, T.; Japundžić-Žigon, N.; Milovanović, M. On Entropy of Probability Integral Transformed Time Series. Entropy 2020, 22, 1146. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lo, B. An Artificial Neural Network Framework for Gait-Based Biometrics. IEEE J. Biomed. Health Inform. 2019, 23, 987–998. [Google Scholar] [CrossRef]
- Vasyltsov, I.; Lee, S. Entropy Extraction from Bio-Signals in Healthcare IoT. In Proceedings of the 1st ACM Workshop on IoT Privacy, Trust, and Security, IoTPTS@AsiaCCS 2015, Singapore, 14 April 2015; Chow, R., Saldamli, G., Eds.; ACM: New York, NY, USA, 2015; pp. 11–17. [Google Scholar] [CrossRef]
- Karimian, N.; Tehranipoor, M.; Woodard, D.; Forte, D. Unlock Your Heart: Next Generation Biometric in Resource-Constrained Healthcare Systems and IoT. IEEE Access 2019, 7, 49135–49149. [Google Scholar] [CrossRef]
- Wan, Y.; Gu, S.; Du, B. A New Image Encryption Algorithm Based on Composite Chaos and Hyperchaos Combined with DNA Coding. Entropy 2020, 22, 171. [Google Scholar] [CrossRef]
- Abd El-Latif, A.A.; Abd-El-Atty, B.; Talha, M. Robust Encryption of Quantum Medical Images. IEEE Access 2018, 6, 1073–1081. [Google Scholar] [CrossRef]
- Hasan, M.K.; Islam, S.; Sulaiman, R.; Khan, S.; Hashim, A.H.A.; Habib, S.; Islam, M.; Alyahya, S.; Ahmed, M.M.; Kamil, S.; et al. Lightweight Encryption Technique to Enhance Medical Image Security on Internet of Medical Things Applications. IEEE Access 2021, 9, 47731–47742. [Google Scholar] [CrossRef]
- Butpheng, C.; Yeh, K.H.; Xiong, H. Security and Privacy in IoT-Cloud-Based e-Health Systems—A Comprehensive Review. Symmetry 2020, 12, 1191. [Google Scholar] [CrossRef]
- Vassilev, A.; Staples, R. Entropy as a Service: Unlocking Cryptography’s Full Potential. Computer 2016, 49, 98–102. [Google Scholar] [CrossRef]
- Salahuddin, M.A.; Al-Fuqaha, A.; Guizani, M.; Shuaib, K.; Sallabi, F. Softwarization of Internet of Things Infrastructure for Secure and Smart Healthcare. Computer 2017, 50, 74–79. [Google Scholar] [CrossRef]
- Tomovic, S.; Lekic, N.; Radusinovic, I.; Gardasevic, G. A New Approach to Dynamic Routing in SDN Networks. In Proceedings of the 2016 18th Mediterranean Electrotechnical Conference (MELECON), Lemesos, Cyprus, 18–20 April 2016; pp. 1–6. [Google Scholar]
- Buratti, C.; Stajkic, A.; Gardasevic, G.; Milardo, S.; Abrignani, M.D.; Mijovic, S.; Morabito, G.; Verdone, R. Testing Protocols for the Internet of Things on the EuWIn Platform. IEEE Internet Things J. 2016, 3, 124–133. [Google Scholar] [CrossRef]
- Li, J.; Cai, J.; Khan, F.; Rehman, A.U.; Balasubramaniam, V.; Sun, J.; Venu, P. A Secured Framework for SDN-Based Edge Computing in IoT-Enabled Healthcare System. IEEE Access 2020, 8, 135479–135490. [Google Scholar] [CrossRef]
- Sood, S.K.; Mahajan, I. IoT-Fog-Based Healthcare Framework to Identify and Control Hypertension Attack. IEEE Internet Things J. 2019, 6, 1920–1927. [Google Scholar] [CrossRef]
- Wright, C. Bitcoin: A Peer-to-Peer Electronic Cash System. SSRN Electron. J. 2008. [Google Scholar] [CrossRef]
- Srivastava, G.; Crichigno, J.; Dhar, S. A Light and Secure Healthcare Blockchain for IoT Medical Devices. In Proceedings of the 2019 IEEE Canadian Conference of Electrical and Computer Engineering (CCECE), Edmonton, AB, Canada, 5–8 May 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Bahmani, A.; Alavi, A.; Buergel, T.; Upadhyayula, S.; Wang, Q.; Ananthakrishnan, S.; Alavi, A.; Celis, D.; Gillespie, D.; Young, G.; et al. A scalable, secure, and interoperable platform for deep data-driven health management. Nat. Commun. 2021, 12, 5757. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Grim, J.C.; Rosales, A.M.; Watson-Capps, J.J.; Anseth, K.S. Engineering precision biomaterials for personalized medicine. Sci. Transl. Med. 2018, 10, eaam8645. [Google Scholar] [CrossRef]
- Wu, P.Y.; Cheng, C.W.; Kaddi, C.D.; Venugopalan, J.; Hoffman, R.; Wang, M.D. Omic and Electronic Health Record Big Data Analytics for Precision Medicine. IEEE Trans. Biomed. Eng. 2017, 64, 263–273. [Google Scholar] [CrossRef]
- Goyal, S.; Purkayastha, S.; Phillips, T.; Quick, R.; Britt, A. Enabling Secure and Effective Biomedical Data Sharing through Cyberinfrastructure Gateways. arXiv 2020, arXiv:2012.12835. [Google Scholar]
- Hamici, Z. Towards Genetic Cryptography for Biomedical Wireless Sensor Networks Gateways. IEEE J. Biomed. Health Inform. 2018, 22, 1814–1823. [Google Scholar] [CrossRef]
- Sakib, S.; Fouda, M.M.; Al-Mahdawi, M.; Mohsen, A.; Oogane, M.; Ando, Y.; Fadlullah, Z.M. Deep Learning Models for Magnetic Cardiography Edge Sensors Implementing Noise Processing and Diagnostics. IEEE Access 2022, 10, 2656–2668. [Google Scholar] [CrossRef]
- Nashif, S.; Raihan, R.; Islam, M.R.; Imam, M. Heart Disease Detection by Using Machine Learning Algorithms and a Real-Time Cardiovascular Health Monitoring System. World J. Eng. Technol. 2018, 6, 854–873. [Google Scholar] [CrossRef]
- Baktir, A.C.; Tunca, C.; Ozgovde, A.; Salur, G.; Ersoy, C. SDN-Based Multi-Tier Computing and Communication Architecture for Pervasive Healthcare. IEEE Access 2018, 6, 56765–56781. [Google Scholar] [CrossRef]
- Vedaei, S.; Fotovvat, A.; Mohebbian, M.; Rahman, G.; Wahid, K.; Babyn, P.; Marateb, H.; Mansourian, M.; Sami, R. COVID-SAFE: An IoT-based system for automated health monitoring and surveillance in post-pandemic life. IEEE Access 2020, 8, 188538–188551. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C. Communication theory of secrecy systems. Bell Syst. Tech. J. 1949, 28, 656–715. [Google Scholar] [CrossRef]
- Shannon, C. Communication in the Presence of Noise. Proc. IRE 1949, 37, 10–21. [Google Scholar] [CrossRef]
- Shannon, C. General treatment of the problem of coding. Trans. IRE Prof. Group Inf. Theory 1953, 1, 102–104. [Google Scholar] [CrossRef]
- World Health Organization: Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 22 November 2021).
- Jovanović, S.; Jovanović, M.; Škorić, T.; Jokić, S.; Milovanović, B.; Katzis, K.; Bajić, D. A Mobile Crowd Sensing Application for Hypertensive Patients. Sensors 2019, 19, 400. [Google Scholar] [CrossRef]
- Bailey, J.J.; Berson, A.S.; Garson, A.; Horan, L.G.; Macfarlane, P.W.; Mortara, D.W.; Zywietz, C. Recommendations for standardization and specifications in automated electrocardiography: Bandwidth and digital signal processing. A report for health professionals by an ad hoc writing group of the Committee on Electrocardiography and Cardiac Electrophysiology of the Council on Clinical Cardiology, American Heart Association. Circulation 1990, 81, 730–739. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology the North American Society of Pacing and Electrophysiology. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [CrossRef]
- Despotović, M.; Bajić, D. ECG signal compression. In Proceedings of the MEDNET 2001 the 6th World Congress on the Internet in Medicine, Udine, Italy, 29 November–2 December 2001; pp. 471–472. [Google Scholar]
- Moody, G.B.; Mark, R.G.; Goldberger, A.L. Evaluation of the ’TRIM’ ECG data compressor. Comput. Cardiol. 1988, 15, 167–170. [Google Scholar]
- Goldberger, A.; Amaral, L.; Glass, L.; Hausdorff, P.C.I.J.; Mark, R.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Jeong, J.; Kim, H.B.; Kwon, I.H.; Park, S.Y.; Kim, J.E.; Choi, Y. Electrocardiogram Sampling Frequency Range Acceptable for Heart Rate Variability Analysis. Healthc. Inform. Res. 2018, 24, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Jalaleddine, S.; Hutchens, C.; Strattan, R.; Coberly, W. ECG data compression techniques—A unified approach. IEEE Trans. Biomed. Eng. 1990, 37, 329–343. [Google Scholar] [CrossRef]
- Singh, B.; Kaur, A.; Singh, J. A Review of ECG Data Compression Techniques. Int. J. Comput. Appl. 2015, 116, 39–44. [Google Scholar] [CrossRef]
- Cox, J.R.; Nolle, F.M.; Fozzard, H.A.; Oliver, G.C. AZTEC, a Preprocessing Program for Real-Time ECG Rhythm Analysis. IEEE Trans. Biomed. Eng. 1968, BME-15, 128–129. [Google Scholar] [CrossRef] [PubMed]
- Abenstein, J.P.; Tompkins, W.J. A New Data-Reduction Algorithm for Real-Time ECG Analysis. IEEE Trans. Biomed. Eng. 1982, BME-29, 43–48. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.; Chen, X.; Wu, J. ECG data compression using a neural network model based on multi-objective optimization. PLoS ONE 2017, 12, e0182500. [Google Scholar] [CrossRef]
- Ranjeet, K.; Kumar, A.; Pandey, R.K. ECG Signal Compression Using Different Techniques. In Advances in Computing, Communication and Control; Springer: Berlin/Heidelberg, Germany, 2011; pp. 231–241. [Google Scholar]
- Ranjeet, K.; Kuamr, A.; Pandey, R.K. ECG Signal Compression using Optimum Wavelet Filter Bank Based on Kaiser Window. Procedia Eng. 2012, 38, 2889–2902. [Google Scholar] [CrossRef]
- Rebollo-Neira, L. Effective high compression of ECG signals at low level distortion. Sci. Rep. 2019, 9, 4564. [Google Scholar] [CrossRef] [PubMed]
- Melek, M.; Khattab, A. ECG compression using wavelet-based compressed sensing with prior support information. Biomed. Signal Process. Control 2021, 68, 102786. [Google Scholar] [CrossRef]
- Abo-Zahhad, M.; Ahmed, S.M.; Zakaria, A. An Efficient Technique for Compressing ECG Signals Using QRS Detection, Estimation, and 2D DWT Coefficients Thresholding. Model. Simul. Eng. 2012, 2012, 742786. [Google Scholar] [CrossRef]
- Zigel, Y.; Cohen, A.; Katz, A. The weighted diagnostic distortion (WDD) measure for ECG signal compression. IEEE Trans. Biomed. Eng. 2000, 47, 1422–1430. [Google Scholar] [CrossRef]
- Hilbel, T.; Brown, B.; de Bie, J.; Lux, R.; Katus, H. Innovation and advantage of the DICOM ECG standard for viewing, interchange and permanent archiving of the diagnostic electrocardiogram. In Proceedings of the 2007 Computers in Cardiology, Durham, NC, USA, 30 September–3 October 2007; pp. 633–636. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Lo, H.C. The clinical application of a PACS-dependent 12-lead ECG and image information system in E-medicine and telemedicine. J. Digit. Imaging 2010, 23, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Sinclair, A.; Morrison, A.; Hafizi, D.; Pyke, L. The Canadian Medical Imaging Inventory 2019–2020. CADTH 2021, 1, 1–215. [Google Scholar] [CrossRef]
- Liu, F.; Hernandez-Cabonero, M.; Sanchez, V.; Marcellin, M.; Bilgin, A. The Current Role of Image Compression Standards in medical Imaging. Information 2017, 8, 131. [Google Scholar] [CrossRef]
- DICOM PS3.1 2021e. Available online: https://www.dicomstandard.org/ (accessed on 30 November 2021).
- DICOM PS3.5 2021e. Available online: https://www.dicomstandard.org (accessed on 30 November 2021).
- RCR. The Adoption of Lossy Image Data Compression for the Purpose of Clinical Interpretation. 2008. Available online: https://www.rcr.ac.uk/publication/it-guidance-adoption-lossy-image-data-compression-purpose-clinical-interpretation (accessed on 25 November 2021).
- CAR. Standards for Irreversible Compression in Digital Diagnostic Imaging within Radiology. 2011. Available online: http://www.car.ca/uploads/standards20guidelines/Standard_Lossy_Compression_EN.pdf (accessed on 28 November 2021).
- Koff, D.; Bak, P.; Brownrigg, P.; Hosseinyadeh, D.; Khademi, A.; Kiss, A.; Lepanto, L.; Michalak, T.; Shulman, H.; Volkening, A. Pan-Canadian Evaluation of Irreversible Compression Ratios (“Losy” Compression) for Development of National Guidelines. J. Digit. Imaging 2009, 22, 569–578. [Google Scholar] [CrossRef]
- Loose, R.; Braunschweig, R.; Kotter, E.; Mildenberger, P.; Simmler, R.; Wucherer, M. Compression of Digital Images in Radiology—Results of a Consensus Conference. Rofo 2009, 181, 32–37. [Google Scholar] [CrossRef]
- ESR. Usability of irreversible image compression in radiological imaging. A position paper by the European Society of Radiology (ESR). Insights Imaging 2011, 2, 103–115. [Google Scholar] [CrossRef] [PubMed]
- ACR–AAPM–SIIM. Technical Standard for Electronic Practice of Medical Imaging—Resolution 41. 2017. Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/elec-practice-medimag.pdf (accessed on 28 November 2021).
- Rahmat, R.; Andreas, T.; Fahmi, F.; Pasha, M.; Alyahrani, M.; Budiarto, R. Analysis of DICOM Image Compression Alternative Using Huffman Coding. J. Healthc. Eng. 2019, 2019, 5810540. [Google Scholar] [CrossRef]
- Ahmadi, H.; Katzis, K.; Shakir, M.; Arvaneh, M.; Gatherer, A. Wireless Communication and the Pandemic: The Story So Far. IEEE ComSoc Technology News (CTN). 2020. Available online: https://www.comsoc.org/publications/ctn/wireless-communication-and-pandemic-story-so-far (accessed on 28 November 2021).
- Shakir, M.; Ramzan, N. AI for Emerging Verticals: Human-Robot Computing, Sensing and Networking; IET: London, UK, 2020. [Google Scholar]
- Dohler, M.; Mahmoodi, T.; Lema, M.; Condoluci, M.; Sardis, F.; Antonakoglou, K.; Aghvami, A. Internet of skills, where robotics meets AI, 5G and the Tactile Internet. In Proceedings of the 2017 European Conference on Networks and Communications (EuCNC), Oulu, Finland, 12–15 June 2017; pp. 1–5. [Google Scholar] [CrossRef]
- Ren, H.; Pan, C.; Deng, Y.; Elkashlan, M.; Nallanathan, A. Resource Allocation for URLLC in 5G Mission-Critical IoT Networks. In Proceedings of the ICC 2019—2019 IEEE International Conference on Communications (ICC), Shanghai, China, 20–24 May 2019; pp. 1–6. [Google Scholar] [CrossRef]


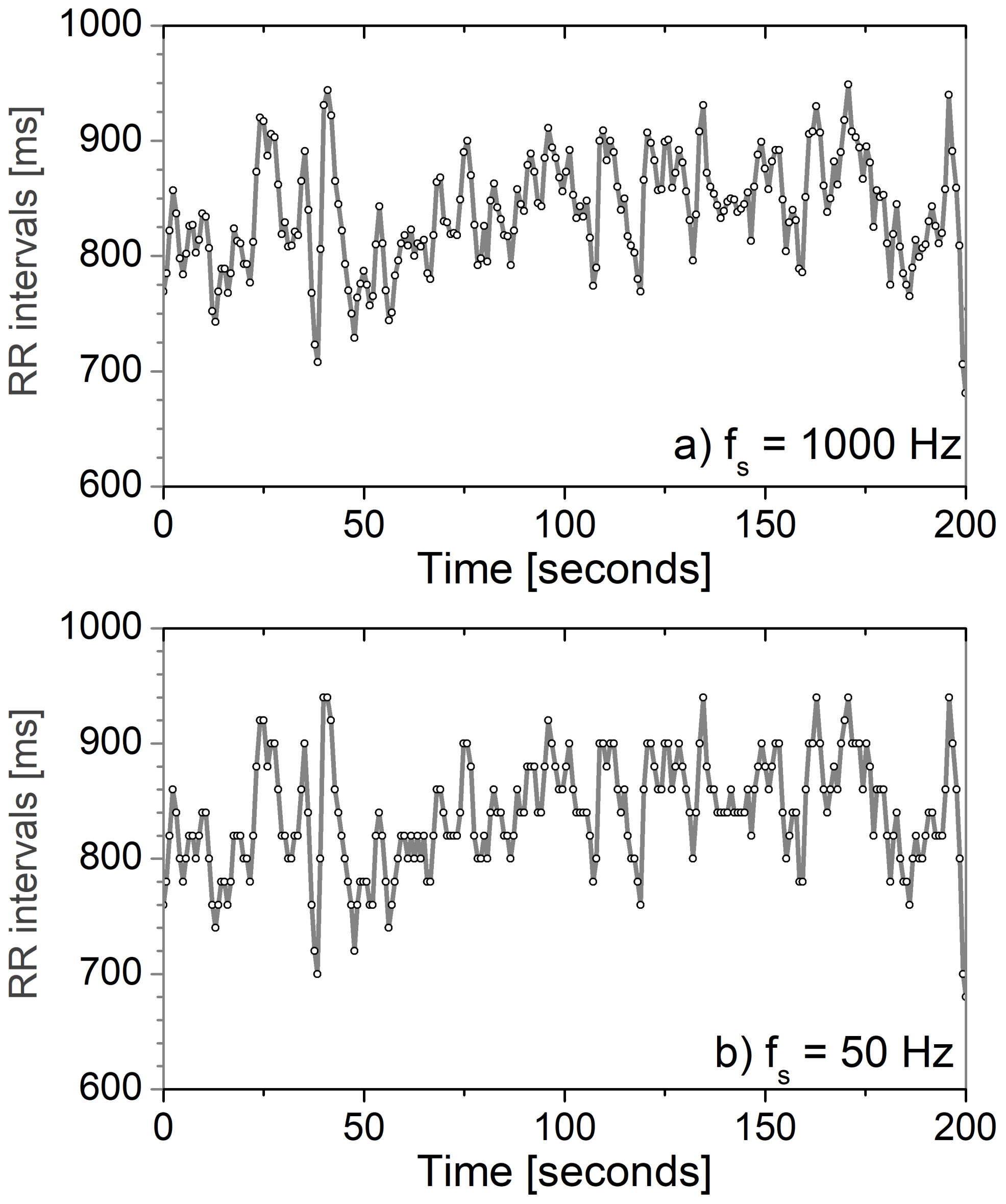
| Sensor Type | Message Size [Byte] | Rate [msg/Day] |
|---|---|---|
| Glucose Sensor [21] | 18 | 288 |
| 24H ECG Rec [22] | 40 | Real Time |
| Temperature [23] | 8 | 6 |
| Heart Rate [24] | 4 | 6 |
| Blood Pressure [25] | 8 | 6 |
| Blood Oxygen [25] | 8 | 6 |
| Sensors | Healthcare Application | IoT Communication Technology | Data Rate | Range | Advanced Technology | Benefits |
|---|---|---|---|---|---|---|
| Electrocardiography (ECG), Magnetocardiography (MCG) | Heart Activity Monitoring, Remote Health Monitoring, Arrhythmia Detection | Bluetooth/BLE Zigbee NB-IoT | 3 Mbps/1 Mbps 20–250 kbps 200 kbps | 10–100 m 100 m 1–10 km | AI-aided model for next-generation ultra-edge IoT sensors [82] | Remote patient monitoring for a prolonged period, especially in aging urban populations and under-served regions. |
| Heart Rate, Blood Pressure, Temperature | Heart Disease Detection, Remote Health Monitoring | Bluetooth/BLE LoRaWAN | 3 Mbps/1 Mbps 50 kbps | 10–100 m 2–20 km | Cloud-based heart disease prediction system using ML techniques [83] | Intelligent cloud-based network for analysis, planning and decision making. Provides continuous supervisions for patient’s safety. |
| Accelerometer, Gyroscope, Magnetometer | Fall Risk Prevention, Pervasive Healthcare Applications | Bluetooth/BLE EC-GSM-IoT | 3 Mbps/1 Mbps 70–240 kbps | 10–100 m 15 km | SDN-based multitier computing and communication architecture [84] | Real-time healthcare services. Performance advantages over traditional cloud-based approaches. |
| Blood Oxygen, Temperature, Heart Rate, Photoplethysmogram (PPG) | Remote Health Monitoring | Bluetooth/BLE LoRaWAN NB-IoT EC-GSM-IoT | 3 Mbps/1 Mbps 50 kbps 200 kbps 70–240 kbps | 10–100 m 2–20 km 1–10 km 15 km | Fog-based ML tools for data analysis and diagnosis [85] | Automated health monitoring and a COVID-safe framework that minimizes a coronavirus exposure risk. Smartphone application. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katzis, K.; Berbakov, L.; Gardašević, G.; Šveljo, O. Breaking Barriers in Emerging Biomedical Applications. Entropy 2022, 24, 226. https://doi.org/10.3390/e24020226
Katzis K, Berbakov L, Gardašević G, Šveljo O. Breaking Barriers in Emerging Biomedical Applications. Entropy. 2022; 24(2):226. https://doi.org/10.3390/e24020226
Chicago/Turabian StyleKatzis, Konstantinos, Lazar Berbakov, Gordana Gardašević, and Olivera Šveljo. 2022. "Breaking Barriers in Emerging Biomedical Applications" Entropy 24, no. 2: 226. https://doi.org/10.3390/e24020226
APA StyleKatzis, K., Berbakov, L., Gardašević, G., & Šveljo, O. (2022). Breaking Barriers in Emerging Biomedical Applications. Entropy, 24(2), 226. https://doi.org/10.3390/e24020226








