Photosensitive Control and Network Synchronization of Chemical Oscillators
Abstract
:1. Introduction
2. Methods
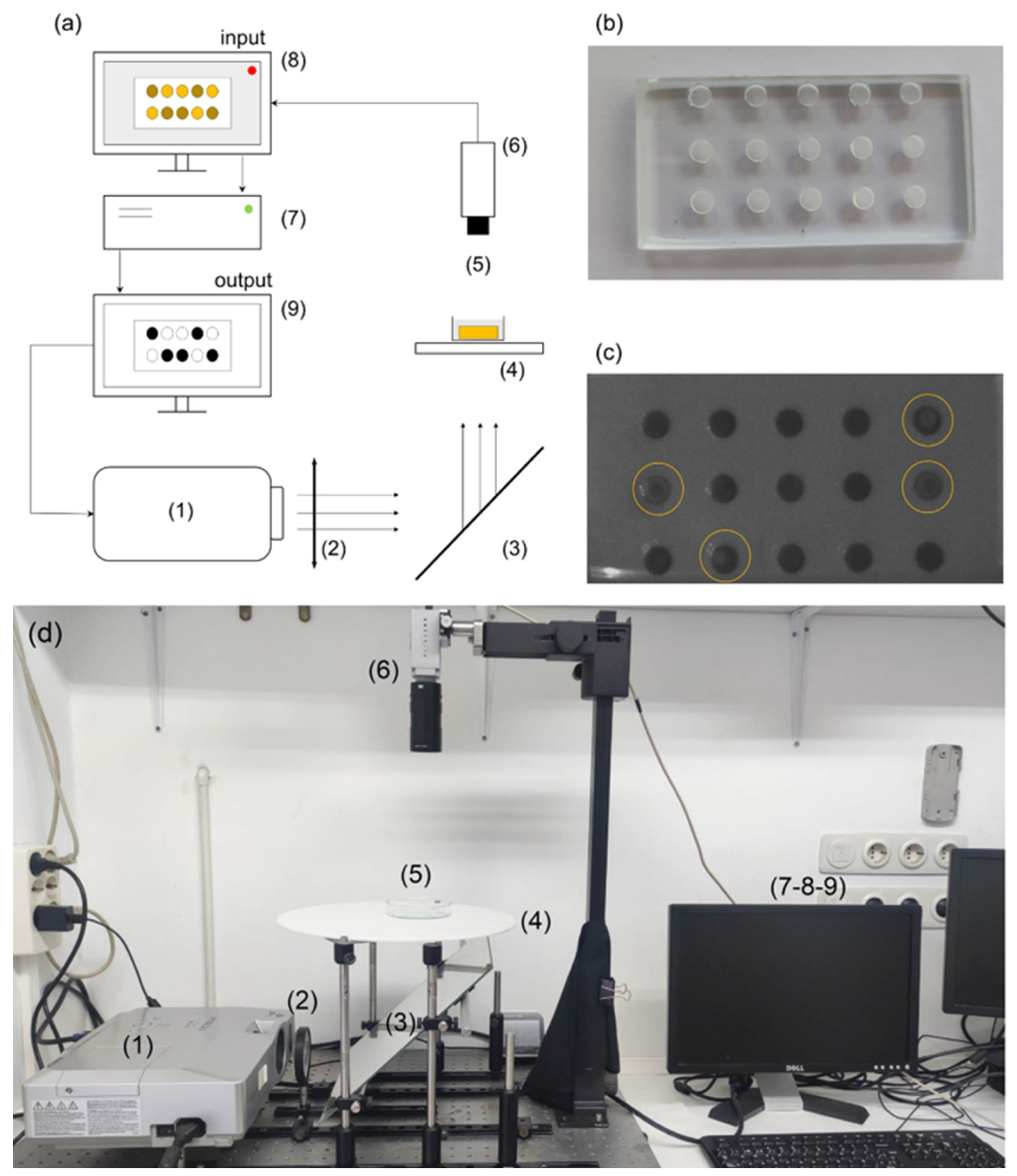
2.1. Fabrication of the Reactors Using the SLIPAA Technique
2.2. Experimental Setup for Control Experiments
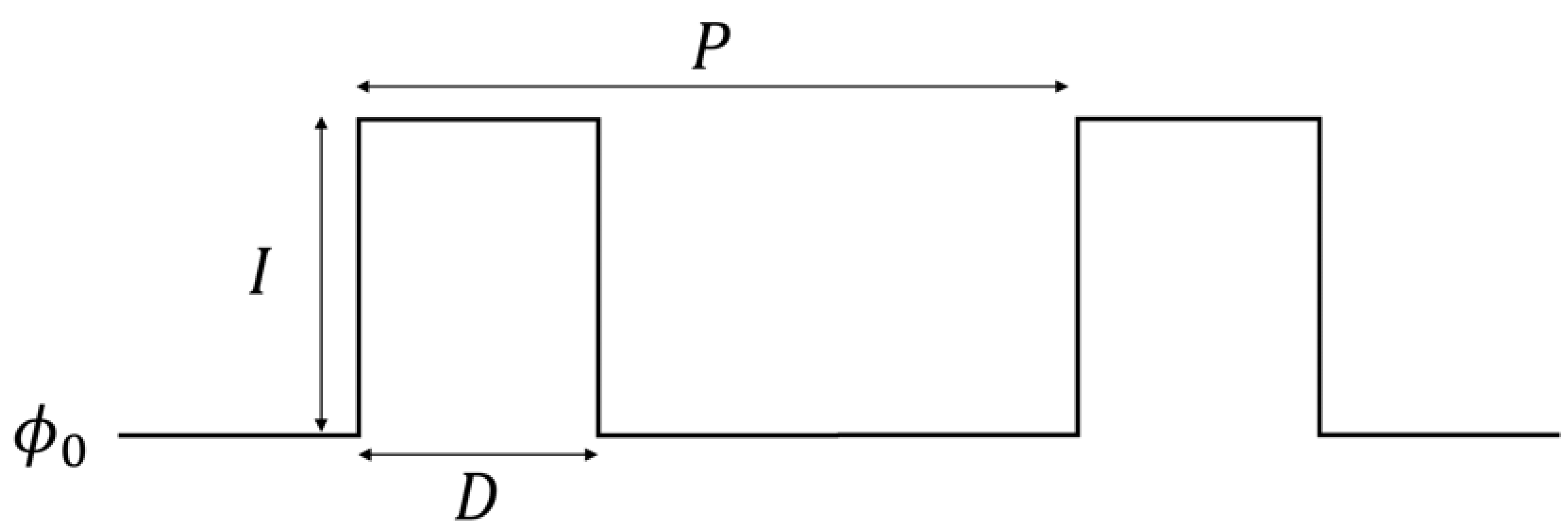
2.3. Periodic Pulses and Coupled Networks
3. Results
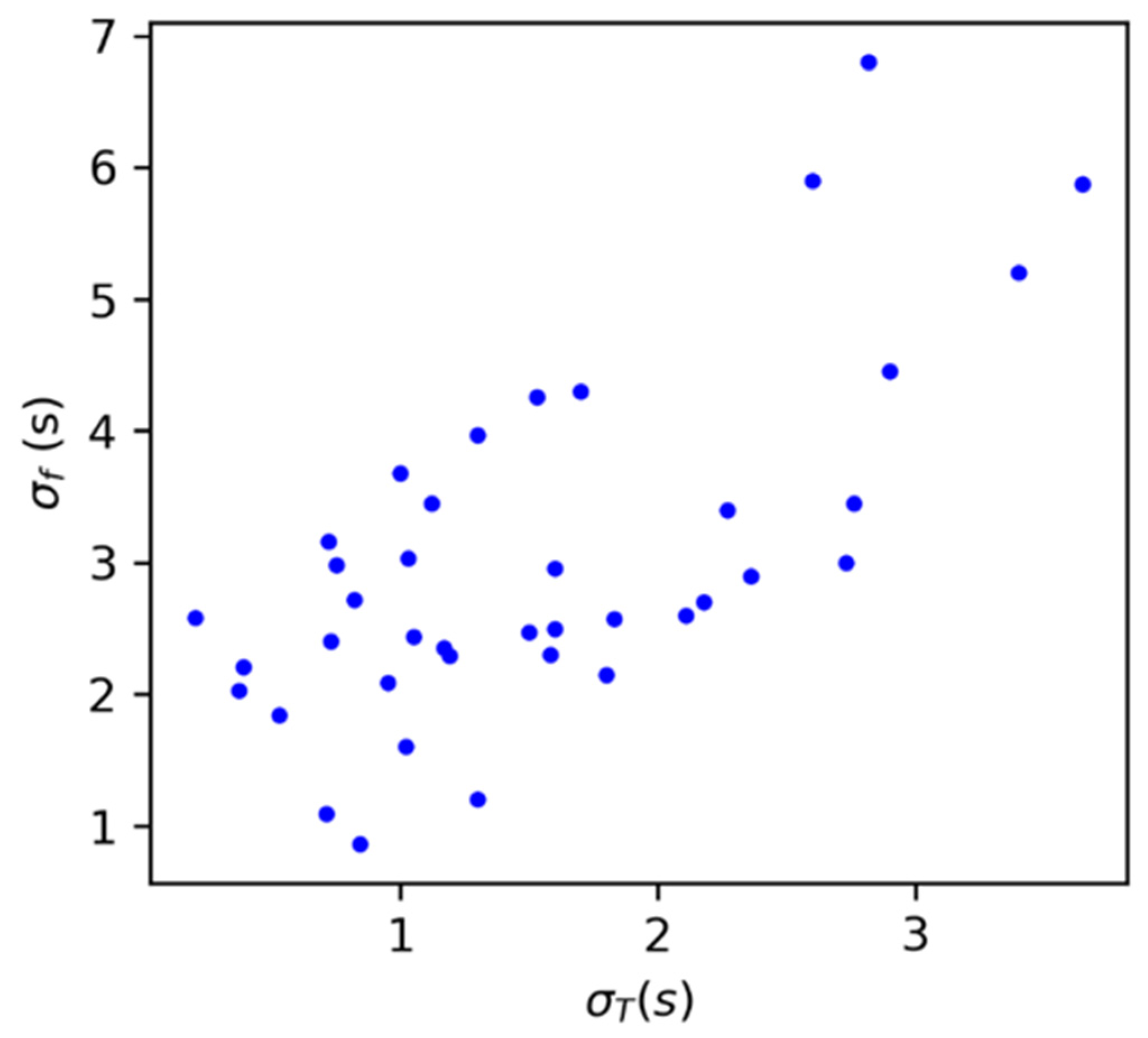
3.1. Characterization of the Experimental Setup
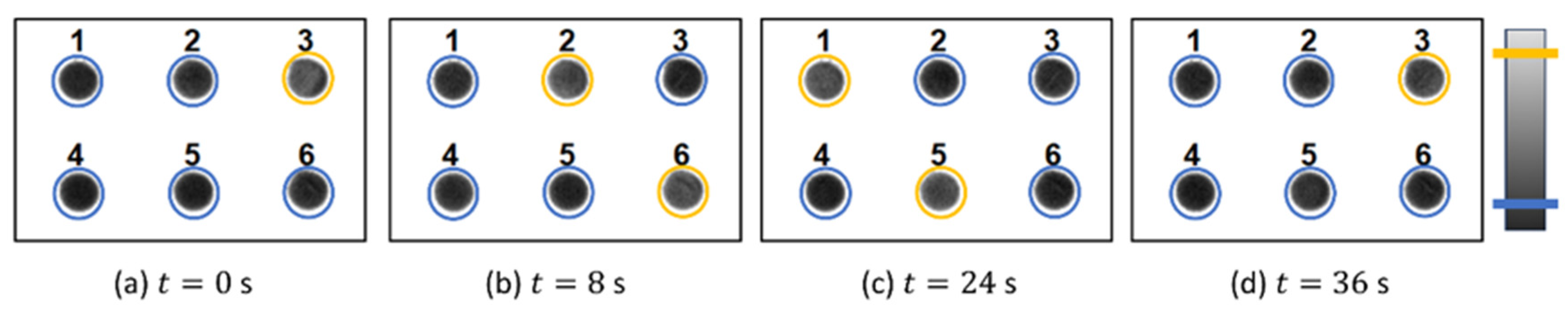
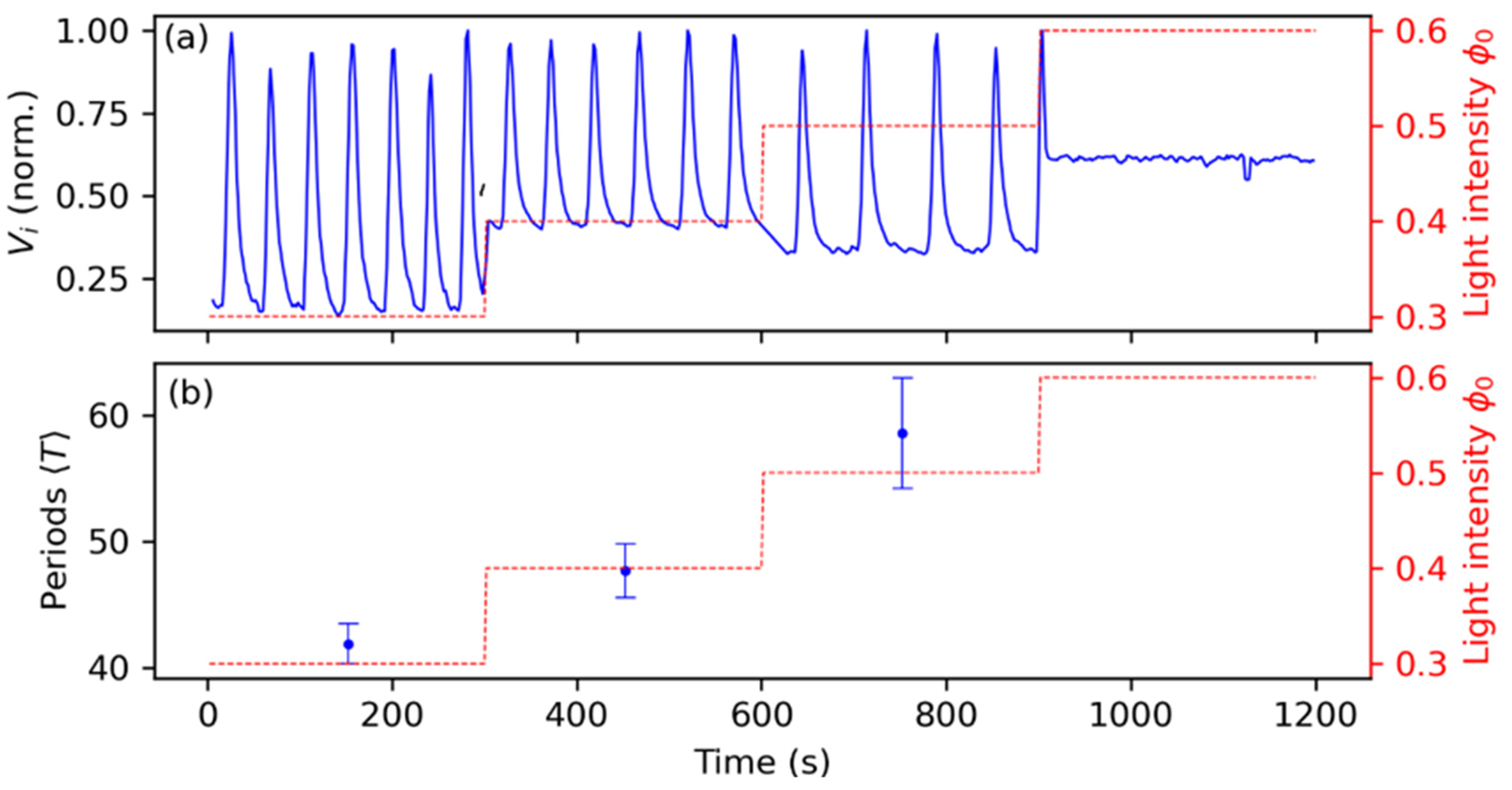
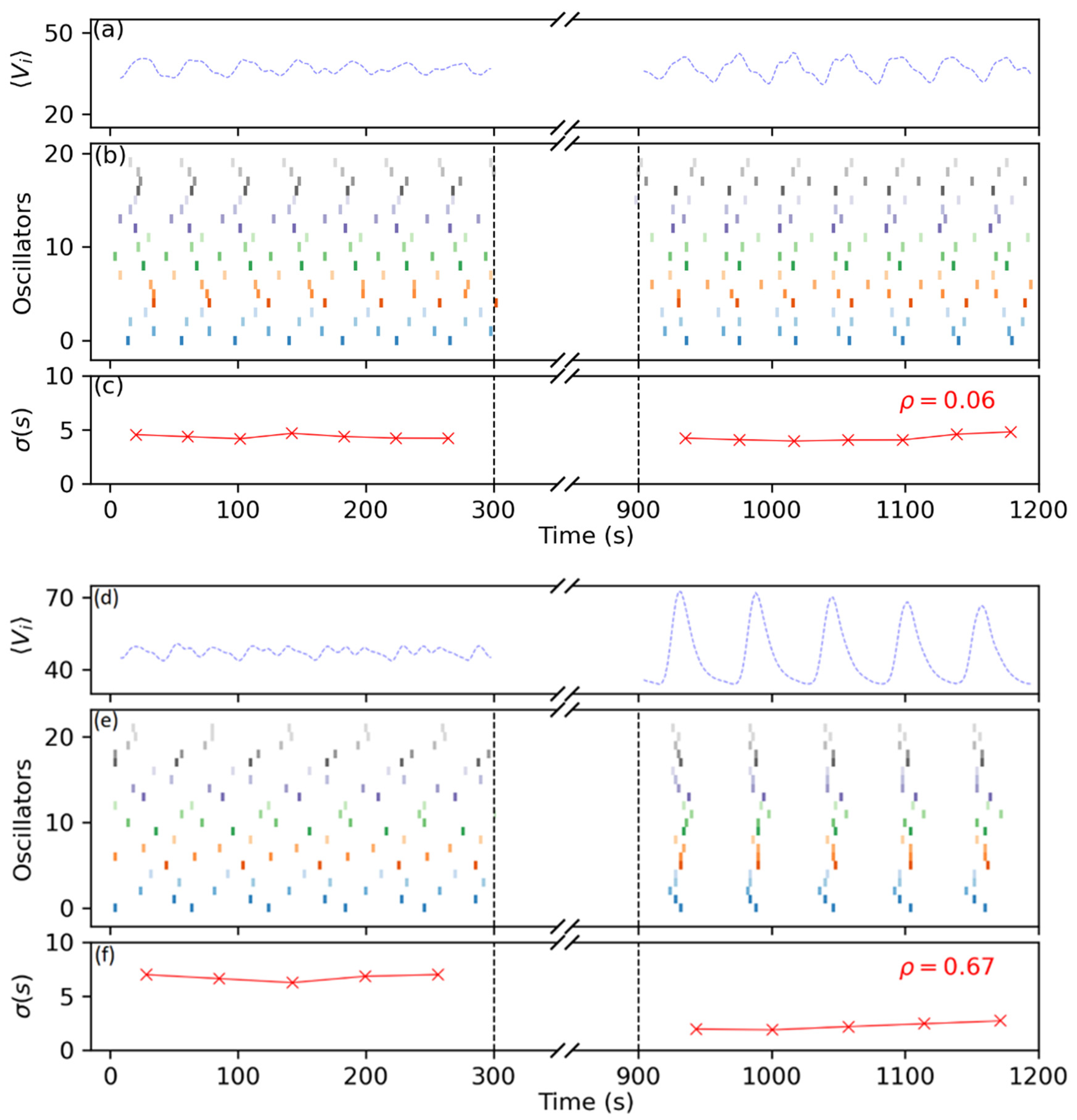
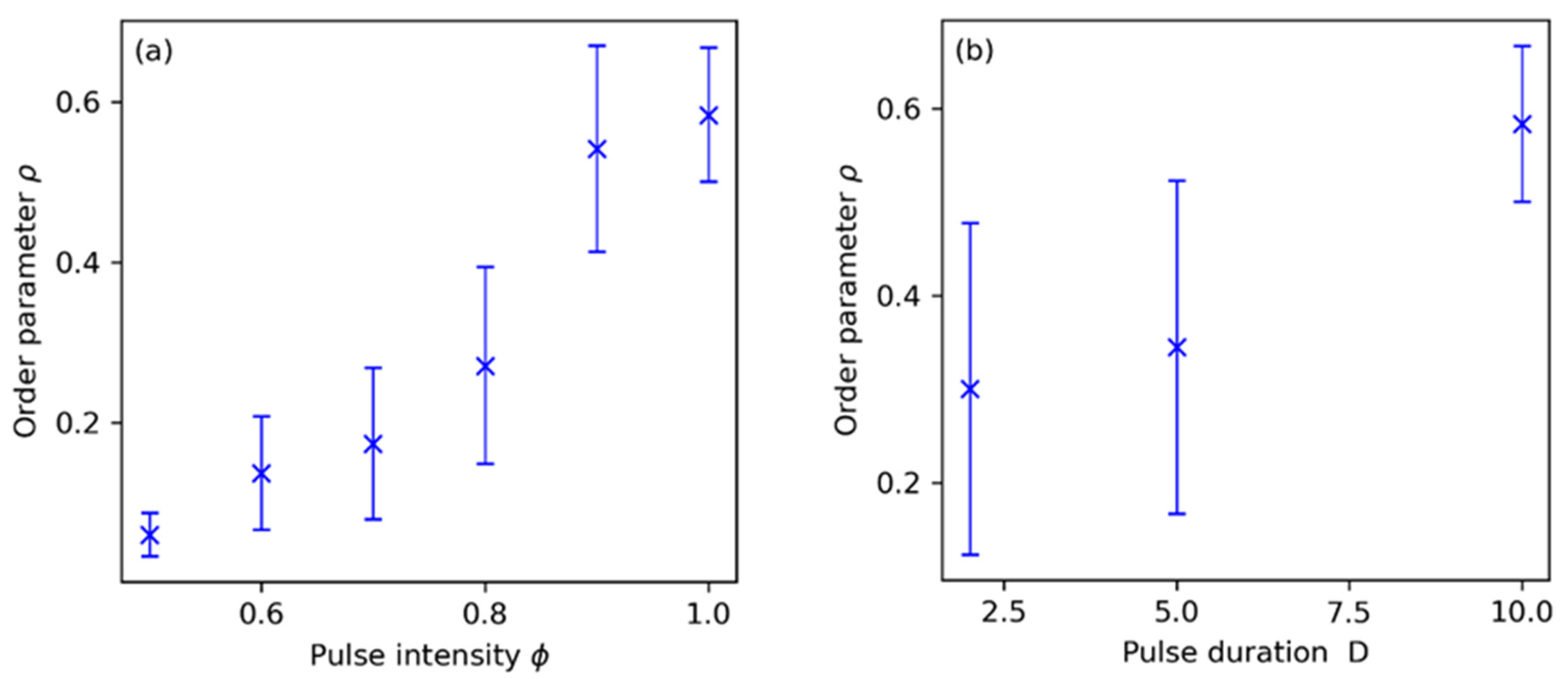
3.2. Synchronization Control by Means of Periodic Pulses
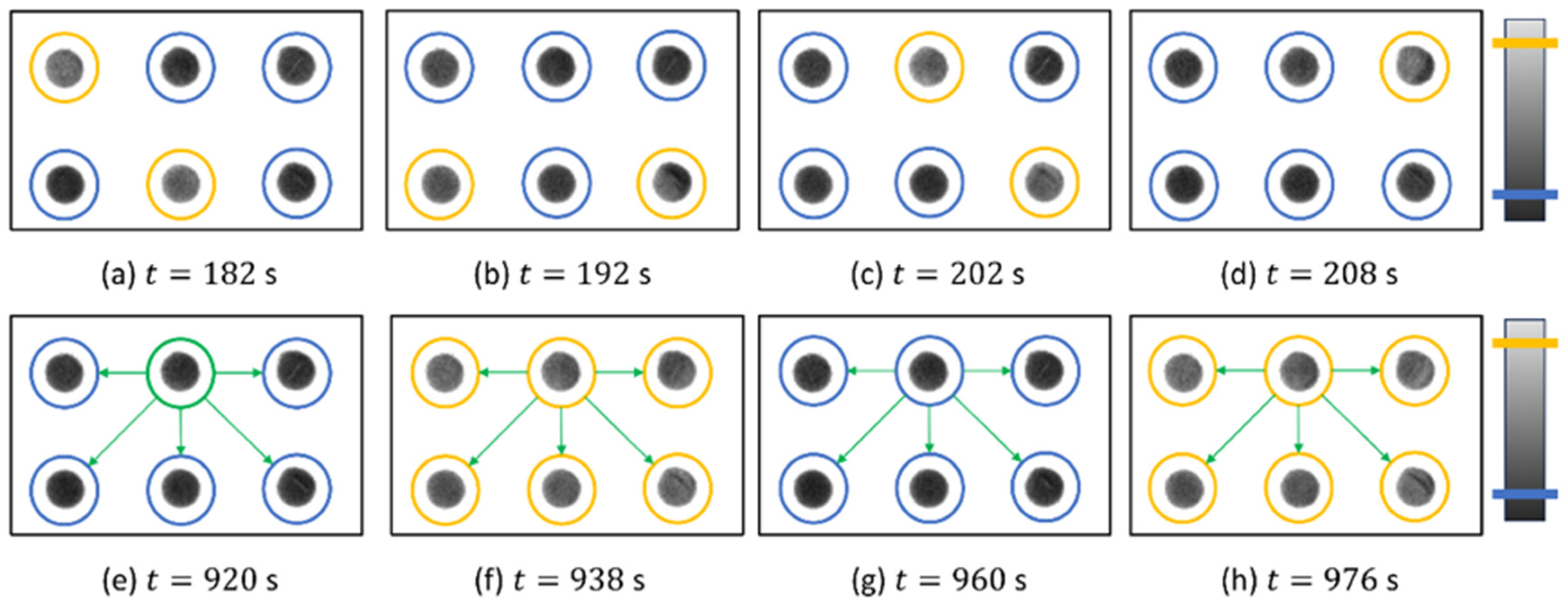
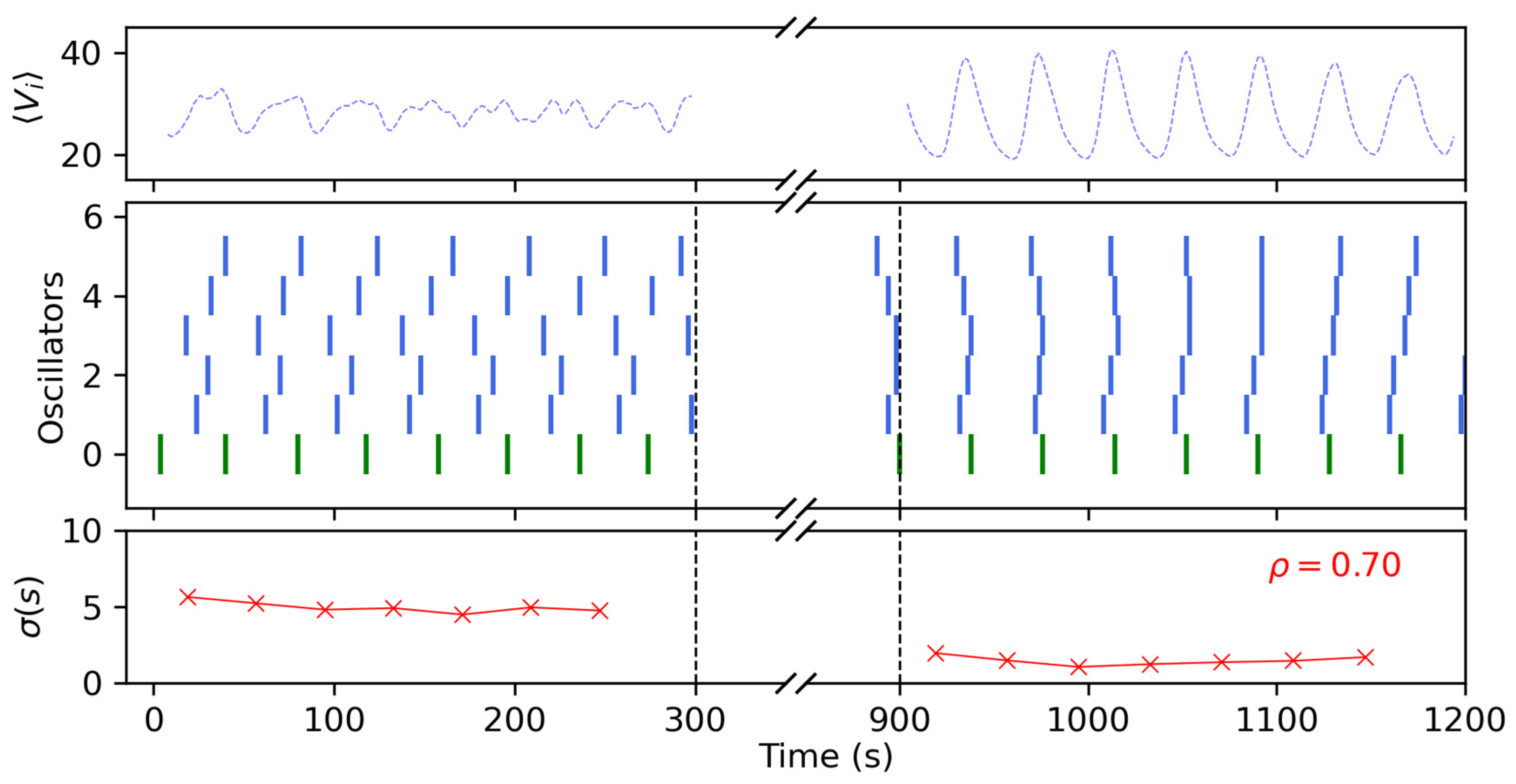
3.3. Networks of Coupled Oscillators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Masè, M.; Faes, L.; Antolini, R.; Scaglione, M.; Ravelli, F. Quantification of synchronization during atrial fibrillation by Shannon entropy: Validation in patients and computer model of atrial arrhythmias. Physiol. Meas. 2005, 26, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.; Jalife, J. Spiral waves and the heart. Int. J. Bifurc. Chaos 1996, 6, 415. [Google Scholar] [CrossRef]
- Lehnertz, K.; Elger, C.E. Can Epileptic Seizures be Predicted? Evidence from Nonlinear Time Series Analysis of Brain Electrical Activity. Phys. Rev. Lett. 1998, 80, 5019–5022. [Google Scholar] [CrossRef]
- Lehnertz, K.; Bialonski, S.; Horstmann, M.-T.; Krug, D.; Rothkegel, A.; Staniek, M.; Wagner, T. Synchronization phenomena in human epileptic brain networks. J. Neurosci. Methods 2009, 183, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Good, L.B.; Sabesan, S.; Marsh, S.T.; Tsakalis, K.; Treiman, D.; Iasemidis, L. Control of synchronization of brain dynamics leads to control of epileptic seizures in rodents. Int. J. Neural Syst. 2009, 19, 173–196. [Google Scholar] [CrossRef]
- Pecora, L.M.; Carroll, T.L. Synchronization in chaotic systems. Phys. Rev. Lett. 1990, 64, 821–824. [Google Scholar] [CrossRef]
- Pecora, L.M.; Carroll, T.L. Driving systems with chaotic signals. Phys. Rev. A 1991, 44, 2374–2383. [Google Scholar] [CrossRef]
- Boccaletti, S.; Kurths, J.; Osipov, G.; Valladares, D.L.; Zhou, C.S. The synchronization of chaotic systems. Phys. Rep. 2002, 366, 1–101. [Google Scholar] [CrossRef]
- Kocarev, L.; Halle, K.S.; Eckert, K.; Chua, L.O.; Parlitz, U. Experimental demonstration of secure communications via chaotic synchronization. Int. J. Bifurc. Chaos 1992, 2, 709–713. [Google Scholar] [CrossRef]
- Chua, L.O.; Itoh, M.; Kocarev, L.; Eckert, K. Chaos synchronization in chua’s circuit. J. Circuits Syst. Comput. 1993, 3, 93–108. [Google Scholar] [CrossRef]
- Cuomo, K.M.; Oppenheim, A.V. Circuit implementation of synchronized chaos with applications to communications. Phys. Rev. Lett. 1993, 71, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Tachikawa, M.; Tsukamoto, T.; Shimizu, T. Observation of synchronization in laser chaos. Phys. Rev. Lett. 1994, 72, 3502–3505. [Google Scholar] [CrossRef]
- VanWiggeren, G.D.; Roy, R. Communication with Chaotic Lasers. Science 1998, 279, 1198–1200. [Google Scholar] [CrossRef]
- Sorrentino, F.; di Bernardo, M.; Garofalo, F.; Chen, G. Controllability of complex networks via pinning. Phys. Rev. E 2007, 75, 046103. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.; Sendiña-Nadal, I.; Zanin, M.; Papo, D.; Boccaletti, S. Targeting the dynamics of complex networks. Sci. Rep. 2012, 2, 396. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Materassi, M.; Focardi, S.; Boccaletti, S. Steering complex networks toward desired dynamics. Sci. Rep. 2020, 10, 20744. [Google Scholar] [CrossRef] [PubMed]
- Zhabotinskii, A.M. Periodic course of the oxidation of malonic acid in a solution (studies on the kinetics of beolusov’s reaction). Biofizika 1964, 9, 306–311. [Google Scholar] [PubMed]
- Crowley, M.F.; Epstein, I.R. Experimental and theoretical studies of a coupled chemical oscillator: Phase death, multi-stability and in-phase and out-of-phase entrainment. J. Phys. Chem. 1989, 93, 2496–2502. [Google Scholar] [CrossRef]
- Marek, M.; Stuchl, I. Synchronization in two interacting oscillatory systems. Biophys. Chem. 1975, 3, 241–248. [Google Scholar]
- Nishiyama, N. Experimental observation of dynamical behaviors of four, five and six oscillators in rings and nine oscillators in a branched network. Phys. D Nonlinear Phenom. 1995, 80, 181–185. [Google Scholar] [CrossRef]
- Taylor, A.F.; Tinsley, M.R.; Wang, F.; Huang, Z.; Showalter, K. Dynamical Quorum Sensing and Synchronization in Large Populations of Chemical Oscillators. Science 2009, 323, 614–617. [Google Scholar] [CrossRef]
- Toiya, M.; González-Ochoa, H.O.; Vanag, V.K.; Fraden, S.; Epstein, I.R. Synchronization of Chemical Micro-oscillators. J. Phys. Chem. Lett. 2010, 1, 1241–1246. [Google Scholar] [CrossRef]
- Makki, R.; Muñuzuri, A.P.; Perez-Mercader, J. Periodic Perturbation of Chemical Oscillators: Entrainment and Induced Synchronization. Chemistry 2014, 20, 14213–14217. [Google Scholar] [CrossRef] [PubMed]
- Muñuzuri, A.P.; Pérez-Mercader, J. Noise-Induced and Control of Collective Behavior in a Population of Coupled Chemical Oscillators. J. Phys. Chem. A 2017, 121, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Totz, J.F.; Rode, J.; Tinsley, M.R.; Showalter, K.; Engel, H. Spiral wave chimera states in large populations of coupled chemical oscillators. Nat. Phys. 2018, 14, 282–285. [Google Scholar] [CrossRef]
- Sharma, A.; Ng, M.T.-K.; Gutierrez, J.M.P.; Jiang, Y.; Cronin, L. A programmable hybrid digital chemical information processor based on the Belousov-Zhabotinsky reaction. Nat. Commun. 2024, 15, 1984. [Google Scholar] [PubMed]
- Bao-Varela, C.; Isabel-Gomez, A.I.; Sanchez-Cruz, R.; Carnero-Groba, B.; Flores-Arias, M.T. Procedimiento Para Fabricar Canales, Pocillos y/o Estructuras Complejas en Vidrio (Spanish Patent). Spain Patent ES2912039B2, 3 April 2023. [Google Scholar]
- Norton, M.M.; Tompkins, N.; Blanc, B.; Cambria, M.C.; Held, J.; Fraden, S. Dynamics of Reaction-Diffusion Oscillators in Star and other Networks with Cyclic Symmetries Exhibiting Multiple Clusters. Phys. Rev. Lett. 2019, 123, 148301. [Google Scholar] [CrossRef]
- Mersing, D.; Tyler, S.A.; Ponboonjaroenchai, B.; Tinsley, M.R.; Showalter, K. Novel modes of synchronization in star networks of coupled chemical oscillators. Chaos Interdiscip. J. Nonlinear Sci. 2021, 31, 093127. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kuhnert, L.; Nagy-Ungvarai, Z.; Mueller, S.C.; Hess, B. Gel systems for the Belousov-Zhabotinskii reaction. J. Phys. Chem. 1991, 95, 5831–5837. [Google Scholar] [CrossRef]
- Sendiña-Nadal, I.; Muñuzuri, A.P.; Vives, D.; Pérez-Muñuzuri, V.; Casademunt, J.; Ramírez-Piscina, L.; Sancho, J.M.; Sagués, F. Wave Propagation in a Medium with Disordered Excitability. Phys. Rev. Lett. 1998, 80, 5437–5440. [Google Scholar] [CrossRef]
- Flores-Arias, M.T.; Gómez-Varela, A.I.; Munuzuri, A.P.; Carballosa, A.; Bao-Varela, C. Micro-reactors fabricated by Subaquatic indirect Laser-Induced Plasma-Assisted Ablation on soda-lime glass substrates. EPJ Web Conf. 2022, 266, 13012. [Google Scholar] [CrossRef]
- Amemiya, T.; Yamamoto, T.; Ohmori, T.; Yamaguchi, T. Experimental and Model Studies of Oscillations, Photoinduced Transitions, and Steady States in the Ru(bpy)32+-Catalyzed Belousov-Zhabotinsky Reaction under Different Solute Compositions. J. Phys. Chem. A 2002, 106, 612–620. [Google Scholar] [CrossRef]
- Kadar, S.; Amemiya, T.; Showalter, K. Reaction Mechanism for Light Sensitivity of the Ru(bpy)32+-Catalyzed Bel-ousov-Zhabotinsky Reaction. J. Phys. Chem. A 1997, 101, 8200–8206. [Google Scholar] [CrossRef]
- Kuramoto, Y. Chemical Oscillations, Waves, and Turbulence; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Reddy, M.K.R.; Nagy-Ungvarai, Z.; Mueller, S.C. Effect of Visible Light on Wave Propagation in the Ruthenium-Catalyzed Belousov-Zhabotinsky Reaction. J. Phys. Chem. 1994, 98, 12255–12259. [Google Scholar] [CrossRef]
- Masia, M.; Marchettini, N.; Zambrano, V.; Rustici, M. Effect of temperature in a closed unstirred Belousov–Zhabotinsky system. Chem. Phys. Lett. 2001, 341, 285–291. [Google Scholar] [CrossRef]
- García-Selfa, D.; Muñuzuri, A.P.; Pérez-Mercader, J.; Simakov, D.S.A. Resonant Behavior in a Periodically Forced Non-isothermal Oregonator. J. Phys. Chem. A 2019, 123, 8083–8088. [Google Scholar] [CrossRef]
- Ruoff, P. Antagonistic balance in the oregonator: About the possibility of temperature-compensation in the Bel-ousov-Zhabotinsky reaction. Phys. D Nonlinear Phenom. 1995, 84, 204–211. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carballosa, A.; Gomez-Varela, A.I.; Bao-Varela, C.; Flores-Arias, M.T.; Muñuzuri, A.P. Photosensitive Control and Network Synchronization of Chemical Oscillators. Entropy 2024, 26, 475. https://doi.org/10.3390/e26060475
Carballosa A, Gomez-Varela AI, Bao-Varela C, Flores-Arias MT, Muñuzuri AP. Photosensitive Control and Network Synchronization of Chemical Oscillators. Entropy. 2024; 26(6):475. https://doi.org/10.3390/e26060475
Chicago/Turabian StyleCarballosa, Alejandro, Ana I. Gomez-Varela, Carmen Bao-Varela, Maria Teresa Flores-Arias, and Alberto P. Muñuzuri. 2024. "Photosensitive Control and Network Synchronization of Chemical Oscillators" Entropy 26, no. 6: 475. https://doi.org/10.3390/e26060475




