The Effect of Water Supply on Sweet Cherry Phytochemicals in Bud, Leaf and Fruit
Abstract
:1. Introduction
2. Results
2.1. Sugars and Organic Acids
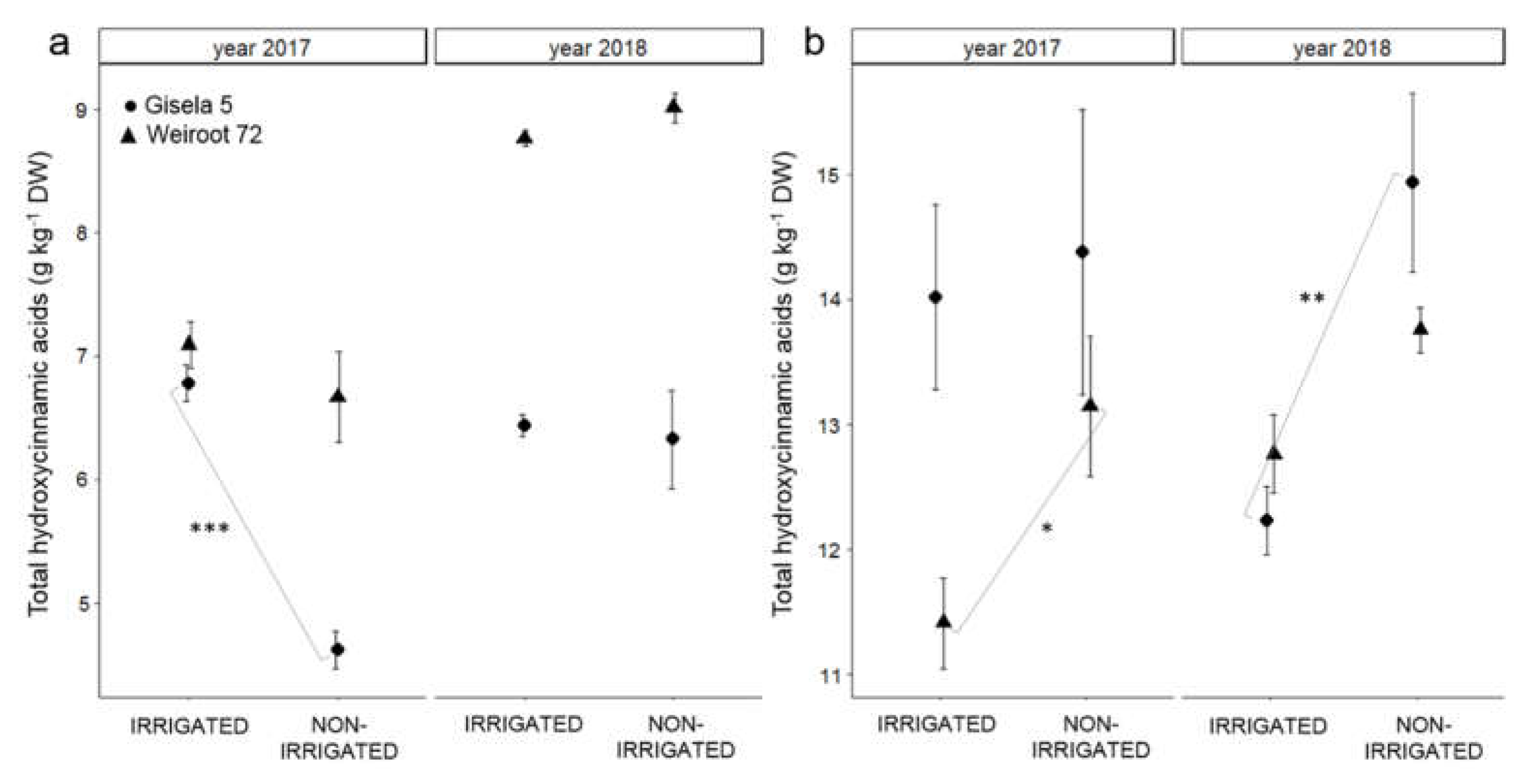
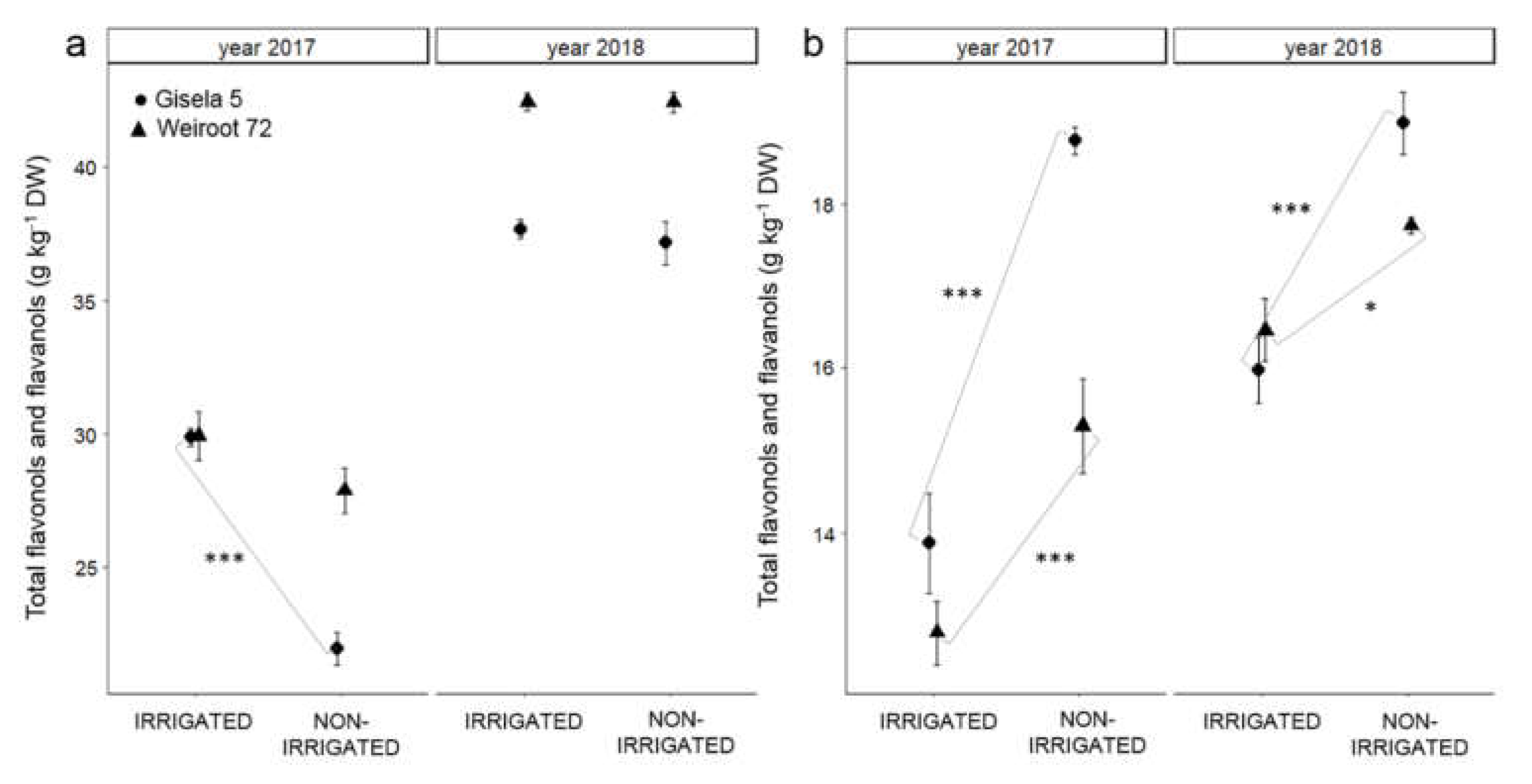
2.2. Phenolics
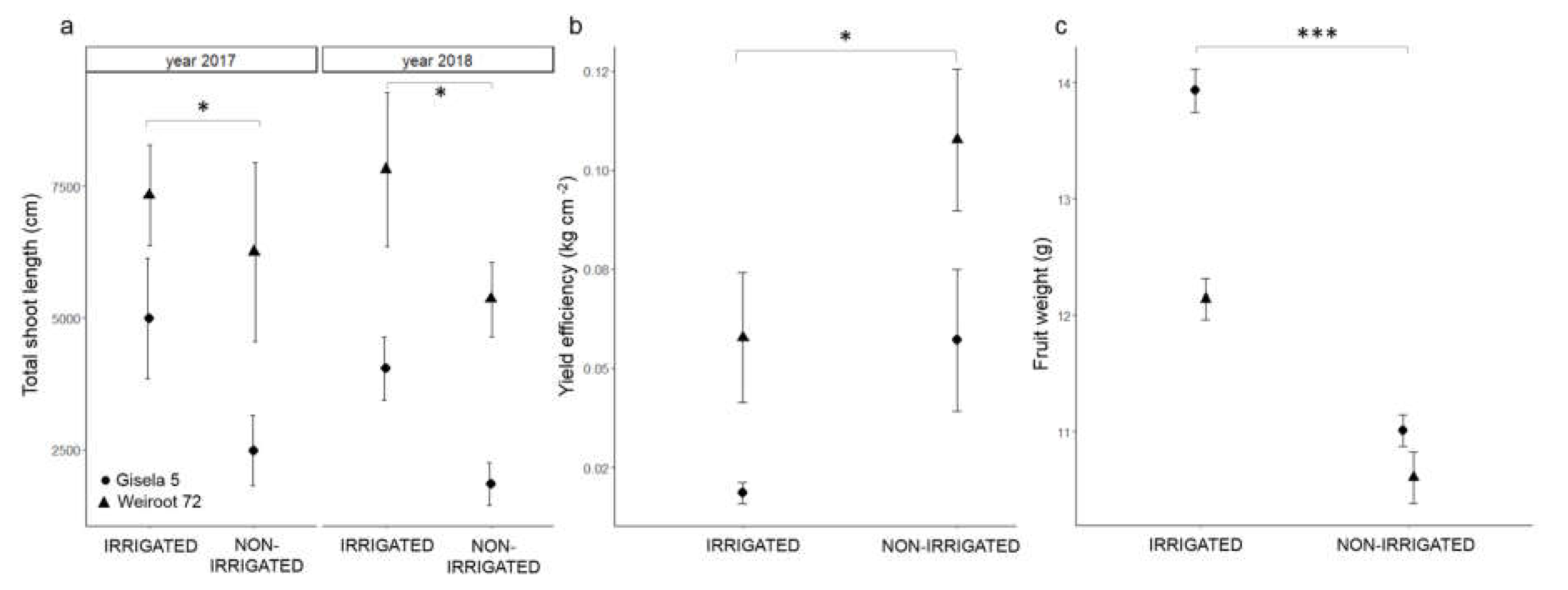
2.3. Shoot Length, Yield Efficiency and Fruit Weight Measurements
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Sampling and Sample Preparation for Biochemical Analysis
4.3. Sugars and Organic Acids
4.4. Phenolics
4.5. Chemicals
4.6. Shoot Length, Yield Efficiency and Fruit Weight Measurements
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blazkova, J.; Hlusickova, I. Cultivar and rootstock response to drip irrigation in sweet cherry tree vigour and start of bearing during the first three years after planting. Hortic. Sci. 2008, 35, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Candogan, B.N.; Yazgan, S. The effects of different irrigation levels on vegetative growth of young dwarf cherry trees in a sub-humid climate. Pak. J. Bot. 2010, 42, 3399–3408. [Google Scholar]
- Demirtas, C.; Erturk, U.; Yazgan, S.; Candogan, B.N.; Soylu, A. Effects of different irrigation levels on the vegetative growth, flower bud formation and fruit quality of sweet cherry in western part of Turkey. J. Food Agric. Environ. 2008, 6, 173–177. [Google Scholar]
- Livellara, N.; Saavedra, F.; Salgado, E. Plant based indicators for irrigation scheduling in young cherry trees. Agric. Water Manag. 2011, 98, 684–690. [Google Scholar] [CrossRef]
- Podestá, L.N.; Vallone, R.C.; Morabito, J.A.; Sanchez, E.E. Long-term effect of water deficit irrigation on cherry vigor and precocity. Acta Hortic. 2011, 889, 439–444. [Google Scholar] [CrossRef]
- Aglar, E.; Yildiz, K.; Long, L.E. The effects of rootstocks and training systems on the early performance of ‘0900 Ziraat’ sweet cherry. Not. Bot. Horti Agrobot. Cluj Napoca 2016, 44, 573–578. [Google Scholar] [CrossRef] [Green Version]
- Predieri, S.; Dris, R.; Sekse, L.; Rapparini, F. Influence of environmental factors and orchard management on yield and quality of sweet cherry. J. Food Agric. Environ. 2003, 1, 263–266. [Google Scholar]
- Gospodinova, M.; Kolev, K. Effect of rootstocks and irrigation rate on vigor and productivity of sweet cherry. Acta Hortic. 2011, 981, 263–268. [Google Scholar] [CrossRef]
- Jiménez, S.; Dridi, J.; Gutiérrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef]
- Tavarini, S.; Gil, M.; Tomas-Barberan, F.; Buendia, B.; Remorini, D.; Massai, R.; Degl’Innocenti, E.; Guidi, L. Effects of water stress and rootstocks on fruit phenolic composition and physical/chemical quality in Suncrest peach. Ann. Appl. Biol. 2011, 158, 226–233. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock–scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hortic. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranney, T.G.; Bassuk, N.L.; Whitlow, T.H. Osmotic adjustment and solute constituents in leaves and roots of water-stressed cherry (Prunus) trees. J. Am. Soc. Hortic. Sci. 1991, 116, 684–688. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Li, C.; Zhang, Z.; Ma, F.; Li, M. Response of sugar metabolism in apple leaves subjected to short-term drought stress. Plant Physiol. Biochem. 2019, 141, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Ebtedaie, M.; Shekafandeh, A. Antioxidant and carbohydrate changes of two pomegranate cultivars under deficit irrigation stress. Span. J. Agric. Res. 2016, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Bolat, I.; Dikilitas, M.; Ercisli, S.; Ikinci, A.; Tonkaz, T. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Dehghanisanij, H.; Naseri, A.; Anyoji, H.; Eneji, A.E. Effects of deficit irrigation and fertilizer use on vegetative growth of drip irrigated cherry trees. J. Plant Nutr. 2007, 30, 411–425. [Google Scholar] [CrossRef]
- Beauvieux, R.; Wenden, B.; Ballias, P.; Christmann, H.; Gibon, Y.; Dirlewanger, E. Dynamics of bud metabolites during dormancy in sweet cherry genotypes with contrasted chilling requirements. Acta Hortic. 2017, 1235, 407–412. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Adamakis, I.-D.; Karamanoli, K.; Martens, S.; Molassiotis, A. Metabolic mechanisms underpinning vegetative bud dormancy release and shoot development in sweet cherry. Environ. Exp. Bot. 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Usenik, V.; Stampar, F.; Petkovsek, M.M.; Kastelec, D. The effect of fruit size and fruit colour on chemical composition in ‘Kordia’ sweet cherry (Prunus avium L.). J. Food Compost. Anal. 2015, 38, 121–130. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Demir, N. Physicochemical characteristics, antioxidant activity, organic acid and sugar contents of 12 sweet cherry (Prunus avium L.) cultivars grown in Turkey. J. Food Sci. 2015, 80, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, S.; Homann, T.; Neugart, S.; Chmielewski, F.-M.; Götz, K.-P.; Gödeke, K.; Huschek, G.; Morlock, G.E.; Rawel, H.M. Selected plant metabolites involved in oxidation-reduction processes during bud dormancy and ontogenetic development in sweet cherry buds (Prunus avium L.). Molecules 2018, 23, 1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oszmiański, J.; Wojdyło, A. Influence of cherry leaf-spot on changes in the content of phenolic compounds in sour cherry (Prunus cerasus L.) leaves. Physiol. Mol. Plant Pathol. 2014, 86, 28–34. [Google Scholar] [CrossRef]
- Gonçalves, B.; Landbo, A.-K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef]
- Holzapfel, B.P.; Smith, J.P. Developmental stage and climatic factors impact more on carbohydrate reserve dynamics of Shiraz than cultural practice. Am. J. Enol. Vitic. 2012, 63, 333–342. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Yu, J.-G.; Wu, C.-S.; Wang, Z.-S.; Wang, Y.-K.; Zhu, D.-L.; Wang, M. Comparison of drip, pipe and surge spring root irrigation for jujube (Ziziphus jujuba Mill.) fruit quality in the Loess Plateau of China. PLoS ONE 2014, 9, 1–7. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Lozano, M.; Bernalte, M.J.; Ayuso, M.C.; López-Corrales, M.; González-Gómez, D. Physicochemical and bioactive properties evolution during ripening of ‘Ambrunés’ sweet cherry cultivar. LWT 2011, 44, 199–205. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, F.; Basile, B.; Morelli, G.; d’Andria, R.; Tonutti, P. Effects of irrigation on fruit ripening behavior and metabolic changes in olive. Sci. Hortic. 2012, 144, 201–207. [Google Scholar] [CrossRef]
- Mahhou, A.; DeJong, T.M.; Cao, T.; Shackel, K.S. Water stress and crop load effects on vegetative and fruit growth of ‘Elegant Lady’peach [Prunus persica (L.) Batch] trees. Fruits 2005, 60, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Whiting, M.D.; Ophardt, D. Comparing novel sweet cherry crop load management strategies. Hort. Sci. 2005, 40, 1271–1275. [Google Scholar] [CrossRef] [Green Version]
- Razouk, R.; Ibijbijen, J.; Kajji, A.; Karrou, M. Response of peach, plum and almond to water restrictions applied during slowdown periods of fruit growth. Am. J. Plant Sci. 2013, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Naor, A.; Stern, R.; Peres, M.; Greenblat, Y.; Gal, Y.; Flaishman, M.A. Timing and severity of postharvest water stress affect following-year productivity and fruit quality of field-grown Snow Queen nectarine. J. Am. Soc. Hortic. Sci. 2005, 130, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. FAO Irrig. Drain. Pap. 1998, 56, 1–50. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing Computer Program, Version 3.6.1; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.r-project.org (accessed on 21 January 2021).

| Irrigated | Non-irrigated | ANOVA a | |||||
|---|---|---|---|---|---|---|---|
| Gisela 5 | Weiroot 72 | Gisela 5 | Weiroot 72 | I | R | I × R | |
| Total sugars | 740.3 ± 19.8 Y | 810.4 ± 21.8 X | 724.5 ± 17.4 Y | 765.5 ± 25.8 X | NS | *** | NS |
| Total organic acids | 70.9 ± 3.4 AY | 78.0 ± 2.2 AX | 62.7 ± 1.7 BY | 68.5 ± 0.9 BX | *** | ** | NS |
| Total hydroxycinnamic acids | 1.01 ± 0.02 ab | 1.10 ± 0.02 a | 1.01 ± 0.03 ab | 1.22 ± 0.04 b | * | *** | * |
| Total flavonols and flavanols | 0.95 ± 0.04 B | 0.92 ± 0.03 B | 1.01 ± 0.03 A | 1.07 ± 0.04 A | ** | NS | NS |
| Total anthocyanins | 2.6 ± 0.1 Y | 3.1 ± 0.1 X | 2.5 ± 0.1 Y | 3.1 ± 0.1 X | NS | *** | NS |
| Sugar/acid ratio | 10.4 ± 0.7 | 10.3 ± 0.4 | 11.6 ± 0.4 | 11.2 ± 0.4 | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vosnjak, M.; Mrzlic, D.; Hudina, M.; Usenik, V. The Effect of Water Supply on Sweet Cherry Phytochemicals in Bud, Leaf and Fruit. Plants 2021, 10, 1131. https://doi.org/10.3390/plants10061131
Vosnjak M, Mrzlic D, Hudina M, Usenik V. The Effect of Water Supply on Sweet Cherry Phytochemicals in Bud, Leaf and Fruit. Plants. 2021; 10(6):1131. https://doi.org/10.3390/plants10061131
Chicago/Turabian StyleVosnjak, Matej, Davor Mrzlic, Metka Hudina, and Valentina Usenik. 2021. "The Effect of Water Supply on Sweet Cherry Phytochemicals in Bud, Leaf and Fruit" Plants 10, no. 6: 1131. https://doi.org/10.3390/plants10061131






