Evaluation of the Antidiabetic and Antihyperlipidemic Activity of Spondias purpurea Seeds in a Diabetic Zebrafish Model
Abstract
:1. Introduction
2. Results
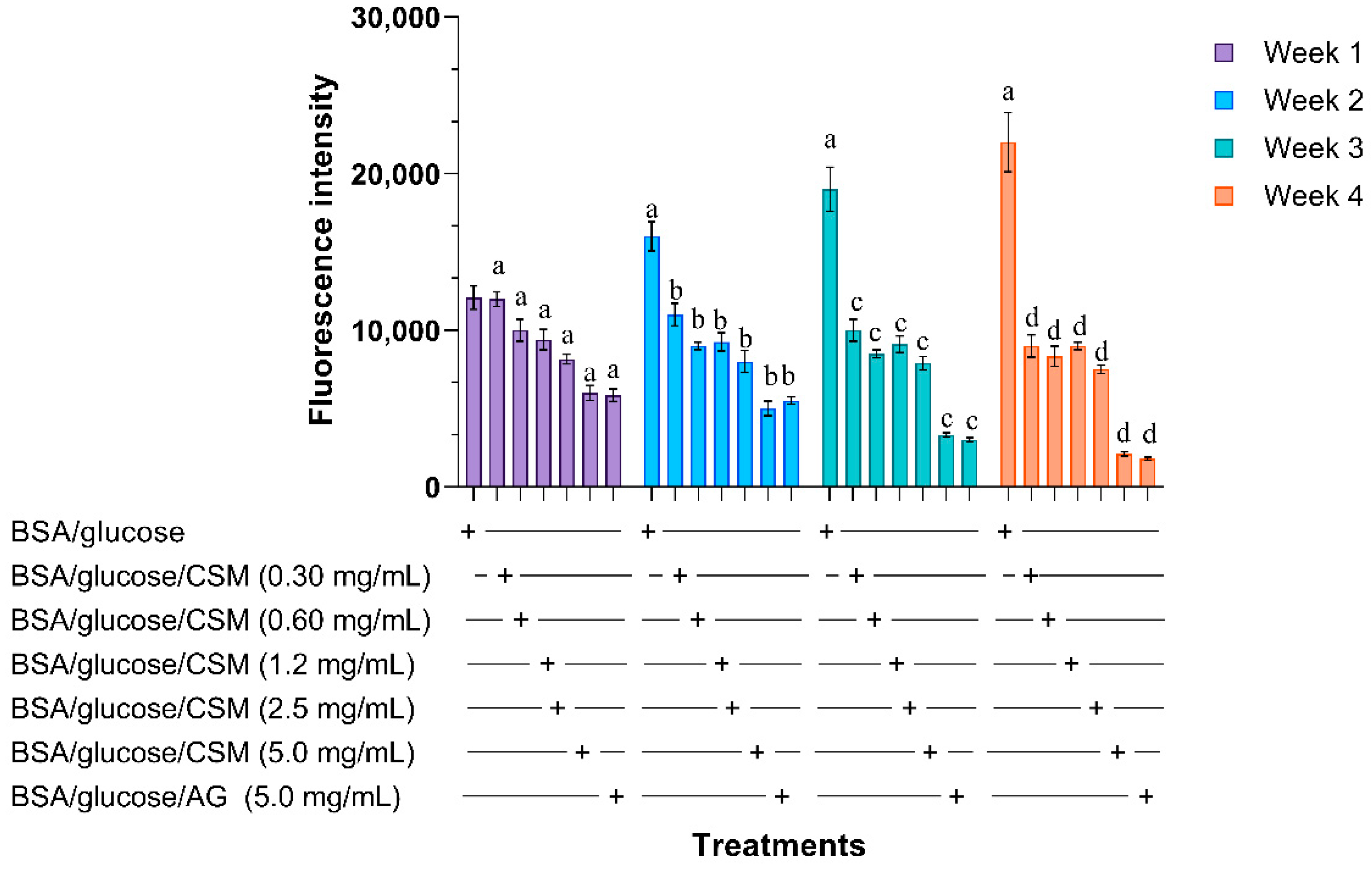
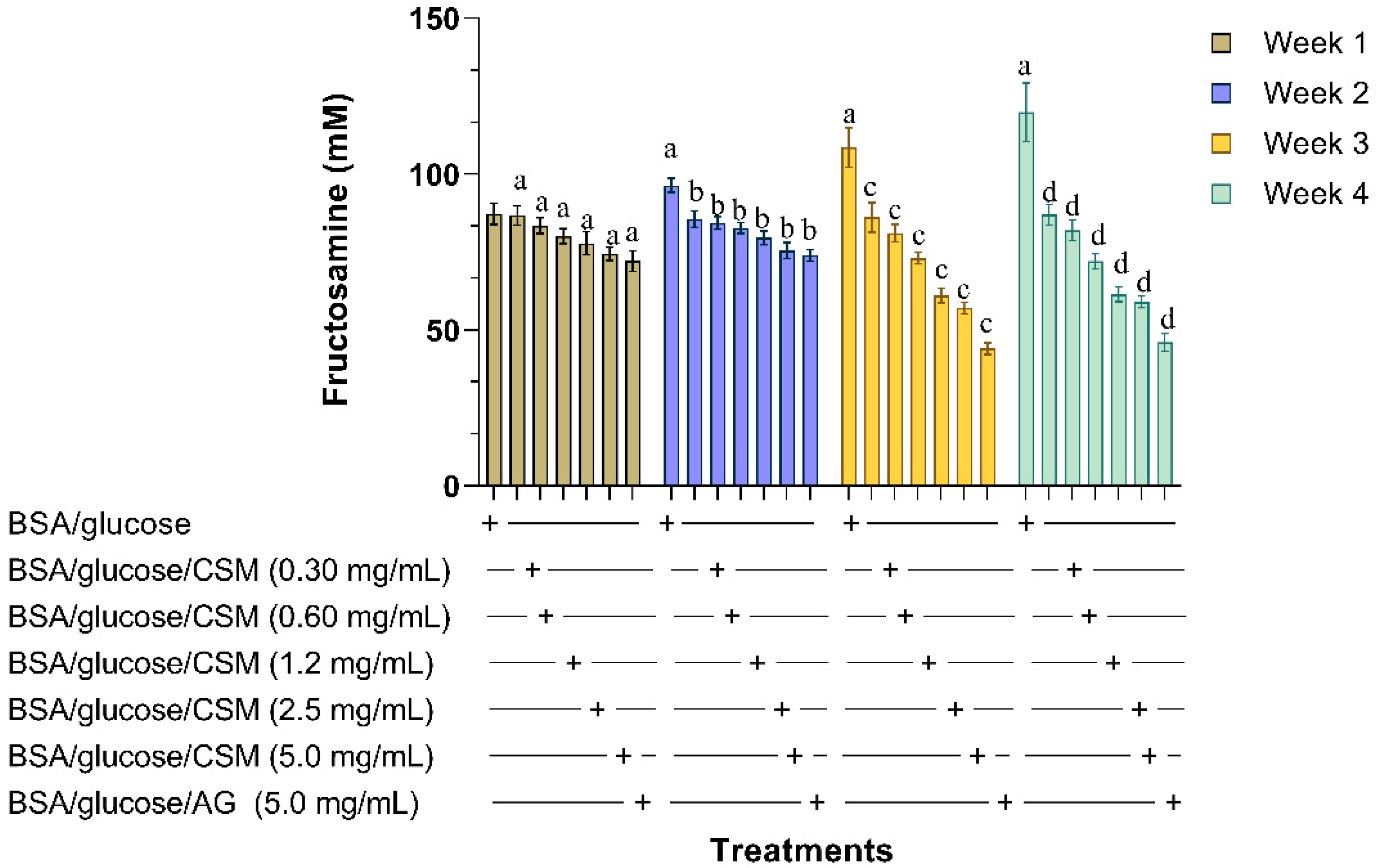
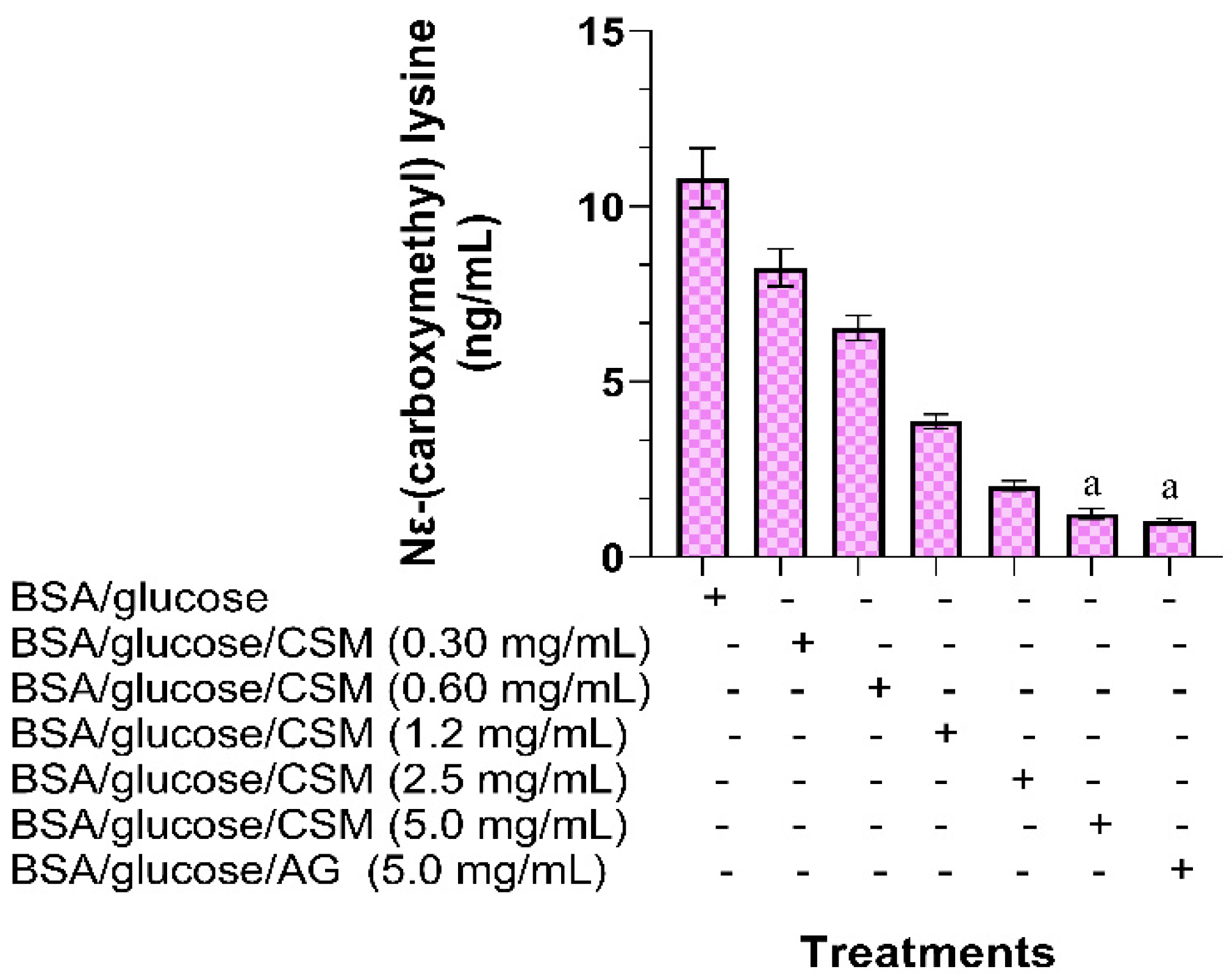
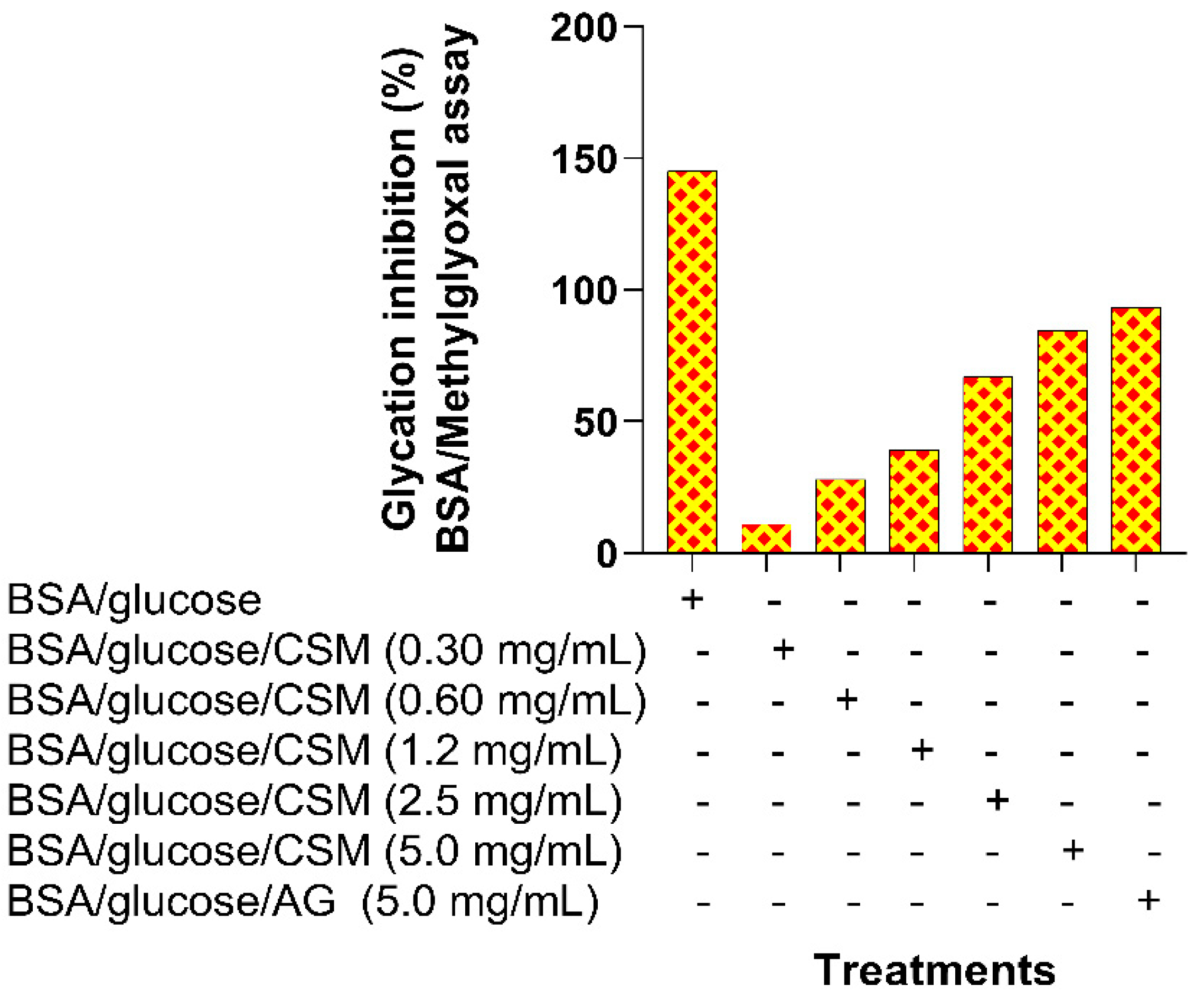
2.1. Inhibition Tests for the Formation of Advanced Glycation End Products In Vitro
2.1.1. Glycation of Bovine Albumin
2.1.2. Determination of Fructosamine
2.1.3. Determination of Nɛ-(carboxymethyl) Lysine
2.1.4. BSA-Methylglyoxal Assay
2.2. In Vivo Experiments
2.2.1. Toxicity Test of the Methanolic Extract of Spondias purpurea
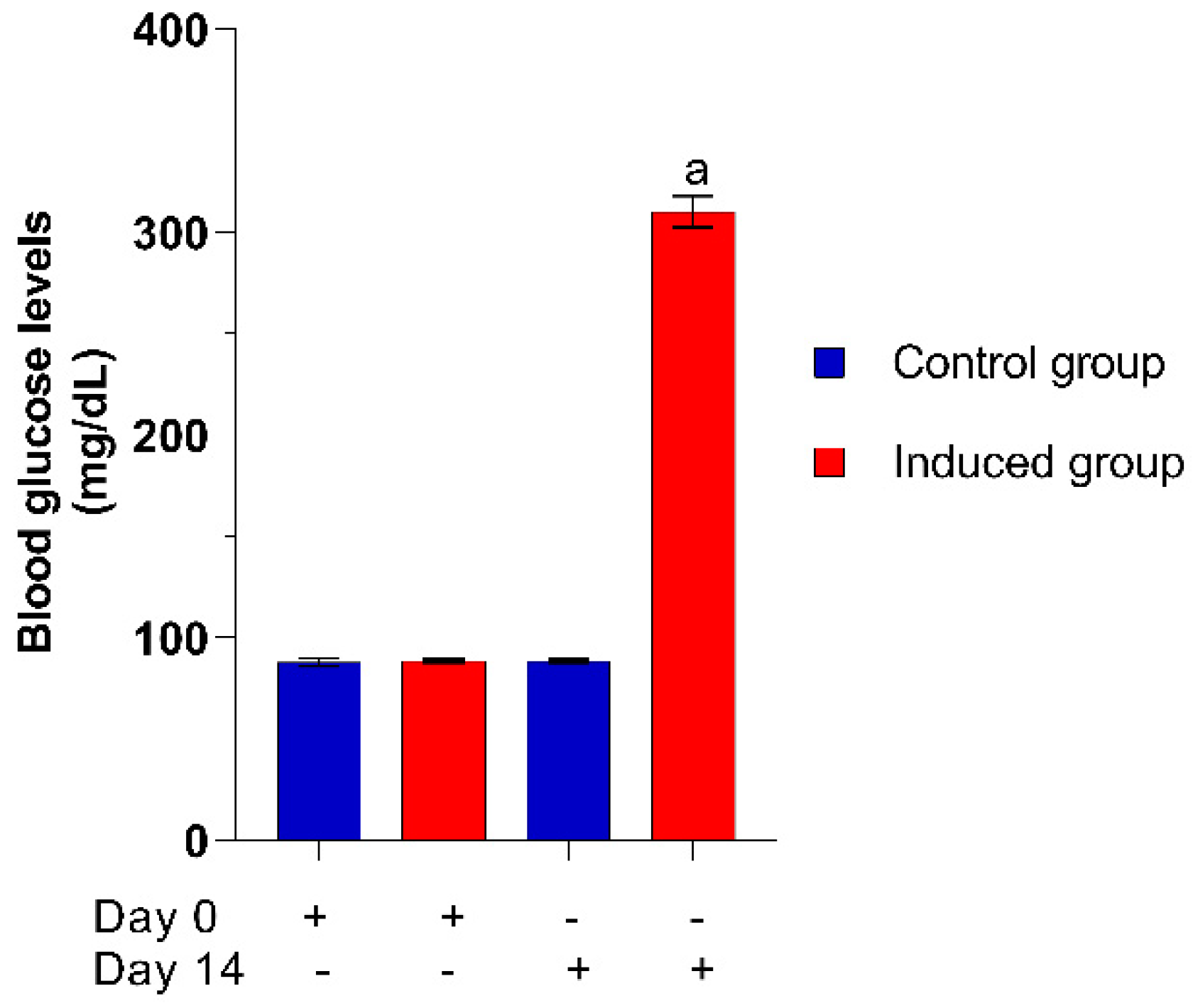
2.2.2. Hyperglycemia Induction
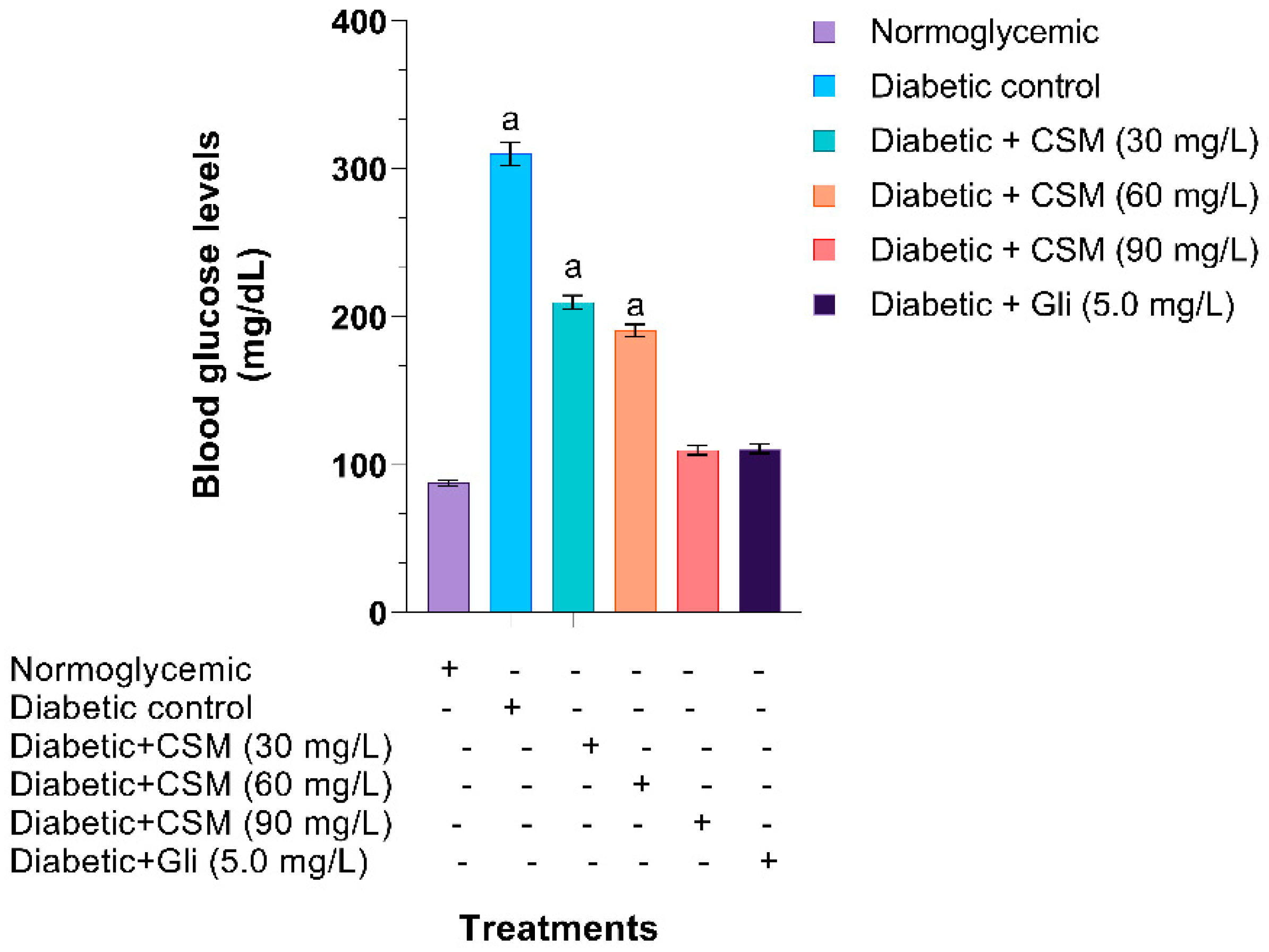
2.2.3. Effect of CSM in Blood Glucose Levels
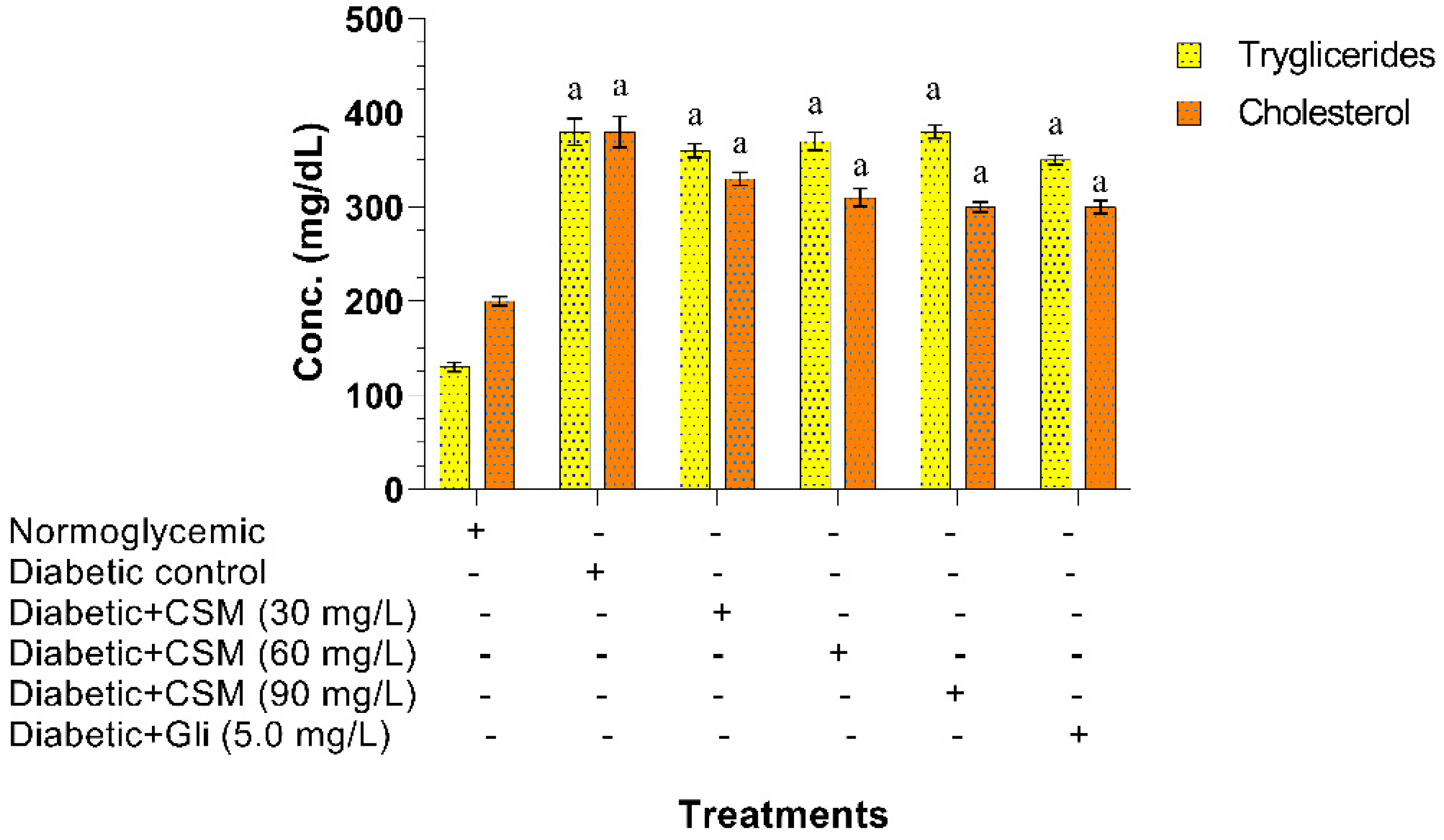
2.2.4. Effect of CSM in Triglyceride and Cholesterol Levels
2.2.5. Effect of CSM in the Inhibition of AGEs In Vivo
3. Discussion
4. Materials and Methods
4.1. Raw Material Conditioning and Extract Preparation
4.2. In Vitro Experiments
4.2.1. In Vitro Glycation of Bovine Albumin
4.2.2. Fructosamine Concentration
4.2.3. BSA-Methylglyoxal Assay
4.2.4. Determination of Nɛ-(carboxymethyl) Lysine
4.3. In Vivo Experiments
4.3.1. Conditioning of Adult Zebrafish
4.3.2. Toxicity Test of the Methanolic Extract of Spondias purpurea
4.3.3. Hyperglycemia Induction
4.3.4. Administration of the Methanol Extract of Spondias purpurea
Experimental Design
- -
- group 1: normoglycemic fish (without treatment administration).
- -
- group 2 (negative control): glucose-induced diabetic fish (without treatment administration).
- -
- group 3: glucose-induced diabetic fish administered with 30 mg/L CSM.
- -
- group 4: glucose-induced diabetic fish administered with 60 mg/L CSM.
- -
- group 5: glucose-induced diabetic fish administered with 90 mg/L CSM.
- -
- group 6 (positive control): glucose-induced diabetic fish administered with 5 mg/L glibenclamide.
4.3.5. Anesthesia and Sacrifice
4.3.6. Analysis of Blood Glucose, Triglycerides, and Total Cholesterol Levels
4.3.7. Evaluation of the Inhibition of Advanced Glycation End Products
4.3.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Shamah, T.; Cuevas, L.; Rivera, J.M.H. Encuesta Nacional de Salud y Nutrición-MC 2016; ENSANUT: Morelos, México, 2016; pp. 1–149. Available online: https://ensanut.insp.mx/encuestas/ensanut2016/descargas.php (accessed on 14 February 2021).
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes Am. Diabetes Assoc. 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronald, P.; Guohua, C. Antioxidant capacity and polyphenolic components of teas: Implications for altering in vivo antioxidant status. Soc. Exp. Biol. Med. 1999, 220, 255–261. [Google Scholar]
- Rahman, T.; Hosen, I.; Islam, M.M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997–1019. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, S.; Matsui, T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxidative Med. Cell. Longev. 2010, 3, 101–108. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Ozougwu, O. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol. Pathophysiol. 2013, 4, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga-Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.K.; Bansal, M. MPPT Techniques—A Review. Adv. Mater. Res. 2014, 1055, 182–187. [Google Scholar] [CrossRef]
- Altemimi, A.; Lightfoot, D. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Synthesis of different-sized silver nanoparticles by simply varying reaction conditions with leaf extracts of Bauhinia variegata L. IET Nanobiotechnol. 2012, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Schaal, B. Domestication of a Mesoamerican cultivated fruit tree, Spondias purpurea. Proc. Natl. Acad. Sci. USA 2005, 102, 12801–12806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz-León, A.; Pita-Duque, Á.; Rodríguez-Haros, B. Jocotes, Jobos, Abales o Ciruelas Mexicanas; Universidad Autónoma Chapingo: Texcoco, Mexico, 2010; Volume 1, pp. 77–101. [Google Scholar]
- Villa-Hernández, J.M.; Mendoza-Cardoso, G.; Mendoza-Espinoza, J.A.; Vela-Hinojosa, C.; Díaz de León-Sánchez, F.; Rivera-Cabrera, F.; Alia-Tejacal, I.; Pérez-Flores, L.J. Antioxidant capacity in vitro and in vivo of various ecotypes of Mexican plum (Spondias purpurea L.). J. Food Sci. 2017, 82, 2576–2582. [Google Scholar] [CrossRef]
- Álvarez-Vargas, J.E.; Alia-Tejacal, I.; Chávez-Franco, S.H.; Colinas-León, M.T.; Nieto-Ángel, D.; Rivera-Cabrera, F.A. Ciruelas Mexicanas (Spondias purpurea L.) de clima húmedo y seco: Calidad, metabolitos funcionales y actividad antioxidante. Interciencia 2017, 42, 653–660. [Google Scholar]
- Alia-Tejacal, I.; Astudillo-Maldonado, Y.I.; Núñez-Colín, C.A.; Valdez-Aguilar, L.A.; Bautista-Baños, S.; García-Vázquez, E.; Ariza-Flores, R.; Rivera-Cabrera, F. Caracterización de frutos de ciruela mexicana (Spondias purpurea L.) del sur de México. Nota científica Rev. Fitotec. Mex. 2012, 35, 21–26. [Google Scholar]
- Miller, A.J.; Schaal, B.A. Domestication and the distribution of genetic variation in wild and cultivated populations of the Mesoamerican fruit tree Spondias purpurea L. (Anacardiaceae). Mol. Ecol. 2006, 15, 1467–1480. [Google Scholar] [CrossRef]
- Ceva-Antunes, P.M.N.; Bizzo, H.R.; Silva, A.S.; Carvalho, C.P.S.; Antunes, O.A.C. Analysis of volatile composition of siriguela (Spondias purpurea L.) by solid phase microextraction (SPME). LWT Food Sci. Technol. 2006, 39, 437–443. [Google Scholar] [CrossRef]
- Kozioł, M.J.; Macía, M.J. Chemical composition, nutritional evaluation, and economic prospects of Spondias purpurea (Anacardiaceae). Econ. Bot. 1998, 52, 373–380. [Google Scholar] [CrossRef]
- Baldosano, H.; Castillo, M.G.; Elloran, C.; Bacani, F.T. Effect of particle size, solvent and extraction time on tannin extract from Spondias purpurea bark through soxhlet extraction. Proc. DLSU Res. Congr. 2015, 3, 4–9. [Google Scholar]
- Olufunke, M.D.; Kasali, A.A.; Olusegun, E. Constituents of the Spondias mombin linn and the comparison between its fruit and leaf essential oils. J. Essent. Oil-Bear. Plants 2003, 6, 148–152. [Google Scholar] [CrossRef]
- Silva, R.V.; Costa, S.C.C.; Branco, C.R.C.; Branco, A. In vitro photoprotective activity of the Spondias purpurea L. peel crude extract and its incorporation in a pharmaceutical formulation. Ind. Crops Prod. 2016, 83, 509–514. [Google Scholar] [CrossRef]
- Ednaldo, Q.D.L.; Elisabeth, D.O.; Helio, R.D.B. Extraction and characterization of the essential oils from Spondias mombin L. (Caj), Spondias purpurea L. (Ciriguela) and Spondia ssp. (Cajarana do serto). Afr. J. Agric. Res. 2016, 11, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Sameh, S.; Al-Sayed, E.; Labib, R.M.; Singab, A.N. Genus Spondias: A phytochemical and pharmacological review. Evid. Based Complement. Altern. Med. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ragasa, C.Y.; Poblete, A.T.; Navida, E.C. Antimicrobial compounds from Spondias purpurea. Manila J. Sci. 2001, 4, 24–28. [Google Scholar]
- Garduño, C.P.; Barrera, L.L.N.; Rios, Y.G. Evaluation of the fungicidal of leaves powders and extracts of fifteen mexican plants against Fusarium oxysporum f. sp. gadioli (Massey) Snyder and Hansen. Plant Pathol. J. 2010, 9, 103–111. [Google Scholar]
- Ferreira, C.L.A.; Alves, S.B.; Italo, T.S.; Alves, H.B.C.; Helder-Reis, C.C.; Vanusa, M.S.; Leone, L.A.; Araújo, L.R.; Dos Santos, V.L.; Gonçalves, A.; et al. Spondias purpurea L. (Anacardiaceae): Antioxidant and antiulcer activities of the leaf hexane extract. Oxidative Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar]
- Muñiz, A.; Garcia, E.; Gonzalez, D.; Zuñiga, L. Antioxidant activity and in vitro antiglycation of the fruit of Spondias purpurea. Evid. Based Complement. Altern. Med. 2018, 5, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Sharma, S.; Patial, V.; Singh, D.; Padwad, Y.S. Zebrafish (Danio rerio): A potential model for nephroprotective drug screening. Clin. Queries Nephrol. 2014, 3, 97–105. [Google Scholar] [CrossRef]
- Kang, M.C.; Lee, S.H.; Lee, W.W.; Kang, N.; Kim, E.A.; Kim, S.Y.; Lee, D.H.; Kim, D.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Ishige okamurae against high-glucose induced oxidative stress in human umbilical vein endothelial cells and zebrafish model. J. Funct. Foods 2014, 11, 304–312. [Google Scholar] [CrossRef]
- Wang, J.J.; Li, Y.J.; Lai, K.; Zhong, Q.M.; Demin, K.A.; Kalueff, A.V.; Song, C. High-glucose/high-cholesterol diet in zebrafish evokes diabetic and affective pathogenesis: The role of peripheral and central inflammation, microglia and apoptosis. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2020, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bradford, Y.M.; Toro, S.; Ramachandran, S.; Ruzicka, L.; Howe, D.G.; Eagle, A.; Kalita, P.; Martin, R.; Moxon, S.A.T.; Schaper, K.; et al. Zebrafish models of human disease: Gaining insight into human disease at ZFIN. ILAR J. 2017, 58, 4–16. [Google Scholar] [CrossRef] [Green Version]
- Benchoula, K.; Khatib, A.; Quzwain, F.M.C.; Che Mohamad, C.A.; Wan Sulaiman, W.M.A.; Wahab, R.A.; Ahmed, Q.U.; Ghaffar, M.A.; Saiman, M.Z.; Alajmi, M.F.; et al. Optimization of hyperglycemic induction in zebrafish and evaluation of its blood glucose level and metabolite fingerprint treated with Psychotria malayana Jack leaf extract. Molecules 2019, 24, 1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchoula, K.; Khatib, A.; Jaffar, A.; Ahmed, Q.U.; Sulaiman, W.M.A.W.; Wahab, R.A.; El-Seedi, H.R. The promise of zebrafish as a model of metabolic syndrome. Exp. Anim. 2019, 68, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M.; et al. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front. 2019, 9, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Castillo, H.A.; Brown, J.; Kaslin, J.; Dwyer, K.M.; Gibert, Y. High glucose levels affect retinal patterning during zebrafish embryogenesis. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Heckler, K.; Kroll, J. Zebrafish as a model for the study of microvascular complications of diabetes and their mechanisms. Int. J. Mol. Sci. 2017, 18, 2002. [Google Scholar] [CrossRef] [Green Version]
- Garcia, H.; Perez, R.; Manriquez, G.; Muñiz, A. Protection of silver nanoparticles using Eysenhardtia polystachya in peroxide-induced pancreatic β-cell damage and their antidiabetic properties in zebrafish. Int. J. Nanomed. 2018, 13, 2601–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Guidelines for the Testing of Chemicals: 203—Fish, Acute Toxicity Test; OECD Library, 1992; pp. 1–8. [Google Scholar] [CrossRef]
- Hao, K.; Li, Y.; Feng, J.; Zhang, W.; Zhang, Y.; Ma, N.; Zeng, Q.; Pang, H.; Wang, C.; Xiao, L.; et al. Ozone promotes regeneration by regulating the inflammatory response in zebrafish. Int. Immunopharmacol. 2015, 28, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Amatruda, J.F.; Shepard, J.L.; Stern, H.M.; Zon, L.I. Zebrafish as a cancer model system. Cancer Cell. 2002, 1, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Belyaeva, N.F.; Kashirtseva, V.N.; Medvedeva, N.V.; Khudoklinova, Y.Y.; Ipatova, O.M.; Archakov, A.I. Zebrafish as a model system for biomedical studies. Biochem. Suppl. Ser. B Biomed. Chem. 2009, 3, 343–350. [Google Scholar] [CrossRef]
- Gerhard, G.S. Comparative aspects of zebrafish (Danio rerio) as a model for aging research. Exp. Gerontol. 2003, 38, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarras, M.P.; Leontovich, A.A.; Intine, R.V. Use of zebrafish as a model to investigate the role of epigenetics in propagating the secondary complications observed in diabetes mellitus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 178, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Elo, B.; Villano, C.M.; Govorko, D.; White, L.A. Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J. Mol. Endocrinol. 2007, 38, 433–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wen, C.; Peng, J.; Korzh, V.; Gong, Z. Generation of living color transgenic zebrafish to trace somatostatin-expressing cells and endocrine pancreas organization. Differentiation 2009, 77, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Capiotti, K.M.; Antonioli, R.; Kist, L.W.; Bogo, M.R.; Bonan, C.D.; Da Silva, R.S. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 171, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, Y.; Chen, K.; Reynolds, A.L.; Waghorne, N.; O’Connor, J.J.; Kennedy, B.N. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. DMM Dis. Model. Mech. 2010, 3, 236–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capiotti, K.; De Moraes, D.; Menezes, F.; Kist, L.; Bogo, M.; Da Silva, R. Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Dario rerio). Behav. Brain Res. 2014, 274, 319–325. [Google Scholar] [CrossRef]
- Tseng, Y.C.; Chen, R.D.; Lee, J.R.; Liu, S.T.; Lee, S.J.; Hwang, P.P. Specific expression and regulation of glucose transporters in zebrafish ionocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R275–R290. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, M.; Connaughton, V.; Arneson, L.S. Induction of hyperglycaemia in zebrafish (Danio rerio) leads to morphological changes in the retina. Acta Diabetol. 2007, 44, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, X.; Xiao, W.; Ma, W.; Li, T.; Huang, J.; Liu, X.; Liang, X.; Tang, S.; Luo, Y. Mitochondria-targeted antioxidant peptide SS31 attenuates high glucose-induced injury on human retinal endothelial cells. Biochem. Biophys. Res. Commun. 2011, 404, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Huo, D.; Wang, S.; Qian, Q. Inhibition of glucose-induced vascular endothelial growth factor expression by Salvia miltiorrhiza hydrophilic extract in human microvascular endothelial cells: Evidence for mitochondrial oxidative stress. J. Ethnopharmacol. 2011, 137, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.; Egan, J. Pharmacological Agents That Directly Modulate Insulin secretion. Pharmacol. Rev. 2003, 55, 105–131. [Google Scholar] [CrossRef]
- Frazier, D.T.; Narahashi, T. Tricaine (MS-222): Effects on ionic conductances of squid axon membranes. Eur. J. Pharmacol. 1975, 33, 313–317. [Google Scholar] [CrossRef]
- Brisson, G.; Camu, F.; Malaisse-Lagae, F.; Malaisse, W. Effect of a local anesthetic upon calcium uptake and insulin secretion by isolated islets of Langerhans. Life Sci. 1971, 10, 445–448. [Google Scholar] [CrossRef]
- Eames, S.C.; Philipson, L.H.; Prince, V.E.; Kinkel, M.D. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish 2010, 7, 205–213. [Google Scholar] [CrossRef]
- Münch, G.; Westcott, B.; Menini, T.; Gugliucci, A. Advanced glycation end products and their pathogenic roles in neurological disorders. Amino Acids 2012, 42, 1221–1236. [Google Scholar] [CrossRef]
- Ortwerth, B.J.; James, H.; Simpson, G.; Linetsky, M. The generation of superoxide anions in glycation reactions with sugars, osones, and 3-deoxyosones. Biochem. Biophys. Res. Commun. 1998, 245, 161–165. [Google Scholar] [CrossRef]
- Mullarkey, C.J.; Edelstein, D.; Brownlee, M. Free radical generation by early glycation products: A mechanism for accelerated atherogenesis in diabetes. Biochem. Biophys. Res. Commun. 1990, 173, 932–939. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. 2014, 53, 10316–10329. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M. Fatty acids and glucolipotoxicity in the pathogenesis of Type 2 diabetes. Biochem. Soc. Trans. 2008, 36, 348–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol metabolism, pancreatic β-cell function and diabetes. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Hannaert, J.C.; Grupping, A.Y.; Pipeleers, D.G. Low density lipoprotein can cause death of islet β-cells by its cellular uptake and oxidative modification. Endocrinology 2002, 143, 3449–3453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Liu, J.; Hou, F.; Liu, Z.; Cao, X.; Seo, H.; Gao, B. Cholesterol induces pancreatic β cell apoptosis through oxidative stress pathway. Cell Stress Chaperones 2011, 16, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kari, G.; Rodeck, U.; Dicker, A.P. Zebrafish: An emerging model system for human disease and drug discovery. Clin. Pharmacol. Ther. 2007, 82, 70–80. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P. Inhibitory Activities of guaianolides from the seeds of Byrsonima crassifolia against protein glycation in vitro. Med. Chem. 2015, 5, 217–225. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-Anun, S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yagiz, Y.; Buran, T.J.; Nunes, C.; Do, N.; Gu, L. Phytochemicals from berries and grapes inhibited the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Res. Int. 2011, 44, 2666–2673. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñiz-Ramirez, A.; Garcia-Campoy, A.H.; Pérez Gutiérrez, R.M.; Garcia Báez, E.V.; Mota Flores, J.M. Evaluation of the Antidiabetic and Antihyperlipidemic Activity of Spondias purpurea Seeds in a Diabetic Zebrafish Model. Plants 2021, 10, 1417. https://doi.org/10.3390/plants10071417
Muñiz-Ramirez A, Garcia-Campoy AH, Pérez Gutiérrez RM, Garcia Báez EV, Mota Flores JM. Evaluation of the Antidiabetic and Antihyperlipidemic Activity of Spondias purpurea Seeds in a Diabetic Zebrafish Model. Plants. 2021; 10(7):1417. https://doi.org/10.3390/plants10071417
Chicago/Turabian StyleMuñiz-Ramirez, Alethia, Abraham Heriberto Garcia-Campoy, Rosa Martha Pérez Gutiérrez, Efrén Venancio Garcia Báez, and José María Mota Flores. 2021. "Evaluation of the Antidiabetic and Antihyperlipidemic Activity of Spondias purpurea Seeds in a Diabetic Zebrafish Model" Plants 10, no. 7: 1417. https://doi.org/10.3390/plants10071417
APA StyleMuñiz-Ramirez, A., Garcia-Campoy, A. H., Pérez Gutiérrez, R. M., Garcia Báez, E. V., & Mota Flores, J. M. (2021). Evaluation of the Antidiabetic and Antihyperlipidemic Activity of Spondias purpurea Seeds in a Diabetic Zebrafish Model. Plants, 10(7), 1417. https://doi.org/10.3390/plants10071417





