Unravelling the Effect of Provitamin A Enrichment on Agronomic Performance of Tropical Maize Hybrids
Abstract
:1. Introduction
2. Results
2.1. Carotenoid Accumulation in Hybrids
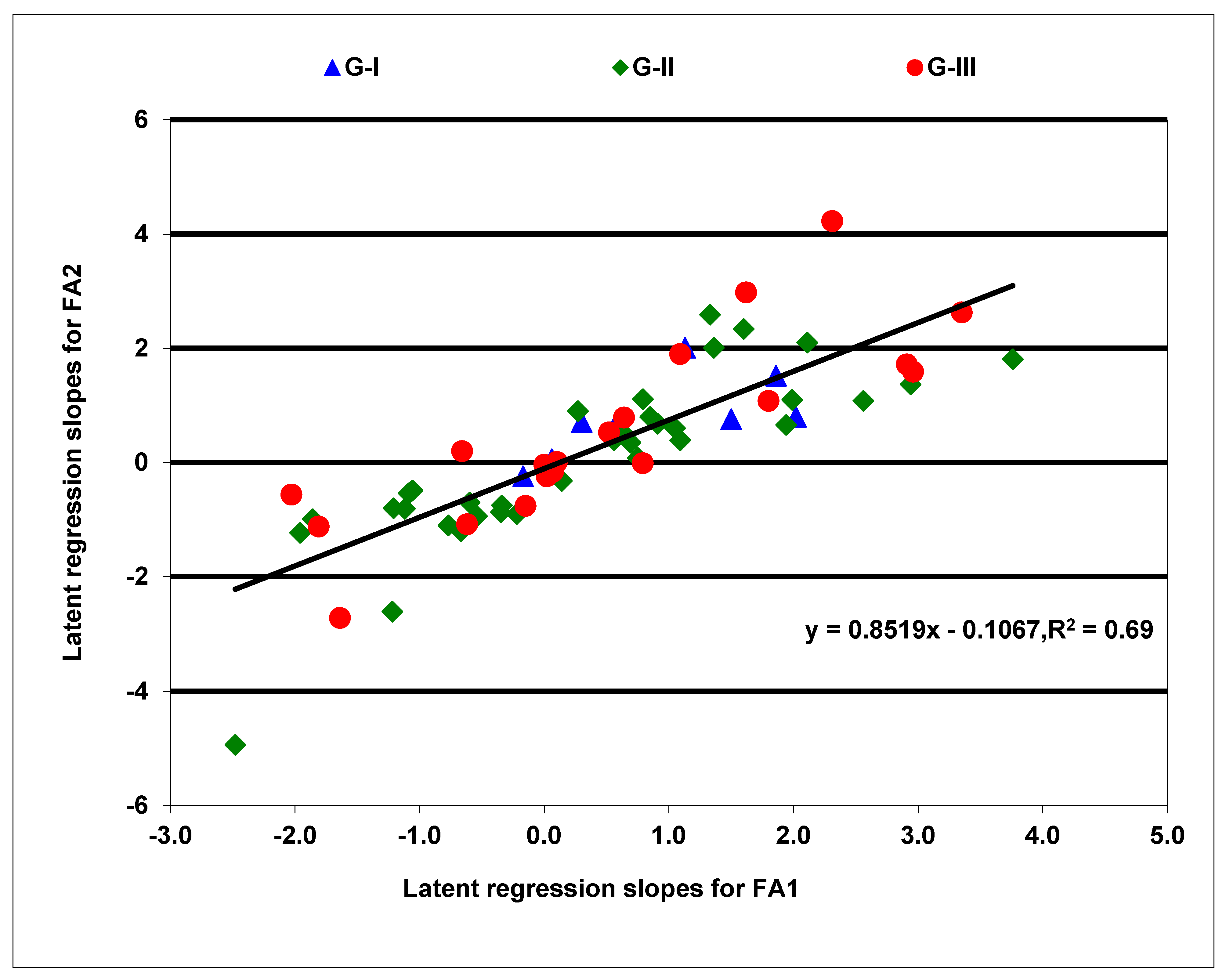
2.2. Stability of Hybrids in Accumulating Provitamin A
2.3. Agronomic Performance of Provitamin A Biofortified Hybrids in Diverse Field Environments
2.4. Yield Stability of Biofortified Maize Hybrids across Diverse Field Environments
2.5. Trait Correlations for Carotenoids and Agronomic Traits
3. Discussion
3.1. Environmental Effects on Carotenoid Composition and Content
3.2. Changes That Occurred in Pro-Vitamin A and Non-Provitamin A Carotenoids
3.3. Adaptability of PVA Hybrids across Diverse Growing Environments
3.4. Grain Yield of PVA Hybrids across Diverse Growing Environments
3.5. Effect of Accumulating PVA Carotenoids on Agronomic Performance of Hybrids
4. Materials and Methods
4.1. Genetic Materials
4.2. Performance Evaluation in Multi-Environment Trials (MET)
4.3. Agronomic Trait Measurements
4.4. Analysis of Carotenoids
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shiferaw, B.; Boddupalli, M.P.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef] [Green Version]
- Ekpa, O.; Palacios-Rojas, N.; Kruseman, G.; Fogliano, V.; Linnemann, A. Sub-Saharan African maize-based foods: Processing practices, challenges and opportunities. Food Rev. Int. 2019, 35, 609–639. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Food Balance Sheets. 2019. Available online: http://www.fao.org/faostat/en/#data/FBS (accessed on 10 April 2019).
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A Paramount Staple Crop in the Context of Global Nutrition. Compr. Rev. Food Sci. Food Saf. 2010, 9, 417–436. [Google Scholar] [CrossRef]
- FAO; OECD; OECD-FAO. Agricultural Outlook 2019–2018; FAO; OECD: Washington, DC, USA, 2019.
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global Maize Production, Utilization, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef]
- Palacios-Rojas, N.; McCulley, L.; Kaeppler, M.; Titcomb, T.J.; Gunaratna, N.S.; Lopez-Ridaura, S.; Tanumihardjo, S.A. Mining maize diversity and improving its nutritional aspects within agro-food systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1–26. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef]
- Wurtzel, E.T.; Cuttriss, A.; Vallabhaneni, R. Maize provitamin A carotenoids, current resources, and future metabolic engineering challenges. Front. Plant Sci. 2012, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H.E.; Saltzman, A. Improving Nutrition through Biofortification: A Review of Evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Pixley, K.; Palacios-Rojas, N.; Babu, R.; Mutale, R.; Surles, R.L.; Simpungwe, E. Biofortification of maize with provitamin A carotenoids. In Carotenoids and Human Health; Tanumihardjo, S.A., Ed.; Springer Science: New York, NY, USA, 2013; pp. 271–292. [Google Scholar]
- Giuliano, G. Provitamin A biofortification of crop plants: A gold rush with many miners. Curr. Opin. Biotechnol. 2017, 44, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Menkir, A.; Maziya-Dixon, B.; Mengesha, W.; Rocheford, T.; Alamu, E.O. Accruing genetic gain in pro-vitamin A enrichment from harnessing diverse maize germplasm. Euphytica 2017, 213, 105. [Google Scholar] [CrossRef]
- Dhliwayo, T.; Palacios-Rojas, N.; Crossa, J.; Pixley, K.V. Effects of S1 recurrent selection for provitamin a carotenoid content for three open-pollinated maize cultivars. Crop Sci. 2014, 54, 2449–2460. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, B.M.; Palacios-Rojas, N.; Hossain, F.; Muthusamy, V.; Menkir, A.; Dhliwayo, T.; Ndhlela, T.; San, V.F.; Nair, S.K.; Vivek, B.S.; et al. Molecular Breeding for Nutritionally Enriched Maize: Status and Prospects. Front. Genet. 2020, 10, 1392. [Google Scholar] [CrossRef] [Green Version]
- Strobbe, S.; De Lepeleire, J.; Der Straeten, D.V. From in planta function to vitamin-rich food crops: The ACE of biofortification. Front. Plant Sci. 2018, 9, 1862. [Google Scholar] [CrossRef]
- Wurtzel, E.T. Changing Form and function through carotenoids and synthetic biology. Plant Phys. 2019, 179, 830–843. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Li, L. Toward the ‘golden’ era: The status in uncovering the regulatory control of carotenoid accumulation in plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef]
- Checker, V.G.; Kushwaha, H.R.; Kumari, P.; Yadav, S. Role of Phytohormones in Plant Defence: Signaling and Cross Talk. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Singapore, 2018; pp. 159–184. ISBN 978-981-10-7370. [Google Scholar]
- Zanga, D.; Capell, T.; Slafer, G.A.; Christou, P.; Savin, R.A. A carotenogenic mini-pathway introduced into white corn does not affect development or agronomic performance. Sci. Rep. 2016, 6, 38288. [Google Scholar] [CrossRef] [Green Version]
- Halilu, A.D.; Ado, S.G.; Aba, D.A.; Usman, I.S. Genetics of carotenoids for provitamin A biofortification in tropical-adapted maize. Crop J. 2016, 4, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Senete, C.T.; de Oliveira Guimaraes, P.E.; Paes, M.C.D.; de Souza, J.C. Diallel analysis of maize inbred lines for carotenoids and grain yield. Euphytica 2011, 182, 395–404. [Google Scholar] [CrossRef]
- Ortiz-Covarrubias, Y.; Dhliwayo, T.; Palacios-Rojas, N.; Ndhlela, T.; Magorokosho, C.; Aguilar-Rincon, V.H. Effects of drought and low nitrogen stress on provitamin A carotenoid content of biofortified maize hybrids. Crop Sci. 2019, 59, 2521–2532. [Google Scholar] [CrossRef] [Green Version]
- Menkir, A.; Gedil, M.; Tanumihardjo, S.A.; Adepoju, A.; Bossey, B. Carotenoid accumulation and agronomic performanca of maize hybrids involving parental combinations from different marker-based groups. Food Chem. 2014, 148, 131–137. [Google Scholar] [CrossRef]
- Suwarno, W.B.; Pixley, K.V.; Palacios-Rojas, N.; Kaeppler, S.M.; Babu, R. Formation of Heterotic Groups and Understanding Genetic Effects in a Provitamin A Biofortified Maize Breeding Program. Crop Sci. 2014, 54, 14–24. [Google Scholar] [CrossRef]
- Khamkoh, W.; Ketthaisong, D.; Lomthaisong, K.; Lertrat, K.; Suriharn, B. Recurrent selection method for improvement of lutein and zeaxanthin in orange waxy corn populations. Aust. J. Crop Sci. 2019, 13, 566–573. [Google Scholar] [CrossRef]
- Othman, R.; Zaifuddin, F.A.M.; Hasan, N.M. Carotenoid Biosynthesis Regulatory Mechanisms in Plants. J. Oleo Sci. 2014, 63, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Keum, Y.S. Significance of genetic, environmental, and pre-and post-harvest factors affecting carotenoid contents in crops: A review. J. Agric. Food Chem. 2018, 66, 5310–5324. [Google Scholar] [CrossRef]
- Taylor, M.; Ramsay, G. Carotenoid biosynthesis in plant storage organs: Recent advances and prospects for improving plant food quality. Physiol. Plant. 2005, 124, 143–151. [Google Scholar] [CrossRef]
- Li, H.; Tsao, R.; Deng, Z. Factors affecting the antioxidant potential and health benefits of plant foods. Can. J. Plant Sci. 2012, 92, 1101–1111. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Bidel, L.P.R.; Urban, L. Carotenoid responses to environmental stimuli: Integrating redox and carbon controls into a fruit model. Plant Cell Environ. 2014, 37, 273–289. [Google Scholar] [CrossRef]
- O’Hare, T.J.; Fanning, K.J.; Martin, I.F. Zeaxanthin biofortification of sweet-corn and factors affecting zeaxanthin accumulation and colour change. Arch. Biochem. Biophys. 2015, 572, 184–187. [Google Scholar] [CrossRef]
- Mengesha, A.W.; Menkir, A.; Meseka, S.K.; Bossey, B.; Afolabi, A.; Burgueno, J.; Crossa, J. Factor analysis to investigate genotype and genotype X environment interaction effects on pro-vitamin A. Euphytica 2019, 215, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.B.; Ganesalingam, A.; Kuchel, H. Factor analytic mixed models for the provision of grower information from national crop variety testing programs. Theor. Appl. Genet. 2015, 128, 55–72. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Moster, J.B.; Quackenbush, F.W. The effects of temperature and light on the carotenoids of seedlings grown from three corn hybrids. Arch. Biochem. Biophys. 1952, 38, 297–303. [Google Scholar] [CrossRef]
- Chenard, C.H.; Kopsell, D.A.; Kopsell, D.E. Nitrogen concentration affects nutrient and carotenoid accumulation in parsley. J. Plant Nutr. 2005, 28, 285–297. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E. Genetic and environmental factors affecting plant lutein/zeaxanthin. Agro Food Ind. Hi-Tech 2008, 19, 44–46. [Google Scholar]
- Ali, Q.; Ashraf, M.; Anwar, F. Seed composition and seed oil antioxidant activity of maize under water stress. J. Am. Oil Chem. Soc. 2010, 87, 1179–1187. [Google Scholar]
- Brunson, A.M.; Quackenbush, F.W. Breeding corn with high provitamin A in the grain. Crop Sci. 1962, 2, 344–347. [Google Scholar] [CrossRef] [Green Version]
- Quackenbush, F.W.; Firch, J.G.; Brunson, A.M.; House, L.R. Carotenoid, oil, and tocopherol content of corn inbreds. Cereal Chem. 1966, 40, 251–259. [Google Scholar]
- Kurilich, A.C.; Juvik, J.A. Quantification of carotenoid and tocopherol antioxidants in Zea mays. J. Agric. Food Chem. 1999, 47, 1948–1955. [Google Scholar] [CrossRef]
- Egesel, C.O.; Wong, J.C.; Lambert, R.J.; Rocheford, T.R. Combining ability of maize inbreds for carotenoids and tocopherols. Crop Sci. 2003, 43, 818–823. [Google Scholar] [CrossRef]
- Menkir, A.; Liu, W.; White, W.S.; Maziya-Dixon, B.; Rocheford, T. Carotenoid diversity in tropical adapted yellow maize inbred lines. Food Chem. 2008, 109, 521–529. [Google Scholar] [CrossRef]
- Burt, A.J.; Grainger, C.M.; Smid, M.P.; Shelp, B.J.; Lee, E.A. Allele mining of exotic maize germplasm to enhance macular carotenoids. Crop Sci. 2011, 51, 991–1004. [Google Scholar] [CrossRef]
- Chander, S.; Meng, Y.; Zhang, Y.; Yan, J.; Li, J. Comparison of nutritional traits variability in selected eighty-seven inbreds from Chinese maize (Zea mays L.) germplasm. J. Agric. Food Chem. 2008, 56, 6506–6511. [Google Scholar] [CrossRef]
- Grogan, C.O.; Blessin, C.W. Stability of carotenoids in the kernels of maize. Can. J. Plant Sci. 1973, 53, 507–510. [Google Scholar] [CrossRef]
- Azmach, G.; Gedil, M.; Menkir, A.; Spillane, C. Marker-trait association analysis of functional gene markers for provitamin A levels across diverse tropical yellow maize inbred lines. BMC Plant Biol. 2013, 13, 227. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.; Zunjare, R.U.; Khan, S.; Muthusamy, V.; Baveja, A.; Das, A.K.; Jaiswal, S.K.; Bhat, J.S.; Guleria, S.K.; Hossain, F. Genetic Variability of Kernel Provitamin-A in Sub-tropically Adapted Maize Hybrids Possessing Rare Allele of β-carotene hydroxylase. Cereal Res. Commun. 2019, 47, 205–215. [Google Scholar] [CrossRef]
- Berardo, N.; Brenna, O.V.; Amato, A.; Valoti, P.; Pisacane, V.; Motto, M. Carotenoid concentrations among maize genotypes measured by near infrared reflectance spectroscopy (NIRS). Innov. Food Sci. Emerg. Technol. 2004, 5, 393–398. [Google Scholar] [CrossRef]
- Owens, B.F.; Lipka, A.E.; Magallanes-Lundback, M.; Tiede, T.; Diepenbrock, C.H.; Kandianis, C.B.; Kim, E.; Cepela, J.; Mateos-Hernandez, M.; Buell, C.R.; et al. A foundation for provitamin A biofortification of maize: Genome-wide association and genomic prediction models of carotenoid levels. Genetics 2014, 198, 1699–1716. [Google Scholar] [CrossRef] [Green Version]
- Muthusamy, V.; Hossain, F.; Thirunavukkarasu, N.; Saha, S.; Agrawal, P.K.; Gupta, H.S. Genetic analyses of kernel carotenoids in novel maize genotypes possessing rare allele of β-carotene hydroxylase gene. Cereal Res. Commun. 2016, 44, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Bahukhandi, A.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S. Variation in polyphenolics and antioxidant activity of traditional apple cultivars from West Himalaya, Uttarakhand. Hortic. Plant J. 2018, 4, 151–157. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Giordano, E.; Quadro, L. Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health. Arch. Biochem. Biophys. 2018, 647, 33–40. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J. An overview of carotenoids, apocarotenoids, and vitamin A in agro-food, nutrition, health, and disease. Mol. Nutr. Food Res. 2019, 63, 1801045. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Concepción, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [Green Version]
- Sauer, L.; Li, B.; Bernstein, P.S. Ocular carotenoid status in health and disease. Annu. Rev. Nutr. 2019, 39, 95–120. [Google Scholar] [CrossRef]
- Kandianis, C.B.; Stevens, R.; Liu, W.; Palacios, N.; Montgomery, K.; Pixley, K.; White, W.S.; Rocheford, T. Genetic architecture controlling variation in grain carotenoid composition and concentrations in two maize populations. Theor. Appl. Genet. 2013, 126, 2879–2895. [Google Scholar] [CrossRef] [Green Version]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of beta-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ji, J.; Wang, G.; Wu, G.; Diao, J.; Li, Z.; Chen, X.; Chen, Y.; Luo, L. Ectopic expression of the Lycium barbarum β-carotene hydroxylase gene (chyb) enhances drought and salt stress resistance by increasing xanthophyll cycle pool in tobacco. Plant Cell Tissue Organ Cult. 2015, 121, 559–569. [Google Scholar] [CrossRef]
- de Almeida Rios, S.; Paes, M.C.D.; Cardoso, W.S.; Borém, A.; Teixeira, F.F. Color of Corn Grains and Carotenoid Profile of Importance for Human Health. Am. J. Plant Sci. 2014, 5, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Muthusamy, V.; Hossain, F.; Nepolean, T.; Saha, S.; Agrawal, P.K.; Guleria, S.K.; Gupta, H.S. Genetic variability and inter-relationship of kernel carotenoids among indigenous and exotic maize (Zea mays L.) inbreds. Cereal Res. Commun. 2015, 43, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Sagare, B.; Reddy, S.S.; Shetti, P.; Surender, M. Enhancing Provitamin A of Maize using functional gene markers. Int. J. Adv. Biotechnol. Res. 2015, 6, 86–95. [Google Scholar]
- Cullis, B.R.; Smith, A.; Coombes, N. On the design of early generation variety trials with correlated data. J. Agric. Biol. Environ. Stat. 2006, 11, 381–393. [Google Scholar] [CrossRef]
- Cullis, B.R.; Jefferson, P.; Thompson, R.; Smith, A.B. Factor analytic and reduced animal models for the investigation of additive genotype-by-environment interaction in outcrossing plant species with application to a Pinus radiata breeding programme. Theor. Appl. Genet. 2014, 127, 2193–2210. [Google Scholar] [CrossRef]
- Zhang, H.; Berger, J.D.; Herrmann, C. Yield stability and adaptability of canola (Brassica napus L.) in multiple environment trials. Euphytica 2017, 213, 155. [Google Scholar] [CrossRef]
- Sukto, S.; Lomthaisong, K.; Sanitchon, J.; Chankaew, S.; Scott, M.P.; Lübberstedt, T.; Lertrat, K.; Suriharn, B. Variability in prolificacy, total carotenoids, lutein, and zeaxanthin of yellow small-ear waxy corn germplasm. Int. J. Agron. 2020, 8818768. [Google Scholar] [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [Green Version]
- Dannehl, D.; Josuttis, M.; Huyskens-Keil, S.; Ulrichs, C.; Schmidt, U. Comparison of different greenhouse systems and their impacts on plant responses of tomatoes. Gesunde Pflanz. 2014, 66, 111–119. [Google Scholar] [CrossRef]
- Muthusamy, V.; Hossain, F.; Thirunavukkarasu, N.; Choudhary, M.; Saha, S.; Bhat, J.S. Development of β-carotene rich maize hybrids through marker-assisted introgression of β-carotene hydroxylase allele. PLoS ONE 2014, 9, e113583. [Google Scholar] [CrossRef] [Green Version]
- Menkir, A.; Kling, J.G.; Jagtap, S.S.; Aliu, B.A. GIS Based Classification of Maize Testing Locations in West and Central Africa. Maydica 2000, 45, 143–150. [Google Scholar]
- Howe, J.A.; Tanumihardjo, S.A. Carotenoid-biofortified maize maintains adequate vitamin A status in Mongolian gerbils. J. Nutr. 2006, 136, 2562–2567. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.B.; Cullis, B.R.; Gilmour, A. The analysis of crop variety evaluation data in Australia. Aust. N. Z. J. Stat. 2001, 43, 129–145. [Google Scholar] [CrossRef]
- Gogel, B.; Smith, A.; Cullis, B. Comparison of a one- and two-stage mixed model analysis of Australia’s National Variety Trial Southern Region wheat data. Euphytica 2018, 214, 44. [Google Scholar] [CrossRef]
- Smith, A.B.; Cullis, B.R. Plant breeding selection tools built on factor analytic mixed models for multi-environment trial data. Euphytica 2018, 214, 143. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 10 June 2021).
- Butler, D. ASREML: Fits the Linear Mixed Model. R Package Version 4.1.0.126. VSN International Ltd., 2 Amberside House, Wood Lane, Hemel Hempstead, HP2 4TP, UK. 2020. Available online: www.vsni.co.uk (accessed on 10 June 2021).
- SAS Institute. Statistical Analysis Software (SAS). In Users Guide; SAS Inst Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Watkins, J.L.; Li, M.; McQuinn, R.P.; Chan, K.X.; McFarlane, H.E.; Ermakova, M.; Furbank, R.T.; Mares, D.; Dong, C.; Chalmers, K.J.; et al. A GDSL esterase/lipase catalyzes the esterification of lutein in bread wheat. Plant Cell 2019, 31, 3092–3112. [Google Scholar] [CrossRef]
- Zheng, X.; Giuliano, G.; Al-Babili, S. Carotenoid biofortification in crop plants: Citius, altius, forties. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158664. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Farré, G.; Zanga, D.; Lloveras, J.; Michelena, A.; Ferrio, J.P.; Christou, P. High-carotenoid maize: Development of plant biotechnology prototypes for human and animal health and nutrition. Phytochem. Rev. 2018, 17, 195–209. [Google Scholar] [CrossRef]



| Carotenoids | Covariance and REMLRT | Hybrid | Environment | Environment × Hybrid |
|---|---|---|---|---|
| Lutein | Covariance | 2.8 **** | 9.4 **** | 0.8 **** |
| REMLRT | 217.2 | 55.6 | 35.3 | |
| Zeaxanthin | Covariance | 13.7 **** | 9.2 **** | 0.5 **** |
| REMLRT | 331.9 | 15.4 | 39.0 | |
| β-cryptoxanthin | Covariance | 1.7 **** | 1.3 **** | 0.1 **** |
| REMLRT | 415.0 | 27.8 | 45.8 | |
| α-carotene | Covariance | 0.1 **** | 0.1 **** | 0.003 **** |
| REMLRT | 317.0 | 8.7 | 38.1 | |
| β- carotene | Covariance | 1.7 **** | 1.9 **** | 0.1 **** |
| REMLRT | 383.4 | 15.3 | 52.2 | |
| Provitamin A | Covariance | 3.7 **** | 3.3 **** | 0.2 **** |
| REMLRT | 465.4 **** | 27.8 **** | 47.8 **** |
| Simple Correlation Coefficients with | ||
|---|---|---|
| Carotenoids | CAN1 | CAN2 |
| Lutein | 0.18 | 0.93 **** |
| Zeaxanthin | 0.58 **** | 0.27 * |
| β-cryptoxanthin | 0.68 **** | −0.27 * |
| α-carotene | 0.80 **** | −0.09 |
| β-carotene | 0.84 **** | −0.33 ** |
| Provitamin-A | 0.86 **** | −0.31 * |
| Variance (%) | 0.73 | 0.27 |
| Canonical correlations (CC) | 0.84 | 0.69 |
| Significant levels for CC | p < 0.0001 | p < 0.0001 |
| Carotenoids | Minimum | Maximum | Mean ± SE |
|---|---|---|---|
| G-I | |||
| Lutein (µg/g) | 8.3 | 11.8 | 10.5 ± 0.5 |
| Zeaxanthin (µg/g) | 13.9 | 17.3 | 15.5 ± 0.4 |
| β-cryptoxanthin (µg/g) | 4.0 | 5.4 | 4.7 ± 0.2 |
| α-carotene (µg/g) | 1.1 | 1.5 | 1.3 ± 0.1 |
| β-carotene (µg/g) | 4.1 | 6.0 | 5.0 ± 0.2 |
| Total Carotenoids (µg/g) | 34.7 | 41.9 | 37.0 ± 1.0 |
| Provitamin-A (µg/g) | 7.6 | 9.5 | 8.4 ± 0.2 |
| G-II | |||
| Lutein (µg/g) | 7.7 | 11.1 | 9.1 ± 0.1 |
| Zeaxanthin (µg/g) | 13.7 | 18.5 | 16.7 ± 0.2 |
| β-cryptoxanthin (µg/g) | 4.2 | 7.1 | 6.1 ± 0.1 |
| α-carotene (µg/g) | 1.2 | 1.9 | 1.6 ± 0.0 |
| β-carotene (µg/g) | 5.9 | 8.8 | 7.0 ± 0.1 |
| Total Carotenoids (µg/g) | 36.2 | 43.8 | 40.6 ± 0.3 |
| Provitamin-A (µg/g) | 9.8 | 12.5 | 10.9 ± 0.1 |
| G-III | |||
| Lutein (µg/g) | 9.3 | 15.0 | 11.3 ± 0.3 |
| Zeaxanthin (µg/g) | 15.2 | 20.5 | 17.9 ± 0.3 |
| β-cryptoxanthin (µg/g) | 5.1 | 8.4 | 6.2 ± 0.2 |
| α-carotene (µg/g) | 1.5 | 2.1 | 1.7 ± 0.0 |
| β-carotene | 6.5 | 8.0 | 7.3 ± 0.1 |
| Total Carotenoids (µg/g) | 40.8 | 48.9 | 44.4 ± 0.5 |
| Provitamin-A (µg/g) | 10.1 | 14.0 | 11.2 ± 0.2 |
| Traits | Covariance and REMLRT | Hybrid | Environment | Environment × Hybrid |
|---|---|---|---|---|
| Anthesis days | Covariance | 1.4 **** | 24.8 **** | 0.7 **** |
| REMLRT | 294 | 601 | 385 | |
| Silking days | Covariance | 1.4 **** | 23.3 **** | 0.8 **** |
| REMLRT | 288 | 560 | 502 | |
| Ear height | Covariance | 12.6 **** | 452.4 **** | 13.8 **** |
| REMLRT | 425 | 411 | 209 | |
| Plant height | Covariance | 30.2 **** | 873.6 **** | 36.0 **** |
| REMLRT | 462 | 470 | 217 | |
| Grain yield | Covariance | 496,206 **** | 1,689,020 **** | 406,147 **** |
| REMLRT | 567 | 337 | 677 |
| Traits | Minimum | Maximum | Mean |
|---|---|---|---|
| G-I | |||
| Anthesis days | 58 | 62 | 59 ± 0.4 |
| Silking days | 60 | 64 | 62 ± 0.5 |
| Ear height (cm) | 85 | 101 | 90 ± 1.9 |
| Plant height (cm) | 159 | 183 | 176 ± 2.9 |
| Grain yield (kg/ha) | 3245 | 4559 | 3846 ± 168 |
| G-II | |||
| Anthesis days | 58 | 61 | 59 ± 0.1 |
| Silking days | 60 | 64 | 61 ± 0.2 |
| Ear height (cm) | 85 | 95 | 90 ± 0.4 |
| Plant height (cm) | 176 | 193 | 183 ± 7.0 |
| Grain yield (kg/ha) | 2254 | 5054 | 3977 ± 103 |
| G-III | |||
| Anthesis days | 58 | 62 | 60 ± 0.2 |
| Silking days | 60 | 65 | 62 ± 0.2 |
| Ear height (cm) | 82 | 95 | 89 ± 0.4 |
| Plant height (cm) | 171 | 192 | 181 ± 1.2 |
| Grain yield (kg/ha) | 1420 | 4812 | 3884 ± 176 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menkir, A.; Dieng, I.; Mengesha, W.; Meseka, S.; Maziya-Dixon, B.; Alamu, O.E.; Bossey, B.; Muhyideen, O.; Ewool, M.; Coulibaly, M.M. Unravelling the Effect of Provitamin A Enrichment on Agronomic Performance of Tropical Maize Hybrids. Plants 2021, 10, 1580. https://doi.org/10.3390/plants10081580
Menkir A, Dieng I, Mengesha W, Meseka S, Maziya-Dixon B, Alamu OE, Bossey B, Muhyideen O, Ewool M, Coulibaly MM. Unravelling the Effect of Provitamin A Enrichment on Agronomic Performance of Tropical Maize Hybrids. Plants. 2021; 10(8):1580. https://doi.org/10.3390/plants10081580
Chicago/Turabian StyleMenkir, Abebe, Ibnou Dieng, Wende Mengesha, Silvestro Meseka, Bussie Maziya-Dixon, Oladeji Emmanuel Alamu, Bunmi Bossey, Oyekunle Muhyideen, Manfred Ewool, and Mmadou Mory Coulibaly. 2021. "Unravelling the Effect of Provitamin A Enrichment on Agronomic Performance of Tropical Maize Hybrids" Plants 10, no. 8: 1580. https://doi.org/10.3390/plants10081580






