Projected Status of the Ghost Orchid (Dendrophylax lindenii) in Florida during the Next Decade Based on Temporal Dynamic Studies Spanning Six Years
Abstract
:1. Introduction
2. Results
2.1. Orchid Numbers
2.2. Fecundity
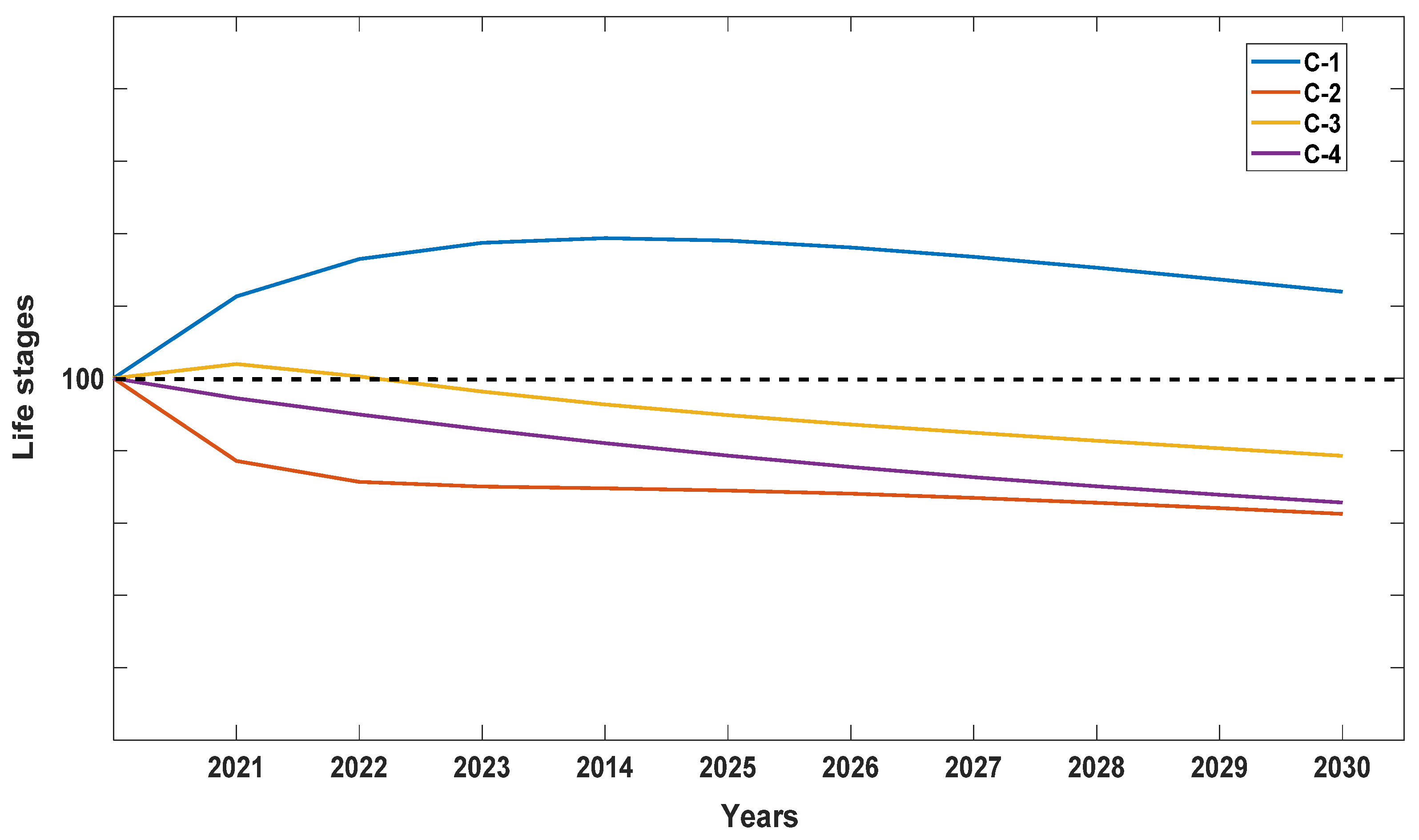
2.3. Demographic Patterns and Population Growth
3. Discussion
3.1. Water, Mycorrhizal Fungi, and Pollinators
3.2. Future Directions
4. Materials and Methods
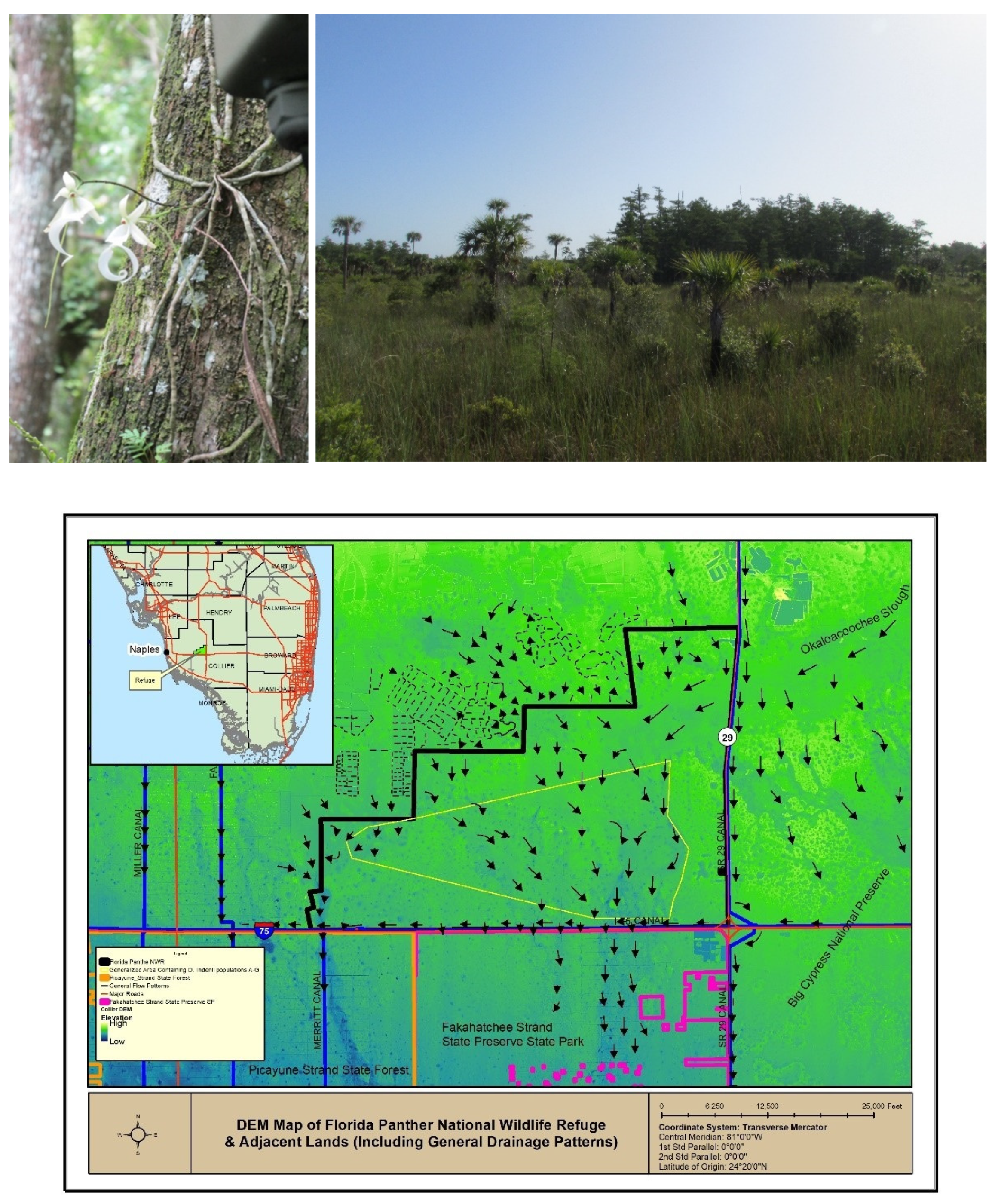
4.1. Study Site
4.2. Field Sampling
4.3. Vital Rate Estimation
4.4. Demographic Modeling
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoang, N.H.; Kane, M.E.; Radcliffe, E.N.; Zettler, L.W.; Richardson, L.W. Comparative seed germination and seedling development of the Ghost Orchid, Dendrophylax lindenii (Orchidaceae), and molecular identification of its mycorrhizal fungus from south Florida. Ann. Bot. 2017, 119, 379–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mújica, E.B.; Mably, J.J.; Skarha, S.M.; Corey, L.L.; Richardson, L.W.; Danaher, M.; González, E.; Zettler, L.W. A comparison of Ghost Orchid (Dendrophylax lindenii) habitats in Florida and Cuba, with special reference to seedling recruitment and mycorrhizal fungi. Bot. J. Linn. Soc. 2018, 186, 572–586. [Google Scholar] [CrossRef]
- Zettler, J.A.; Zettler, L.W.; Richardson, L.W. Pestiferous insects (Hemiptera), on native epiphytic orchids in South Florida: A new threat posed by introduced species. Southeast. Nat. 2012, 11, 127–134. [Google Scholar] [CrossRef]
- Langdon, K.R. The Ghost Orchid, Polyrrhiza lindenii and Endangered Species in Florida; Circular 56; Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 1979.
- Coile, N.C.; Garland, M. Notes on Florida’s Endangered and Threatened Plants, 4th ed.; Contribution No. 38; Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 2003.
- Wiegand, T.; Raventós, J.; Mújica, E.; González, E.; Bonet, A. Spatio-temporal analysis of the effects of Hurricane Ivan on two contrasting orchids in Guanahacabibes, Cuba. Biotropica 2013, 45, 441–449. [Google Scholar] [CrossRef]
- Zettler, L.W.; Kane, M.E.; Mújica, E.B.; Corey, L.L.; Richardson, L.W. The ghost orchid demystified: Biology, ecology and conservation of Dendrophylax lindenii in Florida and Cuba. In Proceedings of the 22nd World Orchid Conference, Guayaquil, Ecuador, 8–12 November 2019; Pridgeon, A.M., Arosemena, A.R., Eds.; Asociación Ecuatoriana de Orquideologia: Guayaquil, Ecuador, 2019; Volume 2, pp. 136–148. [Google Scholar]
- Luer, C.A. The Native Orchids of Florida; The New York Botanical Garden: New York, NY, USA, 1972. [Google Scholar]
- Ackerman, J.D.; Hagsater, E. Orchid Flora of the Greater Antilles; New York Botanical Garden Press: New York, NY, USA, 2014. [Google Scholar]
- Danaher, M.W.; Ward, C.; Zettler, L.W.; Covell, C.V. Pollinia removal and suspected pollination of the endangered ghost orchid (Dendrophylax lindenii) by various hawk moths (Lepidoptera: Sphingidae): Another mystery dispelled. Fla. Entomol. 2019, 102, 1–13. [Google Scholar] [CrossRef]
- Bell, T.J.; Bowles, M.L.; Zettler, L.W.; Pollack, C.A.; Ibberson, J.E. Environmental and management effects on demographic processes in the U.S. threatened Platanthera leucophaea (Nutt.) Lindl. (Orchidaceae). Plants 2021, 10, 1308. [Google Scholar] [CrossRef] [PubMed]
- Crone, E.E.; Menges, E.S.; Ellis, M.M.; Bell, T.; Bierzychudek, P.; Ehrlen, J.; Kaye, T.N.; Knight, T.M.; Lesica, P.; Morris, W.F.; et al. How do plant ecologists use matrix population models? Ecol. Lett. 2011, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. The Various Contrivances by which Orchids are Fertilised by Insects, 2nd ed.; John Murray: London, UK, 1888. [Google Scholar]
- Brown, P.M. Wild Orchids of Florida; University Press of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Stewart, S.L.; Richardson, L.W. Orchid flora of the Florida Panther National Wildlife Refuge. N. Am. Nativ. Orchid J. 2008, 14, 70–104. [Google Scholar]
- Rasmussen, H.N.; Dixon, K.W.; Jersáková, J.; Těšitelová, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore-Landecker, E. Fundamentals of the Fungi, 4th ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1995. [Google Scholar]
- Johnson, L.J.A.N. Investigating Specificity and Diversity of Orchid Mycorrhizal Fungi of Vanilla planifolia and Dendrophylax lindenii. Ph.D. Thesis, Northwestern University, Evanston, IL, USA, 2019. [Google Scholar]
- Sonenshein, R.S. Hydrology of the Florida Panther National Wildlife Refuge. In Proceedings of the PowerPoint Presentation Prepared for the 2008 Greater Everglades Ecosystem Restoration Science Conference, Naples, Italy, 28 July–1 August 2008; Available online: https://conference.ifas.ufl.edu/GEER2008/Presentation_PDFs/Thursday/Rolay%20Palm%20lll/1100%20R%20Sonenshein.pdf (accessed on 15 September 2019).
- Reese, R.S. Hydrologic conditions in the Florida panther national wildlife refuge, 2006–2007. In U.S. Geological Survey Open-File Report 2010-1270; U.S. Geological Survey: Reston, VA, USA, 2008. [Google Scholar]
- Scott, R.; Crone, E.E. Using the right tool for the job: The difference between unsupervised and supervised analysis of multivariate ecological data. Oecologia 2021, 196, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Coopman, J.C.; Kane, M.E. Greenhouse acclimatization methods for field establishment of in vitro-derived ghost orchid (Dendrophylax lindenii) plants. Nativ. Plants J. 2018, 19, 100–108. [Google Scholar] [CrossRef]
- Coopman, J.C.; Kane, M.E. In vitro desiccation tolerance of the epiphytic Ghost Orchid, Dendrophylax lindenii (Lindl.) Benth. ex Rolfe. In Vitro Cell. Dev. Biol. Plant 2019, 55, 60–70. [Google Scholar] [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, K. How to grow the ghost orchid: From seedling to flowering. Orchids 2009, 78, 414–415. [Google Scholar]
- Raventós, J.; González, E.; Mújica, E.; Doak, D. Population viability analysis of the epiphytic ghost orchid (Dendrophylax lindenii) in Cuba. Biotropica 2015, 47, 179–189. [Google Scholar] [CrossRef]
- Easterling, M.R.; Ellner, S.P.; Dixon, P.M. Size-specific sensitivity: Applying a new structured population model. Ecology 2000, 81, 694–708. [Google Scholar] [CrossRef]
- Ellner, S.P.; Rees, M. Integral projection models for species with complex demography. Am. Nat. 2006, 167, 410–428. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, C.J.E.; McMahon, S.M.; Salguero-Roberto, R.; Jongejans, E. IPMpack: An R package for integral projection models. Methods Ecol. Evol. 2013, 4, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Morris, W.F.; Doak, D.F. Quantitative Conservation Biology: The Theories and Practice of Population Viability Analysis; Sinauer: Sunderland, MA, USA, 2002. [Google Scholar]
- Palmer, T.M.; Doak, D.F.; Stanton, M.L.; Bronstein, J.L.; Kiers, T.; Young, T.P.; Goheen, J.R.; Pringle, R.M. Synergy of multiple partners, including freeloaders, increase host fitness in a multispecies mutualism. Proc. Natl. Acad. Sci. USA 2010, 107, 17234–17239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, B.W. Density Estimation for Statistics and Data Analysis; Chapman and Hall: London, UK; New York, NY, USA, 1986; p. 175. [Google Scholar]






| - | Y1 (2015) | Y2 (2016) | Y3 (2017) | Y4 (2018) | Y5 (2019) | Y6 (2020) |
|---|---|---|---|---|---|---|
| Seedling | 45 (24) | 109 (44) | 155 (44) | 130 (32) | 55 (14) | 0 (0) |
| Juvenile | 44 (23) | 39 (16) | 52 (15) | 63 (16) | 165 (41) | 241 (59) |
| Mature | 99 (53) | 97 (40) | 142 (41) | 208 (52) | 183 (45) | 168 (41) |
| Total Orchids | 188 | 245 | 349 | 401 | 403 | 409 |
| Inflorescences | 40 | 59 | 59 | 85 | 92 | 80 |
| Flowers | 56 | 101 | 78 | 123 | 96 | 82 |
| Flowers/Inflor | 1.4 | 1.7 | 1.3 | 1.4 | 1.0 | 1.0 |
| Fruits | 3 (5.4) | 4 (4.0) | 5 (6.4) | 21 (17.1) | 16 (16.7) | 6 (7.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mújica, E.B.; Herdman, A.R.; Danaher, M.W.; González, E.H.; Zettler, L.W. Projected Status of the Ghost Orchid (Dendrophylax lindenii) in Florida during the Next Decade Based on Temporal Dynamic Studies Spanning Six Years. Plants 2021, 10, 1579. https://doi.org/10.3390/plants10081579
Mújica EB, Herdman AR, Danaher MW, González EH, Zettler LW. Projected Status of the Ghost Orchid (Dendrophylax lindenii) in Florida during the Next Decade Based on Temporal Dynamic Studies Spanning Six Years. Plants. 2021; 10(8):1579. https://doi.org/10.3390/plants10081579
Chicago/Turabian StyleMújica, Ernesto B., Adam R. Herdman, Mark W. Danaher, Elaine H. González, and Lawrence W. Zettler. 2021. "Projected Status of the Ghost Orchid (Dendrophylax lindenii) in Florida during the Next Decade Based on Temporal Dynamic Studies Spanning Six Years" Plants 10, no. 8: 1579. https://doi.org/10.3390/plants10081579







