Metabolism, Transport and Drug–Drug Interactions of Silymarin
Abstract
:1. Introduction
2. Metabolism and Transport of Silymarin
2.1. Absorption
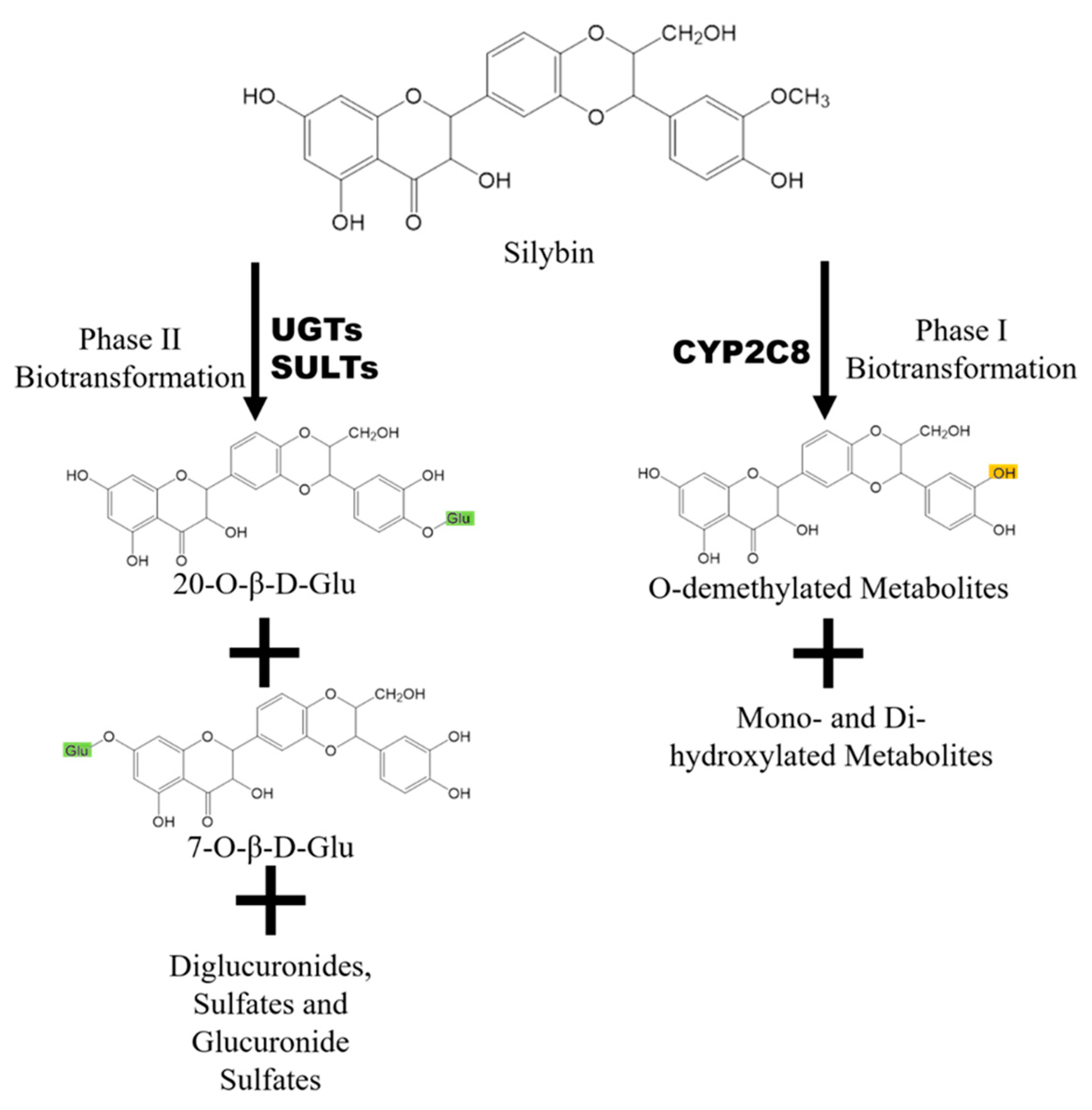
2.2. Metabolism
2.3. Elimination
3. Silybin as a Beneficiary of DDI
3.1. Tangeretin
3.2. Piperine
3.3. Baicalein and Baicalin
4. Silybin as a Perpetrator of DDI
4.1. CYP-Mediated Drug–Drug Interaction
4.2. UGT-Mediated Drug–Drug Interaction
4.3. DDI Mediated by Transporters
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | area under the plasma drug concentration-t curve |
| BCRP | Breast Cancer Resistance Protein |
| Cmax | Maximum plasma drug concentration |
| CYPs | Cytochromes P450 |
| DDI | Drug–drug interaction |
| IC50 | The half maximal inhibitory concentration |
| MDCK cells | Madin-Darby canine kidney cells |
| MRPs | Multidrug resistance-associated protein |
| OATP | Organic-anion-transporting polypeptide |
| P-gp | P-glycoprotein |
| SULTs | sulfotransferases |
| UGT | UDP-glucuronosyltransferase |
References
- Bijak, M. Silybin. a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Rainone, F. Milk thistle. Am. Fam. Physician 2005, 72, 1285–1288. [Google Scholar] [PubMed]
- Lee, J.I.; Narayan, M.; Barrett, J.S. Analysis and comparison of active constituents in commercial standardized silymarin extracts by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 845, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Lou, H.X.; Ren, D.M.; Sun, L.R.; Ma, B.; Ji, M. Stereoselective metabolism of silybin diastereoisomers in the glucuronidation process. J. Pharm. Biomed. Anal. 2004, 34, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Graf, T.N.; Sparacino, C.M.; Wani, M.C.; Wall, M.E. Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org. Biomol. Chem. 2003, 1, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Loguercio, C. Silymarin/Silybin and Chronic Liver Disease: A Marriage of Many Years. Molecules 2017, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Izzo, A.A.; Milic, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxid. (BaselSwitz.) 2015, 4, 204–247. [Google Scholar] [CrossRef] [Green Version]
- Soleimani, V.; Delghandi, P.S.; Moallem, S.A.; Karimi, G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: An updated review. Phytother. Res. 2019, 33, 1627–1638. [Google Scholar] [CrossRef]
- Matsuda, T.; Ferreri, K.; Todorov, I.; Kuroda, Y.; Smith, C.V.; Kandeel, F.; Mullen, Y. Silymarin protects pancreatic beta-cells against cytokine-mediated toxicity: Implication of c-Jun NH2-terminal kinase and janus kinase/signal transducer and activator of transcription pathways. Endocrinology 2005, 146, 175–185. [Google Scholar] [CrossRef]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of addition of silymarin to renin-angiotensin system inhibitors on proteinuria in type 2 diabetic patients with overt nephropathy: A randomized, double-blind, placebo-controlled trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Raina, K.; Rajamanickam, S.; Singh, R.P.; Deep, G.; Chittezhath, M.; Agarwal, R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008, 68, 6822–6830. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Ray, S.K. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7–1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Apoptosis 2016, 21, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Jahanafrooz, Z.; Motamed, N.; Rinner, B.; Mokhtarzadeh, A.; Baradaran, B. Silibinin to improve cancer therapeutic, as an apoptotic inducer, autophagy modulator, cell cycle inhibitor, and microRNAs regulator. Life Sci. 2018, 213, 236–247. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Romanucci, V.; Zarrelli, A.; Monaco, I.; Lolicato, F.; Spinella, N.; Galati, C.; Grasso, G.; D’Urso, L.; Romeo, M.; et al. Inhibition of Aβ Amyloid Growth and Toxicity by Silybins: The Crucial Role of Stereochemistry. ACS Chem. Neurosci. 2017, 8, 1767–1778. [Google Scholar] [CrossRef]
- Sciacca, M.F.M.; Tempra, C.; Scollo, F.; Milardi, D.; La Rosa, C. Amyloid growth and membrane damage: Current themes and emerging perspectives from theory and experiments on Abeta and hIAPP. Biochim. Biophys. Acta Biomembr. 2018. [Google Scholar] [CrossRef]
- Fried, M.W.; Navarro, V.J.; Afdhal, N.; Belle, S.H.; Wahed, A.S.; Hawke, R.L.; Doo, E.; Meyers, C.M.; Reddy, K.R. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: A randomized controlled trial. Jama 2012, 308, 274–282. [Google Scholar] [CrossRef]
- Loguercio, C.; Festi, D. Silybin and the liver: From basic research to clinical practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, H.; Li, Y.Z.; Yuan, Z.W.; Liu, C.X.; Liu, L.; Xie, Y. Baicalein Enhances the Oral Bioavailability and Hepatoprotective Effects of Silybin Through the Inhibition of Efflux Transporters BCRP and MRP2. Front. Pharm. 2018, 9, 1115. [Google Scholar] [CrossRef]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. A J. Clin. Ther. 2011, 16, 239–249. [Google Scholar]
- Wen, Z.; Dumas, T.E.; Schrieber, S.J.; Hawke, R.L.; Fried, M.W.; Smith, P.C. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Schrieber, S.J.; Wen, Z.; Vourvahis, M.; Smith, P.C.; Fried, M.W.; Kashuba, A.D.; Hawke, R.L. The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.W.; Li, Y.Z.; Liu, Z.Q.; Feng, S.L.; Zhou, H.; Liu, C.X.; Liu, L.; Xie, Y. Role of tangeretin as a potential bioavailability enhancer for silybin: Pharmacokinetic and pharmacological studies. Pharmacol. Res. 2018, 128, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Kren, V.; Marhol, P.; Purchartova, K.; Gabrielova, E.; Modriansky, M. Biotransformation of silybin and its congeners. Curr. Drug Metab. 2013, 14, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Jancova, P.; Anzenbacherova, E.; Papouskova, B.; Lemr, K.; Luzna, P.; Veinlichova, A.; Anzenbacher, P.; Simanek, V. Silybin is metabolized by cytochrome P450 2C8 in vitro. Drug Metab. Dispos. 2007, 35, 2035–2039. [Google Scholar] [CrossRef]
- Gunaratna, C.; Zhang, T. Application of liquid chromatography-electrospray ionization-ion trap mass spectrometry to investigate the metabolism of silibinin in human liver microsomes. J. Chromatogr B Anal. Technol. Biomed. Life Sci. 2003, 794, 303–310. [Google Scholar] [CrossRef]
- Jancova, P.; Siller, M.; Anzenbacherova, E.; Kren, V.; Anzenbacher, P.; Simanek, V. Evidence for differences in regioselective and stereoselective glucuronidation of silybin diastereomers from milk thistle (Silybum marianum) by human UDP-glucuronosyltransferases. Xenobiotica 2011, 41, 743–751. [Google Scholar] [CrossRef]
- Hoh, C.S.; Boocock, D.J.; Marczylo, T.H.; Brown, V.A.; Cai, H.; Steward, W.P.; Berry, D.P.; Gescher, A.J. Quantitation of silibinin, a putative cancer chemopreventive agent derived from milk thistle (Silybum marianum), in human plasma by high-performance liquid chromatography and identification of possible metabolites. J. Agric. Food Chem. 2007, 55, 2532–2535. [Google Scholar] [CrossRef]
- Hoh, C.; Boocock, D.; Marczylo, T.; Singh, R.; Berry, D.P.; Dennison, A.R.; Hemingway, D.; Miller, A.; West, K.; Euden, S.; et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: Silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin. Cancer Res. 2006, 12, 2944–2950. [Google Scholar] [CrossRef]
- Xie, Y.; Miranda, S.R.; Hoskins, J.M.; Hawke, R.L. Role of UDP-Glucuronosyltransferase 1A1 in the Metabolism and Pharmacokinetics of Silymarin Flavonolignans in Patients with HCV and NAFLD. Molecules 2017, 22, 142. [Google Scholar] [CrossRef]
- Lorenz, D.; Lucker, P.W.; Mennicke, W.H.; Wetzelsberger, N. Pharmacokinetic studies with silymarin in human serum and bile. Methods Find. Exp. Clin. Pharm. 1984, 6, 655–661. [Google Scholar]
- Wu, J.W.; Lin, L.C.; Hung, S.C.; Chi, C.W.; Tsai, T.H. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.R.; Lee, J.K.; Brouwer, K.L.; Wen, Z.; Smith, P.C.; Hawke, R.L. Hepatic metabolism and biliary excretion of silymarin flavonolignans in isolated perfused rat livers: Role of multidrug resistance-associated protein 2 (Abcc2). Drug Metab. Dispos. Biol. Fate Chem. 2008, 36, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Yuan, Z.; Qu, B.; Zhou, H.; Liu, Z.; Xie, Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine 2019, 54, 98–108. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R.; Mukerjee, A. Bioavailability enhancers of herbal origin: An overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Hung, W.L.; Chang, W.S.; Lu, W.C.; Wei, G.J.; Wang, Y.; Ho, C.T.; Hwang, L.S. Pharmacokinetics, bioavailability, tissue distribution and excretion of tangeretin in rat. J. Food Drug Anal. 2018, 26, 849–857. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.Y.; Back, S.Y.; Han, H.K. Piperine-mediated drug interactions and formulation strategy for piperine: Recent advances and future perspectives. Expert Opin. Drug Metab. Toxicol. 2018, 14, 43–57. [Google Scholar] [CrossRef]
- Woo, A.Y.; Cheng, C.H.; Waye, M.M. Baicalein protects rat cardiomyocytes from hypoxia/reoxygenation damage via a prooxidant mechanism. Cardiovasc. Res. 2005, 65, 244–253. [Google Scholar] [CrossRef]
- Bie, B.; Sun, J.; Guo, Y.; Li, J.; Jiang, W.; Yang, J.; Huang, C.; Li, Z. Baicalein: A review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharm. 2017, 93, 1285–1291. [Google Scholar] [CrossRef]
- Lai, M.Y.; Hsiu, S.L.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharm. 2003, 55, 205–209. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, G.; Zuo, Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm. Res. 2007, 24, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Beckmann-Knopp, S.; Rietbrock, S.; Weyhenmeyer, R.; Bocker, R.H.; Beckurts, K.T.; Lang, W.; Hunz, M.; Fuhr, U. Inhibitory effects of silibinin on cytochrome P-450 enzymes in human liver microsomes. Pharmacol. Toxicol. 2000, 86, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Lin, L.C.; Tsai, T.H. Drug-drug interactions of silymarin on the perspective of pharmacokinetics. J. Ethnopharmacol. 2009, 121, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zuber, R.; Modriansky, M.; Dvorak, Z.; Rohovsky, P.; Ulrichova, J.; Simanek, V.; Anzenbacher, P. Effect of silybin and its congeners on human liver microsomal cytochrome P450 activities. Phytother. Res. 2002, 16, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Fifer, E.K.; Gardner, Z. Pharmacokinetic herb-drug interactions (part 2): Drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta Med. 2012, 78, 1490–1514. [Google Scholar] [CrossRef] [PubMed]
- Brantley, S.J.; Oberlies, N.H.; Kroll, D.J.; Paine, M.F. Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations. J. Pharm. Exp. 2010, 332, 1081–1087. [Google Scholar] [CrossRef]
- Budzinski, J.W.; Trudeau, V.L.; Drouin, C.E.; Panahi, M.; Arnason, J.T.; Foster, B.C. Modulation of human cytochrome P450 3A4 (CYP3A4) and P-glycoprotein (P-gp) in Caco-2 cell monolayers by selected commercial-source milk thistle and goldenseal products. Can. J. Physiol. Pharm. 2007, 85, 966–978. [Google Scholar] [CrossRef]
- Kosina, P.; Maurel, P.; Ulrichova, J.; Dvorak, Z. Effect of silybin and its glycosides on the expression of cytochromes P450 1A2 and 3A4 in primary cultures of human hepatocytes. J. Biochem. Mol. Toxicol. 2005, 19, 149–153. [Google Scholar] [CrossRef]
- Leber, H.W.; Knauff, S. Influence of silymarin on drug metabolizing enzymes in rat and man. Arzneimittelforschung 1976, 26, 1603–1605. [Google Scholar]
- Piscitelli, S.C.; Formentini, E.; Burstein, A.H.; Alfaro, R.; Jagannatha, S.; Falloon, J. Effect of milk thistle on the pharmacokinetics of indinavir in healthy volunteers. Pharmacotherapy 2002, 22, 551–556. [Google Scholar] [CrossRef]
- Mills, E.; Wilson, K.; Clarke, M.; Foster, B.; Walker, S.; Rachlis, B.; DeGroot, N.; Montori, V.M.; Gold, W.; Phillips, E.; et al. Milk thistle and indinavir: A randomized controlled pharmacokinetics study and meta-analysis. Eur. J. Clin. Pharm. 2005, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Carrier, J.; Khan, I.A.; Edwards, D.J.; Shah, A. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin. Pharm. 2004, 76, 428–440. [Google Scholar]
- Gurley, B.; Hubbard, M.A.; Williams, D.K.; Thaden, J.; Tong, Y.; Gentry, W.B.; Breen, P.; Carrier, D.J.; Cheboyina, S. Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: Comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J. Clin. Pharm. 2006, 46, 201–213. [Google Scholar] [CrossRef] [PubMed]
- van Erp, N.P.; Baker, S.D.; Zhao, M.; Rudek, M.A.; Guchelaar, H.J.; Nortier, J.W.; Sparreboom, A.; Gelderblom, H. Effect of milk thistle (Silybum marianum) on the pharmacokinetics of irinotecan. Clin. Cancer Res. 2005, 11, 7800–7806. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.N.; Srinivas, M.; Kumar, Y.S.; Rao, Y.M. Effect of silymarin on the oral bioavailability of ranitidine in healthy human volunteers. Drug Metab. Drug Interact. 2007, 22, 175–185. [Google Scholar] [CrossRef]
- Gurley, B.J.; Swain, A.; Hubbard, M.A.; Williams, D.K.; Barone, G.; Hartsfield, F.; Tong, Y.; Carrier, D.J.; Cheboyina, S.; Battu, S.K. Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: Effects of milk thistle, black cohosh, goldenseal, kava kava, St. John’s wort, and Echinacea. Mol. Nutr. Food Res. 2008, 52, 755–763. [Google Scholar] [CrossRef]
- DiCenzo, R.; Shelton, M.; Jordan, K.; Koval, C.; Forrest, A.; Reichman, R.; Morse, G. Coadministration of milk thistle and indinavir in healthy subjects. Pharmacotherapy 2003, 23, 866–870. [Google Scholar] [CrossRef]
- Rajnarayana, K.; Reddy, M.S.; Vidyasagar, J.; Krishna, D.R. Study on the influence of silymarin pretreatment on metabolism and disposition of metronidazole. Arzneim. -Forsch. 2004, 54, 109–113. [Google Scholar] [CrossRef]
- Gurley, B.J.; Barone, G.W.; Williams, D.K.; Carrier, J.; Breen, P.; Yates, C.R.; Song, P.F.; Hubbard, M.A.; Tong, Y.; Cheboyina, S. Effect of milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa) supplementation on digoxin pharmacokinetics in humans. Drug Metab. Dispos. 2006, 34, 69–74. [Google Scholar] [CrossRef]
- Fuhr, U.; Beckmann-Knopp, S.; Jetter, A.; Luck, H.; Mengs, U. The effect of silymarin on oral nifedipine pharmacokinetics. Planta Med. 2007, 73, 1429–1435. [Google Scholar] [CrossRef]
- Deng, J.W.; Shon, J.H.; Shin, H.J.; Park, S.J.; Yeo, C.W.; Zhou, H.H.; Song, I.S.; Shin, J.G. Effect of silymarin supplement on the pharmacokinetics of rosuvastatin. Pharm. Res. 2008, 25, 1807–1814. [Google Scholar] [CrossRef]
- Han, Y.; Guo, D.; Chen, Y.; Tan, Z.R.; Zhou, H.H. Effect of continuous silymarin administration on oral talinolol pharmacokinetics in healthy volunteers. Xenobiotica Fate Foreign Compd. Biol. Syst. 2009, 39, 694–699. [Google Scholar] [CrossRef]
- Han, Y.; Guo, D.; Chen, Y.; Tan, Z.R.; Zhou, H.H. Effect of silymarin on the pharmacokinetics of losartan and its active metabolite E-3174 in healthy Chinese volunteers. Eur. J. Clin. Pharm. 2009, 65, 585–591. [Google Scholar] [CrossRef]
- Molto, J.; Valle, M.; Miranda, C.; Cedeno, S.; Negredo, E.; Clotet, B. Effect of milk thistle on the pharmacokinetics of darunavir-ritonavir in HIV-infected patients. Antimicrob. Agents Chemother. 2012, 56, 2837–2841. [Google Scholar] [CrossRef]
- Yamsani, S.K.; Yamsani, M.R. Effect of silymarin pretreatment on the bioavailability of domperidone in healthy human volunteers. Drug Metab. Drug Interact. 2014, 29, 261–267. [Google Scholar] [CrossRef]
- Kawaguchi-Suzuki, M.; Frye, R.F.; Zhu, H.J.; Brinda, B.J.; Chavin, K.D.; Bernstein, H.J.; Markowitz, J.S. The effects of milk thistle (Silybum marianum) on human cytochrome P450 activity. Drug Metab. Dispos. Biol. Fate Chem. 2014, 42, 1611–1616. [Google Scholar] [CrossRef]
- Voruganti, S.; Yamsani, S.K.; Yamsani, M.R. Effect of silibinin on the pharmacokinetics of nitrendipine in rabbits. Eur. J. Drug Metab. Pharm. 2014, 39, 277–281. [Google Scholar] [CrossRef]
- Pan, P.P.; Wang, J.; Luo, J.; Wang, S.H.; Zhou, Y.F.; Chen, S.Z.; Du, Z. Silibinin affects the pharmacokinetics of methadone in rats. Drug Test. Anal. 2018, 10, 557–561. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Ramachandran, V.; Komoroski, B.J.; Zhang, S.; Schiff, P.L.; Strom, S.C. Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metab. Dispos. 2000, 28, 1270–1273. [Google Scholar]
- Sridar, C.; Goosen, T.C.; Kent, U.M.; Williams, J.A.; Hollenberg, P.F. Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metab. Dispos. 2004, 32, 587–594. [Google Scholar] [CrossRef]
- Kock, K.; Xie, Y.; Hawke, R.L.; Oberlies, N.H.; Brouwer, K.L. Interaction of silymarin flavonolignans with organic anion-transporting polypeptides. Drug Metab. Dispos. Biol. Fate Chem. 2013, 41, 958–965. [Google Scholar] [CrossRef]
- Nguyen, H.; Zhang, S.; Morris, M.E. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J. Pharm. Sci. 2003, 92, 250–257. [Google Scholar] [CrossRef]
- Hussain, S.A.; Marouf, B.H. Silibinin improves the cytotoxicity of methotrexate in chemo resistant human rhabdomyosarcoma cell lines. Saudi Med. J. 2013, 34, 1145–1150. [Google Scholar]
- Wang, L.; Collins, C.; Kelly, E.J.; Chu, X.; Ray, A.S.; Salphati, L.; Xiao, G.; Lee, C.; Lai, Y.; Liao, M.; et al. Transporter Expression in Liver Tissue from Subjects with Alcoholic or Hepatitis C Cirrhosis Quantified by Targeted Quantitative Proteomics. Drug Metab. Dispos. 2016, 44, 1752–1758. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.W.; Tsai, T.H. Effect of silibinin on the pharmacokinetics of pyrazinamide and pyrazinoic acid in rats. Drug Metab. Dispos. 2007, 35, 1603–1610. [Google Scholar] [CrossRef]


| Subjects | Silybin Dosing | Probe Drug Dosing | Enzymes or Transporters Involved | Conclusion | ||
|---|---|---|---|---|---|---|
| 1 | 16 healthy volunteers | 3 × 70 mg Legalon® (silymarin), 28 days | Aminopyrine/phenylbutazone | No influence | [49] | |
| 2 | 10 healthy volunteers | 175 mg milk thistle extract, 3 times daily for 3 weeks | indinavir 800 mg/8 h | CYP3A4 | 9% reduction in AUC of indinavir | [50] |
| 3 | 10 healthy volunteers | 160 mg silymarin, 3 times/day | indinavir 800 mg 3 times/day | CYP3A4 | No influence | [57] |
| 4 | 16 healthy volunteers | 450 mg milk thistle extract daily | indinavir | CYP3A4 | No influence | [51] |
| 5 | 12 healthy volunteers | 140 mg silymarin daily for 9 days | 400 mg metronidazole trice daily for 3 days | P-gp, CYP3A4, CYP2C9 | 28% reduction in AUC of metronidazole | [58] |
| 6 | 12 healthy volunteers | 175 mg (containing 80% silymarin) twice daily | midazolam and caffeine, followed 24 h later by chlorzoxazone and debrisoquin | CYP1A2, CYP2D6, CYP2E1, CYP3A4 | No influence | [52] |
| 7 | 6 cancer patients | 200 mg milk thistle (containing 80% silymarin), thrice a day, for 14 consecutive days | irinotecan once a week i.v. 125 mg/m2 | CYP3A4, UGT1A1 | No influence | [54] |
| 8 | 16 healthy volunteers | 900 mg milk thistle (containing 80% silymarin) for 14 days | digoxin 0.4 mg | P-gp | No influence | [59] |
| 9 | 19 healthy volunteers | 900 mg milk thistle (containing 80% silymarin) for 14 days | midazolam | CYP3A | No influence | [53] |
| 10 | 16 young male volunteers | 280 mg silymarin | 10 mg nifedipine | CYP3A4 | No influence | [60] |
| 11 | 12 young male volunteers | 140 mg silymarin thrice daily | 150 mg ranitidine | CYP3A4, P-gp | No influence | [55] |
| 12 | 8 healthy male volunteers | 140 mg silymarin 4 times daily | 10 mg rouvastatin | OATP1B1, BCRP | No influence | [61] |
| 13 | 16 healthy volunteers | 300 mg milk thistle extract (containing 80% silymarin) 3 times daily | 5 mg debrisoquine | CYP2D6 | No influence | [56] |
| 14 | 18 healthy adult men | 140 mg silymarin 3 times daily for 14 days | talinolol | P-gp | Silymarin increased (36%) AUC of talinolol | [62] |
| 15 | 12 healthy adult men | 140 mg silymarin 3 times daily | losartan | CYP2C9 | Inhibition CYP2C9 in a genotype-dependent manner | [63] |
| 16 | 15 HIV-infected patients | 150 mg silymarin 3 times daily | darunavir-ritonavir (600/100 mg twice daily) | CYP3A4, P-gp | Silymarin slightly decreased (15%) the AUC of darunavir-ritonavir | [64] |
| 17 | 8 healthy male volunteers | 500 mg silymarin twice daily for 7 days | 10 mg domperidon | CYP3A4, P-gp | Silymarin pretreatment increased AUC of domperidone by 5-fold. | [65] |
| 18 | 9 healthy volunteers | 175 mg Legalon® (140 mg silymarin) thrice daily for 14 days | caffeine, tolbutamide, dextromethorphan, midazolam | CYP1A2, CYP2C9, CYP2D6, CYP3A4/5 | No influence | [66] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Zhang, D.; Zhang, J.; Yuan, J. Metabolism, Transport and Drug–Drug Interactions of Silymarin. Molecules 2019, 24, 3693. https://doi.org/10.3390/molecules24203693
Xie Y, Zhang D, Zhang J, Yuan J. Metabolism, Transport and Drug–Drug Interactions of Silymarin. Molecules. 2019; 24(20):3693. https://doi.org/10.3390/molecules24203693
Chicago/Turabian StyleXie, Ying, Dingqi Zhang, Jin Zhang, and Jialu Yuan. 2019. "Metabolism, Transport and Drug–Drug Interactions of Silymarin" Molecules 24, no. 20: 3693. https://doi.org/10.3390/molecules24203693
APA StyleXie, Y., Zhang, D., Zhang, J., & Yuan, J. (2019). Metabolism, Transport and Drug–Drug Interactions of Silymarin. Molecules, 24(20), 3693. https://doi.org/10.3390/molecules24203693




