Sprouts and Needles of Norway Spruce (Picea abies (L.) Karst.) as Nordic Specialty—Consumer Acceptance, Stability of Nutrients, and Bioactivities during Storage
Abstract
:1. Introduction
- What is the optimal way to dry sprout raw material to preserve the quality (flavor, color, antioxidative activity, and vitamins)?
- Can the sprouts be replaced by older, i.e., mature, needles, and how do the quality and basic characteristics differ between sprouts and older needles (from herein called ‘needles’)?
- How do the quality factors change during the large trader process and what needs to be considered from a quality point of view?
- How are the sprout-containing gourmet food products (ice-cream and sorbet) accepted by the consumers?
2. Results and Discussion
2.1. Drying Results
2.2. Basic Properties of Spruce Sprouts and Needles
2.2.1. Macro-Components and Carbohydrates
2.2.2. Minerals and Vitamins
2.2.3. Dietary Fiber
2.3. Microbial Quality of Spruce Sprouts and Needles
2.4. Color of Fresh and Dried Spruce Sprouts and Needles
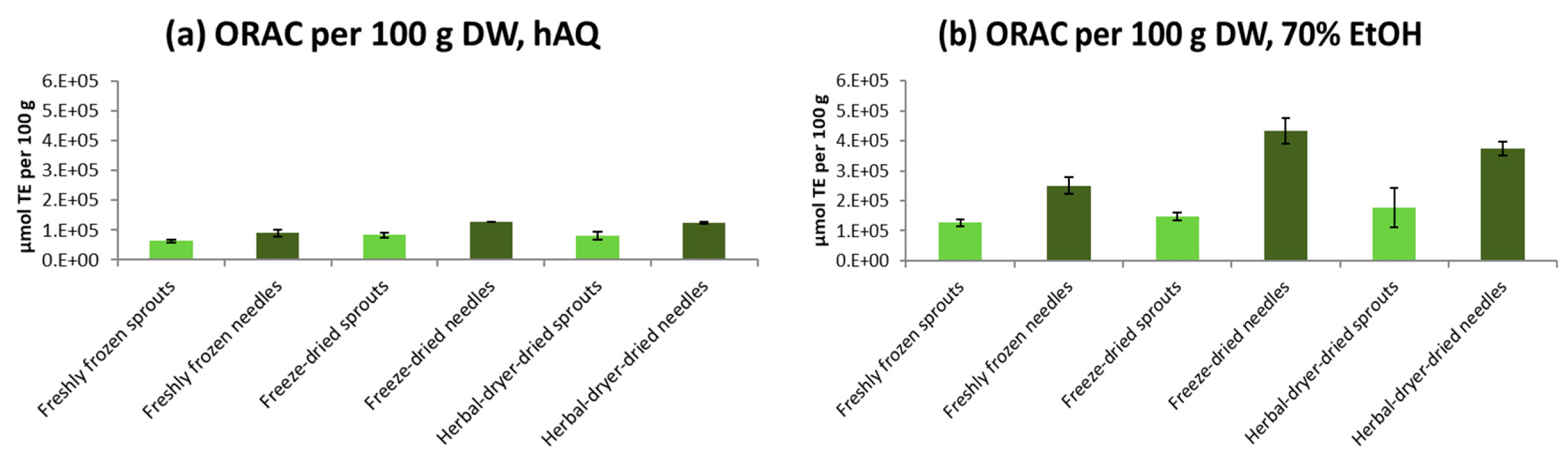
2.5. Antioxidative Activity of Spruce Sprouts and Needles
2.6. Production Scale: Quality of Raw Materials Across the Value-Chain
2.6.1. Microbial Quality
2.6.2. Heavy Metals and Pesticides
2.6.3. Vitamins and Minerals
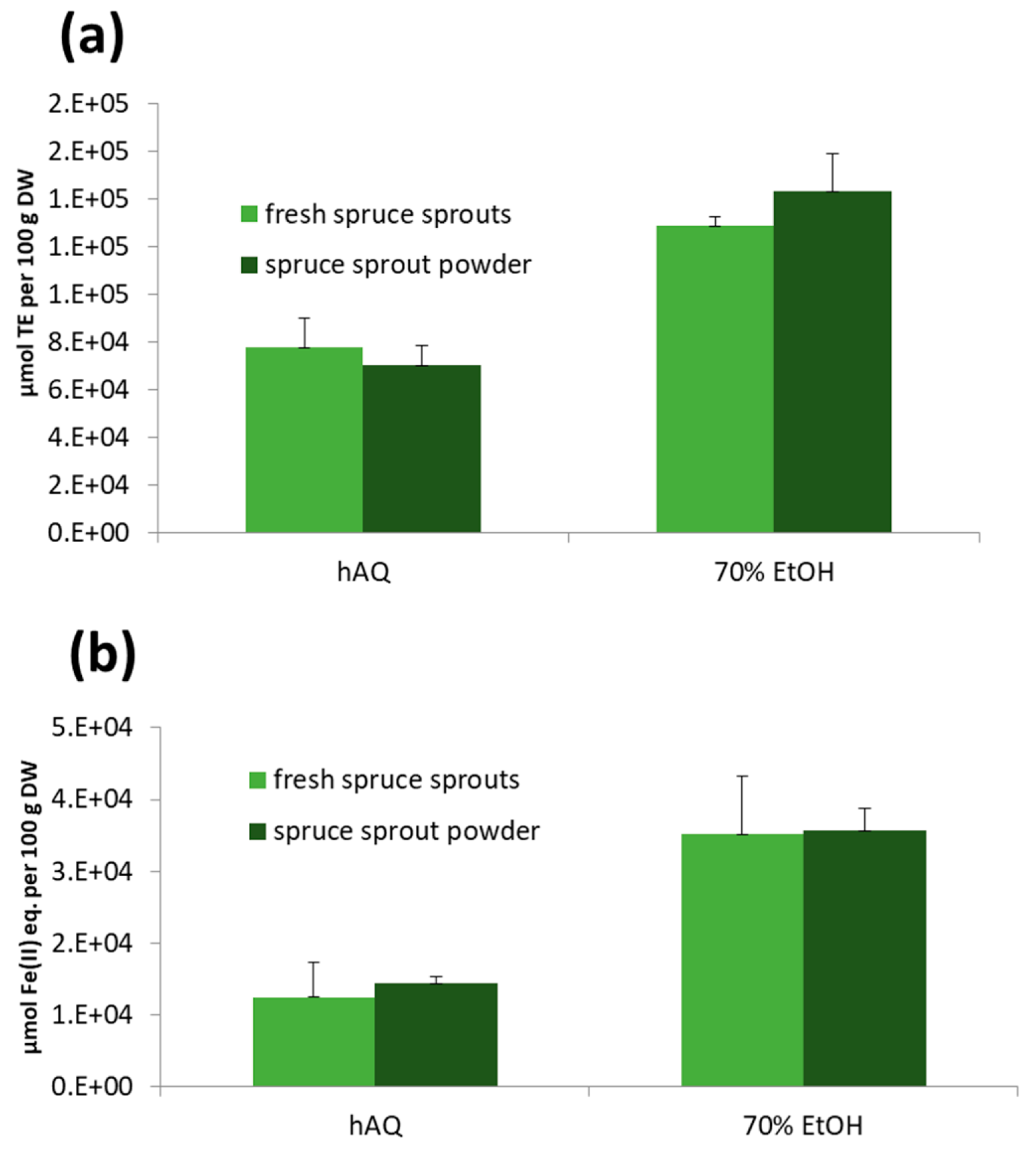
2.6.4. Antioxidant Activity
2.7. Characteristics of Food Product Prototypes with Sprouts and Needle Additions
2.7.1. Sensory Evaluation of Prototypes: Sprout and Needle Syrups and Ice-Creams
2.7.2. Ice-Creams with Fresh Sprouts Powder: Consumer Acceptance
3. Materials and Methods
3.1. Chemicals
3.2. Plant Raw Materials
3.3. Drying Treatments
3.3.1. Freeze-Drying
3.3.2. Condensing Circulating Warm Air-Drying (Warm Air Dryer)
3.4. Microbial Quality
3.5. Basic Properties and Dietary Fiber Content
3.6. Pesticides and Heavy Metals
3.7. Determination of Color
3.8. Analysis of Antioxidative Properties of Spruce Sprouts and Needles
3.8.1. FRAP
3.8.2. ORAC
3.8.3. H2O2 Scavenging
3.9. Preparation of Food Prototype Products with Sprout and Needle Additions
3.10. Sensory Analysis and Consumer Acceptance of Products
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pauli, A.; Schilcher, H. Specific Selection of Essential Oil Compounds for Treatment of Children’s Infection Diseases. Pharmaceuticals 2004, 1, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Piippo, S. Luonnonlääkeyrtit 2; Kustannusosakeyhtiö Tammi: Hämeenlinna, Finland, 2004; pp. 211–212. (In Finnish) [Google Scholar]
- Radulescu, V.; Saviuc, C.; Chifiriuc, C.; Oprea, E.; Ilies, D.C.; Marutescu, L.; Lazar, V. Chemical Composition and Antimicrobial Activity of Essential Oil from Shoots Spruce (Picea abies L). Rev. Chim. 2011, 62, 69–74. [Google Scholar]
- Werkelin, J.; Lindberg, D.K.; Boström, D.; Skrifvars, B.-J.; Hupa, M. Ash-forming elements in four Scandinavian wood species part 3: Combustion of five spruce samples. Biomass Bioenergy 2011, 35, 725–733. [Google Scholar] [CrossRef]
- Radulescu, V.; Ilies, D.-C.; Voiculescu, I.; Iovu-Adrian, B.; Craciunescu, A. Determination of ascorbic acid in shoots from different coniferous species by HPLC. Farmacia 2013, 61, 1158–1166. [Google Scholar]
- Turtola, S.; Sallas, L.; Holopainen, J.K.; Julkunen-Tiitto, R.; Kainulainen, P. Long-term exposure to enhanced UV-B radiation has no significant effects on growth or secondary compounds of outdoor-grown Scots pine and Norway spruce seedlings. Environ. Pollut. 2006, 144, 166–171. [Google Scholar] [CrossRef]
- Virjamo, V.; Julkunen-Tiitto, R. Shoot development of Norway spruce (Picea abies) involves changes in piperidine alkaloids and condensed tannins. Trees 2013, 28, 427–437. [Google Scholar] [CrossRef]
- Ganthaler, A.; Stöggl, W.; Kranner, I.; Mayr, S. Foliar Phenolic Compounds in Norway Spruce with Varying Susceptibility to Chrysomyxa rhododendri: Analyses of Seasonal and Infection-Induced Accumulation Patterns. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef] [Green Version]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.; Rauha, J.-P.; Pihlaja, K.; Kujala, A.T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Routa, J.; Brännström, H.; Anttila, P.; Mäkinen, M.; Jänis, J.; Asikainen, A. Wood Extractives of Finnish Pine, Spruce and Birch—Availability and Optimal Sources of Compounds—A Literature Review. 2017. Natural Resources and Bioeconomy Studies. Report Series 73/2017, p. 55. Available online: https://jukuri.luke.fi/handle/10024/540829 (accessed on 8 August 2020).
- Samieri, C.; Sun, Q.; Townsend, M.K.; Rimm, E.B.; Grodstein, F. Dietary flavonoid intake at midlife and healthy aging in women. Am. J. Clin. Nutr. 2014, 100, 1489–1497. [Google Scholar] [CrossRef]
- Mikkonen, H.; Myllykoski, L.; Pongrácz, E.; Keiski, R.L. Resources use optimization in small-and medium-sized juice plants in Northern Finland: A novel, waste-free utilization of annual shoots of Norway spruce. J. Solid Waste Technol. Manag. 2005, 31, 59–68. [Google Scholar]
- Haapakoski, S. Luonnontuotteiden käyttö kokkien koulutusohjelmassa ja ravintoloissa. Itäsuomen yliopisto. Pro-gradu –tutkielma. 2014. Available online: http://epublications.uef.fi/pub/urn_nbn_fi_uef-20150109/urn_nbn_fi_uef-20150109.pdf (accessed on 23 July 2020). (In Finnish with English abstract).
- Karvonen, R. Luonnontuotteiden käyttö Pohjois-Savon ravitsemisliikkeissä. Savonia Ammattikorkeakoulu. Diploma Thesis, Savonia University of Applied Sciences, Kuopio, Finland, 13 May 2014. Available online: https://www.theseus.fi/bitstream/handle/10024/74787/Karvonen_Riikka.pdf?sequence=1&isAllowed=y. (accessed on 23 July 2020). (In Finnish with English abstract).
- Herttuainen, H.; Mäkelä, S. Ruokaa luonnosta- toimialaselvityksen tulokset 2018. Available online: https://www.4h.fi/wp-content/uploads/2018/02/ruokaa-luonnosta-hankkeen-tulokset.pdf (accessed on 23 July 2020). (In Finnish).
- Siivonen, J. The Use of Spruce Shoots (Picea abies (L.) Karst.) in Helsinki Area Restaurants and Time Consumption of Spruce Shoot Gathering. Master’s Thesis, Department of Forest sciences, Faculty of Agriculture and Forestry, University of Helsinki, Helsinki, Finland, February 2019; 43p. Helsinki University Library– Helda/E-thesis, ethesis.helsinki.fi(In Finnish with English abstract). [Google Scholar]
- Vuorela, E. Luomukeruualueiden sertifiointi Suomessa. 2018. Available online: https://proluomu.fi/wp-content/uploads/sites/11/2018/07/luomukeruu_proluomu_eija-vuorela_net-tiin.pdf (accessed on 30 June 2020).
- Metsäkeskus. Luomusertifiointi kiinnostaa metsänomistajia. 17.3.2015. Available online: https://www.metsakeskus.fi/uutiset/metsien-luomusertifiointi-kiinnostaa-metsanomistajia (accessed on 30 June 2020).
- Miina, J.; Niemistö, P.; Potila, H.; Savonen, E.-M. Kuusentaimikon kerkkäsato ja keruun vaikutus kuusen kasvuun. Metsätieteen aikakauskirja vuosikerta 2018, 2018. (In Finnish) [Google Scholar] [CrossRef]
- Sutinen, S.; Partanen, J.; Viherä-Aarnio, A.; Häkkinen, R. Development and growth of primordial shoots in Norway spruce buds before visible bud burst in relation to time and temperature in the field. Tree Physiol. 2012, 32, 987–997. (In Finnish) [Google Scholar] [CrossRef]
- Hejnowicz, A.; Obarska, E. Structure and development of vegetative buds from the lower crown of Picea abies. Ann. Forest Sci. 1995, 52, 433–447. [Google Scholar] [CrossRef]
- Partanen, J.; Koski, V.; Hänninen, H. Effects of photoperiod and temperature on the timing of bud burst in Norway spruce (Picea abies). Tree Physiol. 1998, 18, 811–816. [Google Scholar] [CrossRef]
- Slaney, M.; Wallin, G.; Medhurst, J.; Linder, S. Impact of elevated carbon dioxide concentration and temperature on bud burst and shoot growth of boreal Norway spruce. Tree Physiol. 2006, 27, 301–312. [Google Scholar] [CrossRef]
- Jyske, T.; Mäkinen, H.; Kalliokoski, T.; Nöjd, P. Intra-annual xylem formation of Norway spruce and Scots pine across latitudinal gradient in Finland. Agric. For. Meteorol. 2014, 194, 241–254. [Google Scholar] [CrossRef]
- Mäkinen, H.; Jyske, T.; Nöjd, P. Dynamics of diameter and height increment of Norway spruce and Scots pine in southern Finland. Ann. For. Sci. 2018, 75, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Fierravanti, A.; Rossi, S.; Kneeshaw, D.; De Grandpré, L.; Deslauriers, A. Low Non-structural Carbon Accumulation in Spring Reduces Growth and Increases Mortality in Conifers Defoliated by Spruce Budworm. Front. For. Glob. Chang. 2019, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Schiestl-Aalto, P.; Ryhti, K.; Mäkelä, A.; Peltoniemi, M.; Bäck, J.; Kulmala, L. Analysis of the NSC Storage Dynamics in Tree Organs Reveals the Allocation to Belowground Symbionts in the Framework of Whole Tree Carbon Balance. Front. For. Glob. Chang. 2019, 2, 17. [Google Scholar] [CrossRef]
- Pendergast, B.A.; Boag, D.A. Nutritional aspects of the diet of spruce grouse in Central Alberta. Condor 1971, 73, 437–443. [Google Scholar] [CrossRef]
- ETL. Elintarvikkeiden mikrobiologisia ohjausarvoja viimeisenä käyttöpäivänä. 2017. Available online: https://www.etl.fi/media/aineistot/suositukset-ja-ohjeet/elintarvikkeiden-mikrobiologisia-ohjausarvoja-viimeisena-kayttopaivana-2017-suositus.pdf (accessed on 11 August 2020). (In Finnish).
- Sarvas, R. Havupuut. 2002. Available online: http://haku.helmet.fi/iii/encore/record/C|Rb1606239 (accessed on 13 August 2020).
- Helsingin yliopisto, Metsätieteiden laitoksen virtuaaliarboretum. 2019. Available online: http://www.helsinki.fi/metsatieteet/arboretum/puulajit/index.html (accessed on 30 August 2019). (In Finnish).
- Barnes, J.D.; Davison, A.W.; Booth, T.A. Ozone accelerates structural degradation of epicuticular wax on Norway spruce needles. New Phytol. 1988, 110, 309–318. [Google Scholar] [CrossRef]
- Virjamo, V.; Julkunen-Tiitto, R.; Henttonen, H.; Hiltunen, E.; Karjalainen, R.; Korhonen, J.; Huitu, O. Differences in vole preference, secondary chemistry and nutrient levels between naturally regenerated and planted Norway spruce seedlings. J. Chem. Ecol. 2013, 39, 1322–1334. [Google Scholar] [CrossRef]
- Virjamo, V.; Sutinen, S.; Julkunen-Tiitto, R. Combined effect of elevated UVB, elevated temperature and fertilization on growth, needle structure and phytochemistry of young Norway spruce (Picea abies) seedlings. Glob. Chang. Biol. 2014, 20, 2252–2260. [Google Scholar] [CrossRef]
- Eisner, T.; Goetz, M.; Aneshansley, D.; Ferstandig-Arnold, G.; Meinwald, J. Defensive alkaloid in blood of Mexican bean beetle (Epilachna varivestis). Experentia 1986, 42, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.J.; Montali, J.A.; Hazen, D.; Stanton, C.E. Alkaloids of Picea. J. Nat. Prod. 1991, 54, 905–909. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Tawara, J.N.; Boeckl, M.; Pomeroy, M.; Foderaro, T.A.; Todd, F.G. Piperidine alkaloid content of Picea (spruce) and Pinus (pine). Phytochemistry 1994, 35, 951–953. [Google Scholar] [CrossRef]
- Shtykova, L.; Masuda, M.; Eriksson, C.; Sjödin, K.; Marling, E.; Schlyter, F.; Nydén, M. Latex coatings containing antifeedants: Formulation, characterization, and application for protection of conifer seedlings against pine weevil feeding. Prog. Org. Coat. 2008, 63, 160–166. [Google Scholar] [CrossRef]
- Fyhrquist, P.J.; Virjamo, V.; Hiltunen, E.; Julkunen-Tiitto, R. Epidihy-dropinidine, the main piperidine alkaloid compound of Norway spruce (Picea abies) shows promising antibacterial and anti-Candida activity. Fitoterapia 2017, 117, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Finnish Food Authority. Nutrition and Health Claim Guide for Food Control Officers and Food Business Operators. Evira Guideline 17052/4. 2017. Available online: https://www.ruokavirasto.fi/globalassets/tietoa-meista/asiointi/oppaat-ja-lomakkeet/yritykset/elintarvikeala/elintarvikealan-oppaat/en/eviran_ohje_17052_4_uk.pdf (accessed on 13 August 2020).
- McCleary, B.V.; De Vries, J.W.; I Rader, J.; Cohen, G.; Prosky, L.; Mugford, D.C.; Champ, M.; Okuma, K.; Abercrombie, L.; Ames, N.; et al. Determination of Total Dietary Fiber (CODEX Definition) by Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study. J. AOAC Int. 2010, 93, 221–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCleary, B.V.; Sloane, N.; Draga, A.; Lazewska, I. Measurement of total dietary fiber using AOAC method 2009.01 (AACC international approved method 32-45.01): Evaluation and updates. Cereal Chem 2013, 90, 396–414. [Google Scholar] [CrossRef]
- Rainakari, A.-I.; Rita, H.; Putkonen, T.; Pastell, H. New dietary fibre content results for cereals in the Nordic countries using AOAC 2011.25 method. J. Food Comp. Anal. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.-Y.; Woollard, A.C.; Wolff, S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990, 268, 69–71. [Google Scholar] [CrossRef] [Green Version]
- de Winter, J.C.F.; Dodou, D. Five-point Likert items: T-test versus Mann-Whitney-Wilcoxon. Pract. Assess. Res. Eval. 2010, 15, 1–7. [Google Scholar]
- Westfall, P.H. Multiple testing of general contrasts using logical constraints and correlations. J. Am. Stat. Assoc. 1997, 92, 299–306. [Google Scholar] [CrossRef]
Sample Availability: No samples but data are available from the authors. |








| Drying Method | Raw Material | Dry Matter % |
|---|---|---|
| none, frozen | sprouts | 16.58 |
| freeze-drying | sprouts | 95.35 |
| warm air dryer | sprouts | 93.18 |
| none, frozen | needles | 54.75 |
| freeze-drying | needles | 96.43 |
| warm air dryer | needles | 84.49 |
| Raw Material | Enterobacteria | Aerobic Plate Counts | Yeasts | Molds |
|---|---|---|---|---|
| sprouts | <10 | 2.4 | 3.6 | 3.0 |
| needles | <10 | 4.2 | 4.4 | 4.3 |
| Vitamin/Mineral | A. Freshly Frozen Spruce Sprouts Analyzed in 2018 | B. Batch 2018 Analyzed in 2020 (Stored at –20 °C) | p-Values of Statistical Comparison of A and B (α = 0.05) |
|---|---|---|---|
| Vitamin K1 (μg/100 g DW) | 140 ± 30 | 65 ± 13 | 0.146 |
| Vitamin C (mg/100 g DW) | 350 ± 40 | 290 ± 30 | 0.517 |
| Beta carotene, vitamin A precursors total (cis + trans) (μg/100 g DW) | 3700 ± 1100 | 4000 ± 1000 | 0.343 |
| Zinc (mg/kg DW) | 38 ± 8 | 37 ± 7 | 0.945 |
| Iron (mg/kg DW) | 23 ± 5 | 20 ± 4 | 0.756 |
| Magnesium (mg/kg DW) | 1100 ± 220 | 1300 ± 300 | 0.211 |
| Calsium (mg/kg DW) | 1640 ± 330 | 1300 ± 300 | 0.103 |
| Potassium (mg/kg DW) | 10,000 ± 2000 | 12,000 ± 3000 | 0.027 |
| Phosphorus (mg/kg DW) | 2600 ± 600 | 3000 ± 600 | 0.174 |
| Ice-cream Prototypes (Samples) | Texture Characteristics | Odor, Taste | Willingness to Purchase | ||
|---|---|---|---|---|---|
| Outlook | Hardness, spoonable | Mouthfeel | |||
| Pine bark flour, 2 g/kg | 3.4 ± 1.0 | 3.5 ± 0.8 | 3.1 ± 0.9 | 3.6 ± 0.9 | 2/10 |
| “Pettu” pine bark as flavor, 2 g/kg | 3.9 ± 0.9 | 3.7 ± 1.1 | 3.7 ± 0.7 | 3.9 ± 0.8 | 9/10 |
| Spruce syrup prototype 2 as ingredient, 100 g/kg | 3.9 ± 0.7 | 3.6 ± 0.7 | 4.1 ± 0.8 | 3.6 ± 0.9 | 6/10 |
| Spruce syrup prototype 3 as ingredient, 100 g/kg | 3.2 ± 0.8 | 3.2 ± 1.1 | 3.2 ± 1.1 | 3.6 ± 0.9 | 3/10 |
| Spruce inner bark as flavor, 2 g/kg | 3.1 ± 1.1 | 3.1 ± 0.9 | 3.5 ± 0.9 | 3.6 ± 1.0 | 3/10 |
| Spruce inner bark as ingredient, 2 g/kg | 3.8 ± 0.9 | 3.9 ± 0.7 | 4.0 ± 0.6 | 3.7 ± 1.1 | 5/10 |
| Commercial Ice-creams (Reference Samples) | |||||
| Pingviini vanilla, 1 L brick | 4.7 ± 0.5 | 4.2 ± 0.7 | 4.5 ± 0.5 | 4.5 ± 0.5 | 10/10 |
| JYMY 1917, vanilla, organic, tub | 4.6 ± 0.5 | 4.6 ± 0.5 | 4.7 ± 0.5 | 4.5 ± 0.9 | 9/10 |
| JYMY 1917, pine extract flavor, tub | 4.3 ± 0.7 | 4.5 ± 0.7 | 4.6 ± 0.7 | 3.6 ± 1.3 | 5/10 |
| Sprout Ice-Cream | Forest Berry Ice-Cream | Sprout Sorbet | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Score | Score | Score | TOTAL | ||||||||||||
| Age years | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 0–6 | 1 | 4 | 3 | 4 | 22 | 2 | 1 | 4 | 22 | 1 | 5 | 7 | 6 | 13 | 95 | |
| 7–13 | 3 | 5 | 14 | 28 | 25 | 1 | 1 | 5 | 12 | 57 | 9 | 10 | 20 | 21 | 15 | 226 |
| 14–17 | 1 | 2 | 2 | 1 | 1 | 2 | 9 | |||||||||
| 18–24 | 1 | 3 | 4 | 4 | 6 | 2 | 3 | 4 | 27 | |||||||
| 25–40 | 2 | 6 | 34 | 28 | 2 | 21 | 48 | 1 | 3 | 19 | 25 | 24 | 213 | |||
| 41–60 | 3 | 18 | 21 | 2 | 15 | 25 | 2 | 2 | 19 | 28 | 135 | |||||
| >60 | 3 | 7 | 10 | 2 | 4 | 14 | 1 | 2 | 11 | 5 | 59 | |||||
| total | 4 | 11 | 31 | 96 | 110 | 1 | 3 | 14 | 60 | 173 | 11 | 21 | 53 | 87 | 89 | 764 |
| Male | Score | Score | Score | |||||||||||||
| Age years | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 45 |
| 0–6 | 1 | 1 | 5 | 14 | 22 | 1 | 4 | 3 | 33 | 3 | 5 | 1 | 11 | 23 | 127 | |
| 7–13 | 1 | 2 | 6 | 16 | 20 | 2 | 8 | 35 | 1 | 3 | 8 | 13 | 20 | 135 | ||
| 14–17 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 10 | ||||||||
| 18–24 | 2 | 1 | 3 | 1 | 2 | 9 | ||||||||||
| 25–40 | 1 | 5 | 7 | 10 | 4 | 21 | 1 | 2 | 8 | 12 | 71 | |||||
| 41–60 | 3 | 7 | 6 | 1 | 7 | 8 | 1 | 2 | 4 | 9 | 48 | |||||
| >60 | 3 | 5 | 3 | 3 | 8 | 4 | 3 | 4 | 33 | |||||||
| total | 2 | 6 | 25 | 51 | 61 | 1 | 0 | 7 | 26 | 110 | 5 | 10 | 17 | 42 | 70 | 433 |
| Observed Result | p-Value |
|---|---|
| ‘Forest berry’ scores did not differ by gender | 0.6498 |
| ‘Sprout’ scores did not differ by gender | 0.7944 |
| ‘Sorbet’ scores differed by gender | 0.0013 |
| Women liked ‘forest berry’ the most | <0.0001 |
| Women liked ‘sprout ice-cream’ more than ‘sorbet’ | <0.0001 |
| Men liked ‘forest berry’ the most | <0.0001 |
| Drying Method | Raw Material | Dry Matter % | Amount for 1.5 L (g) | Added Sugar Types | Amount (g) |
|---|---|---|---|---|---|
| freeze-drying | sprouts | 95.35 | 75 | saccharose | 450 |
| warm air-dryer | sprouts | 93.18 | 78 | saccharose, glucose syrup | 330 441 |
| freeze-drying | needles | 96.43 | 75 | saccharose | 450 |
| warm air-dryer | needles | 84.49 | 84 | saccharose, glucose syrup | 330 441 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jyske, T.; Järvenpää, E.; Kunnas, S.; Sarjala, T.; Raitanen, J.-E.; Mäki, M.; Pastell, H.; Korpinen, R.; Kaseva, J.; Tupasela, T. Sprouts and Needles of Norway Spruce (Picea abies (L.) Karst.) as Nordic Specialty—Consumer Acceptance, Stability of Nutrients, and Bioactivities during Storage. Molecules 2020, 25, 4187. https://doi.org/10.3390/molecules25184187
Jyske T, Järvenpää E, Kunnas S, Sarjala T, Raitanen J-E, Mäki M, Pastell H, Korpinen R, Kaseva J, Tupasela T. Sprouts and Needles of Norway Spruce (Picea abies (L.) Karst.) as Nordic Specialty—Consumer Acceptance, Stability of Nutrients, and Bioactivities during Storage. Molecules. 2020; 25(18):4187. https://doi.org/10.3390/molecules25184187
Chicago/Turabian StyleJyske, Tuula, Eila Järvenpää, Susan Kunnas, Tytti Sarjala, Jan-Erik Raitanen, Maarit Mäki, Helena Pastell, Risto Korpinen, Janne Kaseva, and Tuomo Tupasela. 2020. "Sprouts and Needles of Norway Spruce (Picea abies (L.) Karst.) as Nordic Specialty—Consumer Acceptance, Stability of Nutrients, and Bioactivities during Storage" Molecules 25, no. 18: 4187. https://doi.org/10.3390/molecules25184187
APA StyleJyske, T., Järvenpää, E., Kunnas, S., Sarjala, T., Raitanen, J.-E., Mäki, M., Pastell, H., Korpinen, R., Kaseva, J., & Tupasela, T. (2020). Sprouts and Needles of Norway Spruce (Picea abies (L.) Karst.) as Nordic Specialty—Consumer Acceptance, Stability of Nutrients, and Bioactivities during Storage. Molecules, 25(18), 4187. https://doi.org/10.3390/molecules25184187






