Evaluation of Anti-Mycobacterial Compounds in a Silkworm Infection Model with Mycobacteroides abscessus
Abstract
:1. Introduction
2. Results
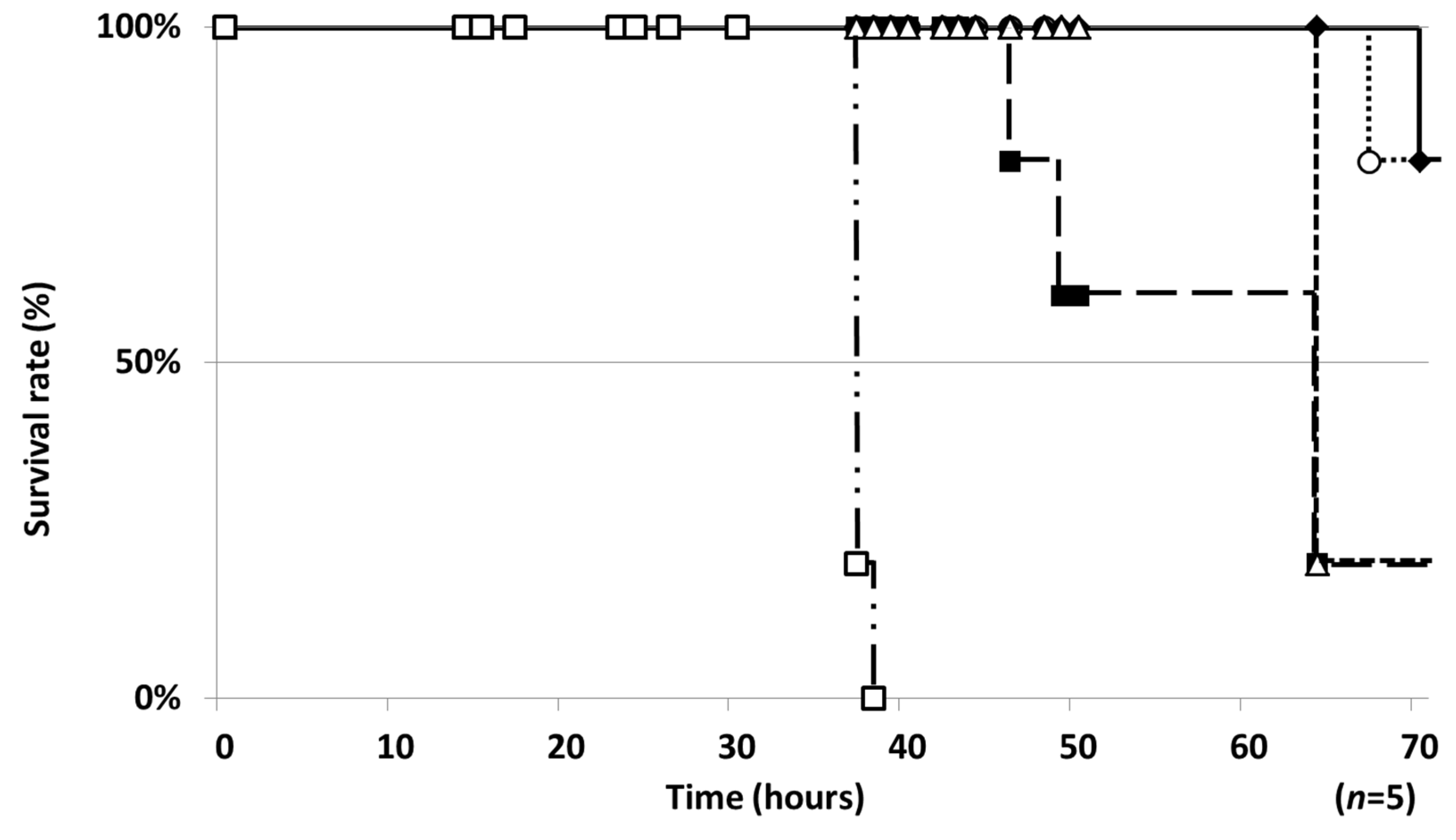
2.1. Establishment of a Silkworm Infection Assay with My. abscessus
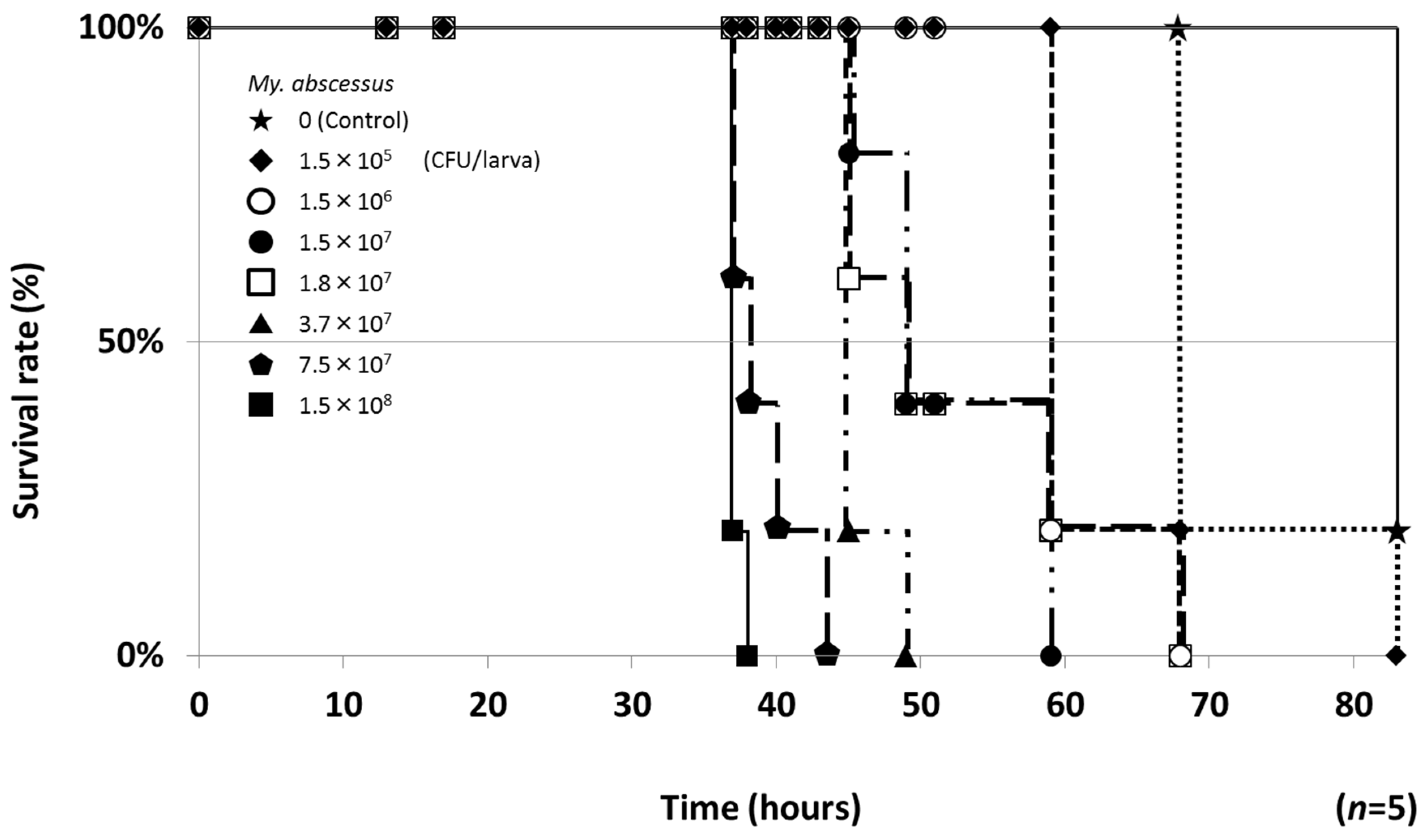
2.2. Therapeutic Efficacies of Drugs in the Silkworm Infection Assay with My. abscessus
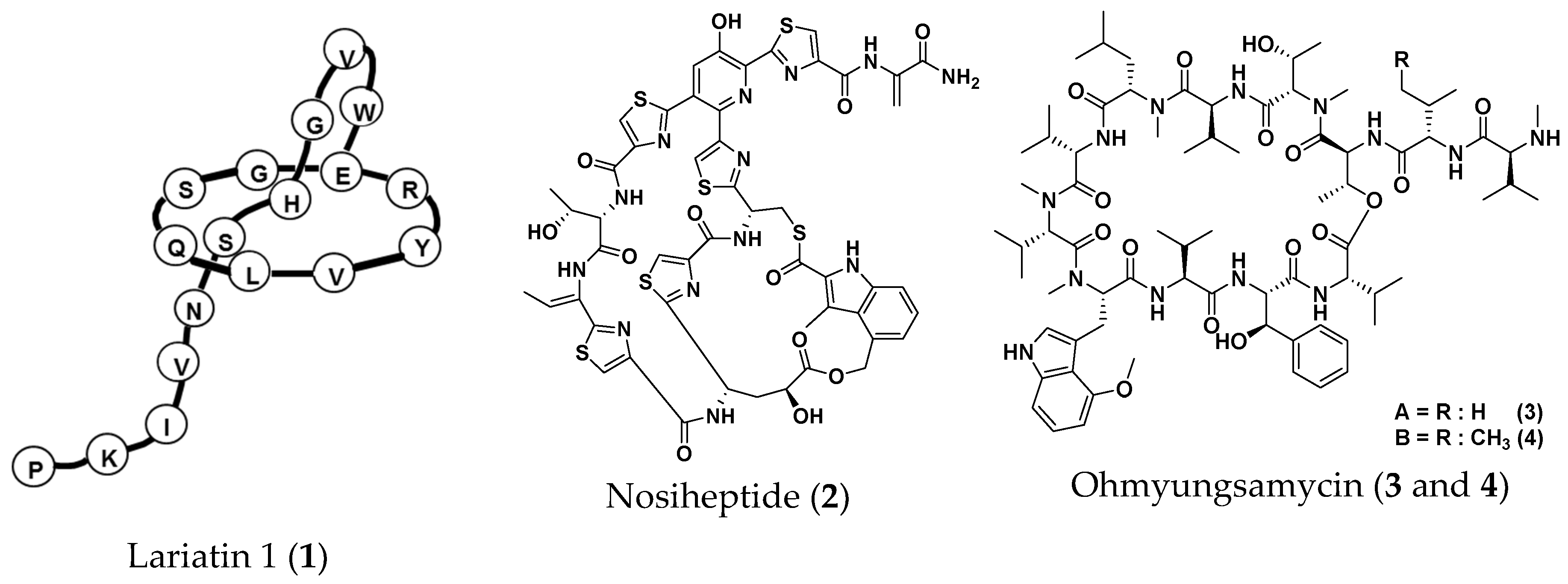
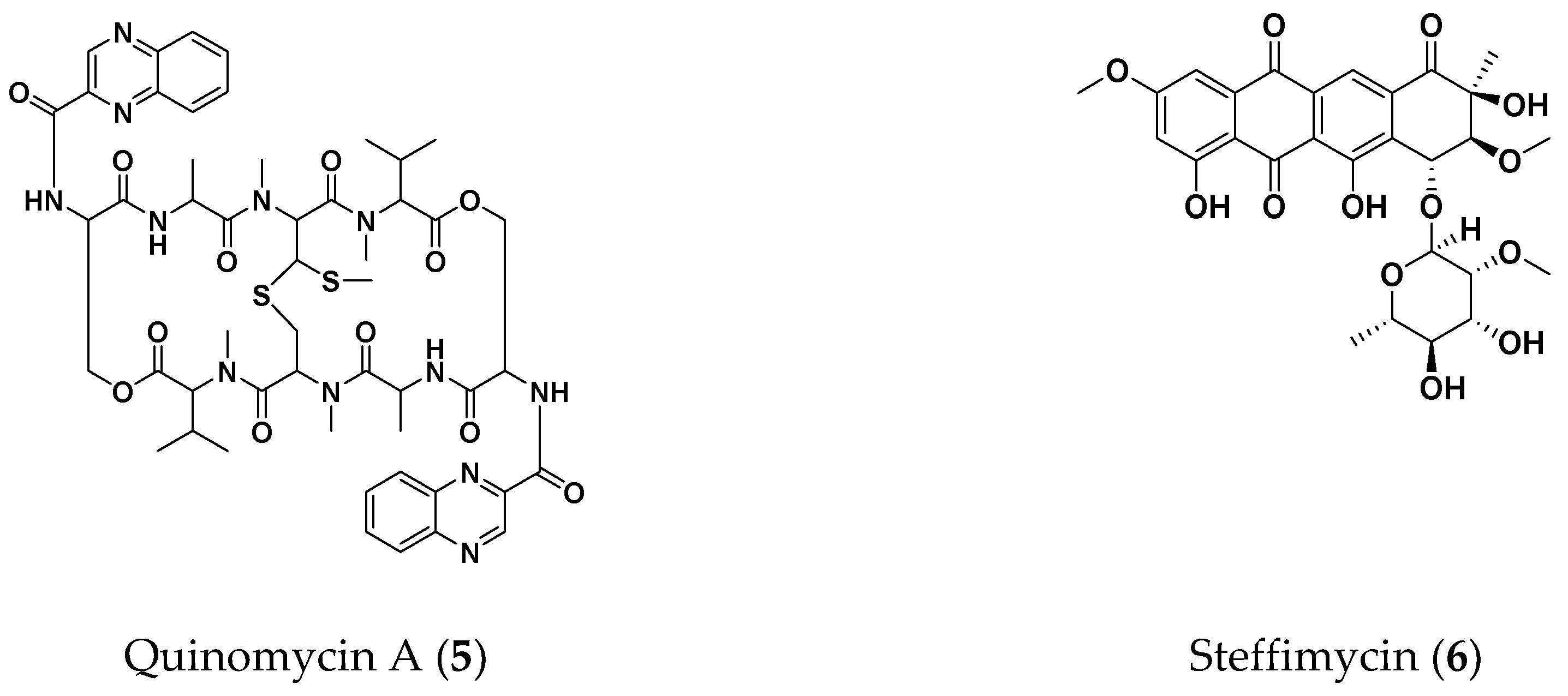
2.3. Screening for Anti-NTM Compounds from a Microbial Product Library
2.4. Therapeutic Efficacies of Microbial Compounds in the Silkworm Infection Assay with My. abscessus
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Mycobacterial Suspension
4.3. MIC Values in the Liquid Microdilution Assay
4.4. Silkworm Infection Assay with Mycobacteria Spp.
4.5. Silkworm Infection Assay with My. abscessus
4.6. ED50 Values in the Silkworm Infection Assay with My. abscessus
Author Contributions
Funding
Conflicts of Interest
References
- Hamamoto, H.; Urai, M.; Ishii, K.; Yasukawa, J.; Paudel, A.; Murai, M.; Kaji, T.; Kuranaga, T.; Hamase, K.; Katsu, T.; et al. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat. Chem. Biol. 2015, 11, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Iwatsuki, M.; Kim, Y.P.; Ohte, S.; Omura, S.; Tomoda, H. Nosokomycins, new antibiotics discovered in an in vivo-mimic infection model using silkworm larvae. I: Fermentation, isolation and biological properties. J. Antibiot. (Tokyo) 2010, 63, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Uchida, R.; Iwatsuki, M.; Kim, Y.P.; Omura, S.; Tomoda, H. Nosokomycins, new antibiotics discovered in an in vivo-mimic infection model using silkworm larvae. II: Structure elucidation. J. Antibiot. (Tokyo) 2010, 63, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, R.; Hanaki, H.; Matsui, H.; Hamamoto, H.; Sekimizu, K.; Iwatsuki, M.; Kim, Y.P.; Tomoda, H. In vitro and in vivo anti-MRSA activities of nosokomycins. Drug Discov. Ther. 2014, 8, 249–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, R.; Namiguchi, S.; Ishijima, H.; Tomoda, H. Therapeutic effects of three trichothecenes in the silkworm infection assay with Candida albicans. Drug Discov. Ther. 2016, 10, 44–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaito, C.; Akimitsu, N.; Watanabe, H.; Sekimizu, K. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 2002, 32, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Kurokawa, K.; Kaito, C.; Kamura, K.; Manitra Razanajatovo, I.; Kusuhara, H.; Santa, T.; Sekimizu, K. Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents. Chemother. 2004, 48, 774–779. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Miyazaki, S.; Fukunaga, D.H.; Shimizu, K.; Kawamoto, S.; Sekimizu, K. Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans. J. Appl. Microbiol. 2012, 112, 138–146. [Google Scholar] [CrossRef]
- Adjemian, J.; Olivier, K.N.; Seitz, A.E.; Holland, S.M.; Prevots, D.R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care. Med. 2012, 185, 881–886. [Google Scholar] [CrossRef] [Green Version]
- Marras, T.K.; Mendelson, D.; Marchand-Austin, A.; May, K.; Jamieson, F.B. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg. Infect. Dis. 2013, 19, 1889–1891. [Google Scholar] [CrossRef]
- Namkoong, H.; Kurashima, A.; Morimoto, K.; Hoshino, Y.; Hasegawa, N.; Ato, M.; Mitarai, S. Epidemiology of Pulmonary Nontuberculous Mycobacterial Disease, Japan. Emerg. Infect. Dis. 2016, 22, 1116–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Adelman, M.H.; Addrizzo-Harris, D.J. Management of nontuberculous mycobacterial pulmonary disease. Curr. Opin. Pulm. Med. 2018, 24, 212–219. [Google Scholar] [CrossRef]
- Iwatsuki, M.; Tomoda, H.; Uchida, R.; Gouda, H.; Hirono, S.; Omura, S. Lariatins, antimycobacterial peptides produced by Rhodococcus sp. K01-B0171, have a lasso structure. J. Am. Chem. Soc. 2006, 128, 7486–7491. [Google Scholar] [CrossRef] [PubMed]
- Pascard, C.; Ducruix, A.; Lunel, J.; Prang, T. Highly modified cysteine-containing antibiotics. Chemical structure and configuration of nosiheptide. J. Am. Chem. Soc. 1977, 99, 6418–6423. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Koyama, N.; Kanamoto, A.; Tomoda, H. Discovery of nosiheptide, griseoviridin, and etamycin as potent anti-mycobacterial agents against Mycobacterium avium complex. Molecules 2019, 24, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Um, S.; Choi, T.J.; Kim, H.; Kim, B.Y.; Kim, S.H.; Lee, S.K.; Oh, K.B.; Shin, J.; Oh, D.C. Ohmyungsamycins A and B: Cytotoxic and antimicrobial cyclic peptides produced by Streptomyces sp. from a volcanic island. J. Org. Chem. 2013, 78, 12321–12329. [Google Scholar] [CrossRef]
- Bergy, M.E.; Reusser, F. A new antibacterial agent (U-20,661) isolated from a Streptomycete strain. Experientia 1967, 23, 254–255. [Google Scholar] [CrossRef]
- Koyama, N.; Shigeno, S.; Kanamoto, A.; Tomoda, H. Steffimycin E, a new anti-mycobacterial agent against Mycobacterium avium complex, produced by Streptomyces sp. OPMA02852. J. Antibiot. (Tokyo) 2020, 73, 581–584. [Google Scholar] [CrossRef]
- Yoshida, T.; Katagiri, K.; Yokozawa, S. Studies on quinoxaline antibiotics. II. Isolation and properties of quinomycins A, B and C. J. Antibiot. (Tokyo) 1961, 14, 330–334. [Google Scholar]
- Yagi, A.; Uchida, R.; Hamamoto, H.; Sekimizu, K.; Kimura, K.I.; Tomoda, H. Anti-Mycobacterium activity of microbial peptides in a silkworm infection model with Mycobacterium smegmatis. J. Antibiot. (Tokyo) 2017, 70, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.A.; Brown-Elliott, B.A.; Wallace, R.J. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob. Agents Chemother. 2009, 53, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Dupont, C.; Viljoen, A.; Dubar, F.; Blaise, M.; Bernut, A.; Pawlik, A.; Bouchier, C.; Brosch, R.; Guèrardel, Y.; Lelièvre, J.; et al. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol. Microbiol. 2016, 101, 515–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riva, C.; Tortoli, E.; Cugnata, F.; Sanvito, F.; Esposito, A.; Rossi, M.; Colarieti, A.; Canu, T.; Cigana, C.; Bragonzi, A.; et al. A New Model of Chronic Mycobacterium abscessus Lung Infection in Immunocompetent Mice. Int. J. Mol. Sci. 2020, 21, 6590. [Google Scholar] [CrossRef]
- Maggioncalda, E.C.; Story-Roller, E.; Mylius, J.; Illei, P.; Basaraba, R.J.; Lamichhane, G. A mouse model of pulmonary Mycobacteroides abscessus infection. Sci. Rep. 2020, 10, 3690. [Google Scholar] [CrossRef] [PubMed]
- Haste, N.M.; Thienphrapa, W.; Tran, D.N.; Loesgen, S.; Sun, P.; Nam, S.J.; Jensen, P.R.; Fenical, W.; Sakoulas, G.; Nizet, V.; et al. Activity of the thiopeptide antibiotic nosiheptide against contemporary strains of methicillin-resistant Staphylococcus aureus. J. Antibiot. (Tokyo) 2012, 65, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.S.; Shin, Y.H.; Lee, H.M.; Kim, J.K.; Choe, J.H.; Jang, J.C.; Um, S.; Jin, H.S.; Komatsu, M.; Cha, G.H.; et al. Ohmyungsamycins promote antimicrobial responses through autophagy activation via AMP-activated protein kinase pathway. Sci. Rep. 2017, 7, 3431. [Google Scholar] [CrossRef] [PubMed]
- Reusser, F. Mode of action of antibiotic U-20,661. J. Bacteriol. 1967, 93, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Yoshida, T.; Katagiri, K. Inhibition of RNA synthesis in E. coli protoplasts by quinomycins A and C. J. Antibiot. (Tokyo) 1967, 20, 188–189. [Google Scholar]
- Kaplan, E.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Tominaga, T.; Uchida, R.; Koyama, N.; Tomoda, H. Anti-Rhizopus activity of tanzawaic acids produced by the hot spring-derived fungus Penicillium sp. BF-0005. J. Antibiot. (Tokyo) 2018, 71, 626–632. [Google Scholar] [CrossRef] [PubMed]



| Test Microorganism | MIC (μg/mL) * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | CAM | AMK | IPM | CPFX | |
| M. avium JCM15430 | 0.78 | 0.02 | 0.19 | 0.19 | <0.01 | 1.56 | 0.19 | 3.12 | >50 | 0.78 |
| M. intracellulare JCM6384 | 1.56 | 0.02 | 0.10 | 0.10 | <0.01 | 0.39 | 0.02 | 0.78 | 1.56 | 0.09 |
| M. bovis BCG Pasteur | 0.78 | 0.01 | N.T. | N.T. | N.T. | 1.56 | 0.12 | 0.78 | >50 | 0.09 |
| My. abscessus ATCC19977 | 1.56 | 1.56 | 12.5 | 12.5 | 0.01 | 25.0 | 0.39 | 12.5 | 12.5 | 1.56 |
| Test Compound | ED50 (μg/larva) 1 | MIC (μg/mL) | ED50/MIC |
|---|---|---|---|
| Lariatin A (1) | 8.84 | 1.56 | 5.67 |
| Nosiheptide (2) | 14.6 | 1.56 | 9.36 |
| Ohmyungsamycin A (3) | 30.0 | 12.5 | 2.40 |
| Ohmyungsamycin B (4) | 30.0 | 12.5 | 2.40 |
| Quinomycin A (5) | >50 | 25.0 | - |
| Steffimycin (6) | >50 | 0.01 | - |
| CAM | 0.22 | 0.39 | 0.56 |
| AMK | 1.48 | 12.5 | 0.12 |
| IPM | 7.82 | 12.5 | 0.63 |
| CPFX | 5.47 * | 1.56 | 3.51 * |
Sample Availability: Samples of compounds 1–6 are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosoda, K.; Koyama, N.; Hamamoto, H.; Yagi, A.; Uchida, R.; Kanamoto, A.; Tomoda, H. Evaluation of Anti-Mycobacterial Compounds in a Silkworm Infection Model with Mycobacteroides abscessus. Molecules 2020, 25, 4971. https://doi.org/10.3390/molecules25214971
Hosoda K, Koyama N, Hamamoto H, Yagi A, Uchida R, Kanamoto A, Tomoda H. Evaluation of Anti-Mycobacterial Compounds in a Silkworm Infection Model with Mycobacteroides abscessus. Molecules. 2020; 25(21):4971. https://doi.org/10.3390/molecules25214971
Chicago/Turabian StyleHosoda, Kanji, Nobuhiro Koyama, Hiroshi Hamamoto, Akiho Yagi, Ryuji Uchida, Akihiko Kanamoto, and Hiroshi Tomoda. 2020. "Evaluation of Anti-Mycobacterial Compounds in a Silkworm Infection Model with Mycobacteroides abscessus" Molecules 25, no. 21: 4971. https://doi.org/10.3390/molecules25214971
APA StyleHosoda, K., Koyama, N., Hamamoto, H., Yagi, A., Uchida, R., Kanamoto, A., & Tomoda, H. (2020). Evaluation of Anti-Mycobacterial Compounds in a Silkworm Infection Model with Mycobacteroides abscessus. Molecules, 25(21), 4971. https://doi.org/10.3390/molecules25214971






