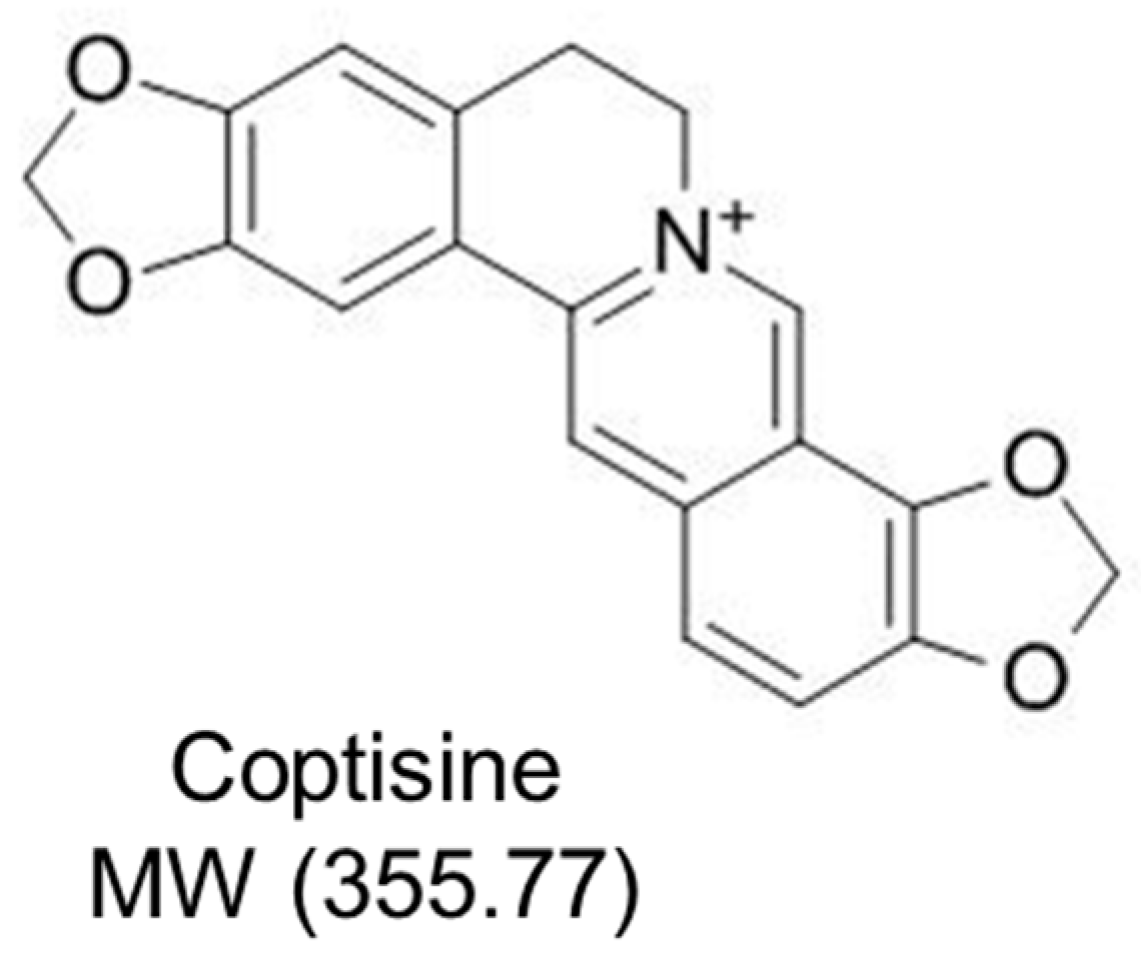
Coptisine Attenuates Diabetes—Associated Endothelial Dysfunction through Inhibition of Endoplasmic Reticulum Stress and Oxidative Stress
Abstract
:1. Background
2. Results
2.1. Coptisine Ameliorates Endothelial Dysfunction Associated with Diabetes
2.2. Coptisine Increases eNOS Phosphorylation and NO Bioavailability
2.3. Coptisine Suppresses ER Stress and Oxidative Stress
3. Methods
3.1. Animals
3.2. Ex Vivo Culture of Mouse Aortas
3.3. Isometric Force Measurement in Wire Myograph
3.4. Western Blotting
3.5. Culture of Human Umbilical Cord Vein Endothelial Cells (HUVECs)
3.6. Detection of Intracellular Oxidant Formation by Dihydroethidium (DHE) Fluorescence
3.7. Determination of NO Generation
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Aminzadeh, A.; Bashiri, H. Myricetin ameliorates high glucose-induced endothelial dysfunction in human umbilical vein endothelial cells. Cell Biochem. Funct. 2019, 38, 12–20. [Google Scholar] [CrossRef]
- Cheang, W.S.; Tian, X.Y.; Wong, W.T.; Lau, C.W.; Lee, S.S.; Chen, Z.Y.; Yao, X.; Wang, N.; Huang, Y. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5’ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arterioscler Thromb Vasc. Biol. 2014, 34, 830–836. [Google Scholar] [CrossRef] [Green Version]
- Cheang, W.S.; Wong, W.T.; Zhao, L.; Xu, J.; Wang, L.; Lau, C.W.; Chen, Z.Y.; Ma, R.C.W.; Xu, A.; Wang, N.; et al. PPARdelta Is Required for Exercise to Attenuate Endoplasmic Reticulum Stress and Endothelial Dysfunction in Diabetic Mice. Diabetes 2017, 66, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.B.; Luo, C.D.; Liang, J.L.; Zhang, Z.B.; Lin, G.S.; Wu, J.Z.; Li, C.L.; Tan, L.H.; Yang, X.B.; Su, Z.R.; et al. Anti-inflammatory activity of coptisine free base in mice through inhibition of NF-kappaB and MAPK signaling pathways. Eur. J. Pharmacol. 2017, 811, 222–231. [Google Scholar] [CrossRef]
- Chen, Z.P.; McConell, G.K.; Michell, B.J.; Snow, R.J.; Canny, B.J.; Kemp, B.E. AMPK signaling in contracting human skeletal muscle: Acetyl-CoA carboxylase and NO synthase phosphorylation. Am. J. Physiol-Endoc. M. 2000, 279, E1202–E1206. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Abou-Mohamed, G.; Caldwell, R.W.; Caldwell, R.B. High glucose-induced tyrosine nitration in endothelial cells: Role of eNOS uncoupling and aldose reductase activation. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3135–3143. [Google Scholar] [CrossRef]
- Gething, M.-J.; Sambrook, J. Protein folding in the cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.-L.; Fang, L.-H.; Qin, H.-L.; Lv, Y.; Du, G.-H. Analysis of the Mechanisms Underlying the Vasorelaxant Action of Coptisine in Rat Aortic Rings. Am. J. Chin. Med. 2012, 40, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kasai, K.; Nakamura, T.; Emoto, T.; Shimoda, S. Effect of glucose and insulin on immunoreactive endothelin-1 release from cultured porcine aortic endothelial cells. Metabolism 1991, 40, 165–169. [Google Scholar] [CrossRef]
- Hink, U.; Tsilimingas, N.; Wendt, M.; Munzel, T. Mechanisms underlying endothelial dysfunction in diabetes mellitus: Therapeutic implications. Treat Endocrinol. 2003, 2, 293–304. [Google Scholar] [CrossRef]
- Hong, J.; Kim, K.; Kim, J.-H.; Park, Y. The Role of Endoplasmic Reticulum Stress in Cardiovascular Disease and Exercise. Int. J. Vasc. Med. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Laight, D.; Carrier, M.; Änggård, E. Antioxidants, diabetes and endothelial dysfunction. Cardiovasc. Res. 2000, 47, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, H.; Si, N.; Ren, W.; Han, L.; Xin, S.; Zuo, R.; Wei, X.; Yang, J.; Zhao, H.; et al. Metabolic profiling analysis of berberine, palmatine, jatrorrhizine, coptisine and epiberberine in zebrafish by ultra-high performance liquid chromatography coupled with LTQ Orbitrap mass spectrometer. Xenobiotica 2015, 45, 302–311. [Google Scholar] [CrossRef]
- Liu, G.; He, W.; Cai, H.; Sun, X.; Hou, W.; Lin, M.; Xie, Z.; Liao, Q. The simultaneous determination of berberine, palmatine, coptisine, epiberberine and jatrorrhizine in rat plasma by LC-MS/MS and a pharmacokinetic comparison after the oral administration of Rhizoma coptidis and Jiao-Tai-Wan extract. Anal. Methods 2014, 6, 2998–3008. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, L.; Lu, Y.; Su, M.; Li, X.; Liu, J.; Zhang, H.; Nasir, K.; A Masoudi, F.; Krumholz, H.M.; et al. Secondary prevention of cardiovascular disease in China. Hear. 2020, 106, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Mahrouf, M.; Ouslimani, N.; Peynet, J.; Djelidi, R.; Couturier, M.; Therond, P.; Legrand, A.; Beaudeux, J.-L. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem. Pharmacol. 2006, 72, 176–183. [Google Scholar] [CrossRef]
- Mori-Quiroz, L.M.; Hedrick, S.L.; Santos, A.R.D.L.; Clift, M.D. A Unified Strategy for the Syntheses of the Isoquinolinium Alkaloids Berberine, Coptisine, and Jatrorrhizine. Org. Lett. 2018, 20, 4281–4284. [Google Scholar] [CrossRef]
- Ooi, B.K.; Goh, B.H.; Yap, W.H. Oxidative Stress in Cardiovascular Diseases: Involvement of Nrf2 Antioxidant Redox Signaling in Macrophage Foam Cells Formation. Int. J. Mol. Sci. 2017, 18, 2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouslimani, N.; Peynet, J.; Bonnefont-Rousselot, D.; Thérond, P.; Legrand, A.; Beaudeux, J.-L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 2005, 54, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.X.C.; Nabeebaccus, A.A.; Shah, A.; Camargo, L.D.L.; Filho, S.V.; Lopes, L. Endoplasmic Reticulum Stress and Nox-Mediated Reactive Oxygen Species Signaling in the Peripheral Vasculature: Potential Role in Hypertension. Antioxid. Redox Signal. 2014, 20, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.L.; Jia, W.H.; Zhang, L.; Xu, C.Y.; Chen, X.; Yin, L.; Wang, N.Q.; Fang, L.H.; Qiang, G.F.; Yang, X.Y.; et al. Glucose consumption assay discovers coptisine with beneficial effect on diabetic mice. Eur. J. Pharmacol. 2019, 859, 172523. [Google Scholar] [CrossRef]
- Sun, Y.; Xiong, Y.-Y.; Wu, H.-Z.; Xiong, W.-C.; Liu, B.; Xie, Z.-T.; Xiao, W.-P.; Huang, B.-S.; Yang, Y.-F. Active Ingredients and Mechanism of Action of Rhizoma Coptidis against Type 2 Diabetes Based on Network-Pharmacology and Bioinformatics. Curr. Med. Sci. 2020, 40, 257–264. [Google Scholar] [CrossRef]
- Suzuki, H.; Tanabe, H.; Mizukami, H.; Inoue, M. Differential Gene Expression in Rat Vascular Smooth Muscle Cells Following Treatment with Coptisine Exerts a Selective Antiproliferative Effect. J. Nat. Prod. 2011, 74, 634–638. [Google Scholar] [CrossRef]
- Tan, H.-L.; Chan, K.-G.; Pusparajah, P.; Duangjai, A.; Saokaew, S.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Rhizoma Coptidis: A Potential Cardiovascular Protective Agent. Front. Pharmacol. 2016, 7, 362. [Google Scholar] [CrossRef] [Green Version]
- Vlassara, H. Receptor-Mediated Interactions of Advanced Glycosylation End Products With Cellular Components Within Diabetic Tissues. Diabetes 1992, 41, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.K. The role of inflammation and oxidative stress in depression and cardiovascular disease. Cardiovasc. Implic. Stress Depress. 2020, 175–209. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Z.; Chen, Y.; Hou, B.; Liu, K.; Qin, H.; Fang, L.; Du, G. Coptisine Alleviates Pristane-Induced Lupus-Like Disease and Associated Kidney and Cardiovascular Complications in Mice. Front. Pharmacol. 2020, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Ye, X.; Wang, D.; He, K.; Yang, Y.; Liu, X.; Li, X. Safety evaluation of main alkaloids from Rhizoma Coptidis. J. Ethnopharmacol. 2013, 145, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nat. Cell Biol. 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]

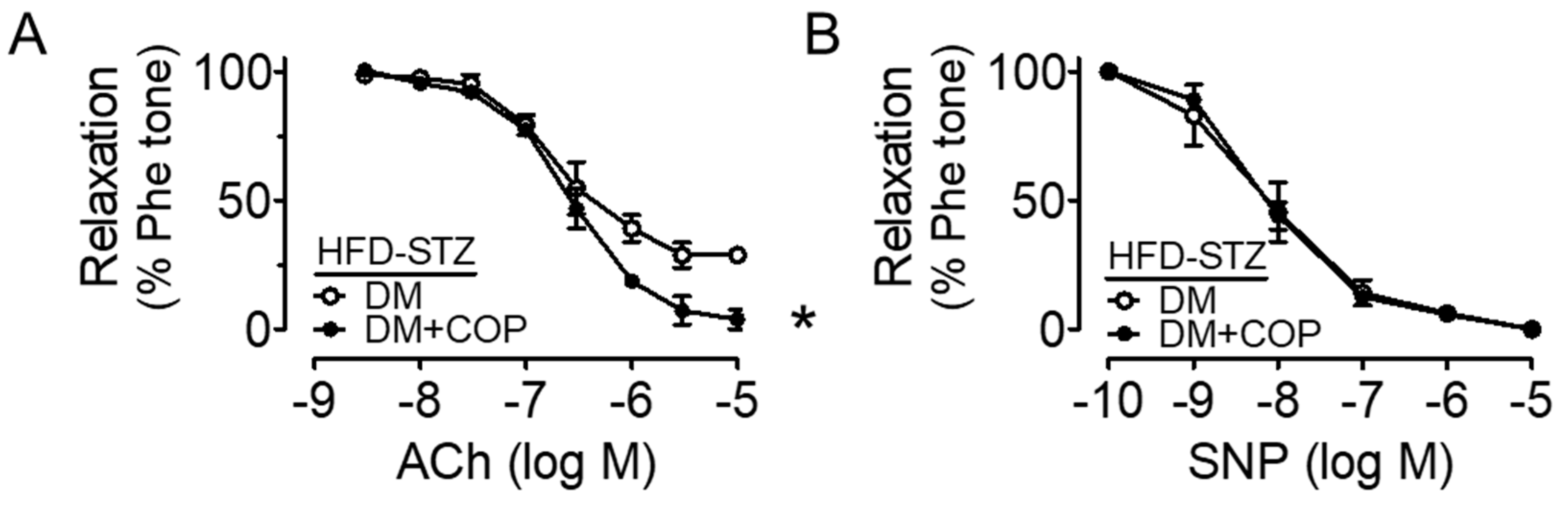
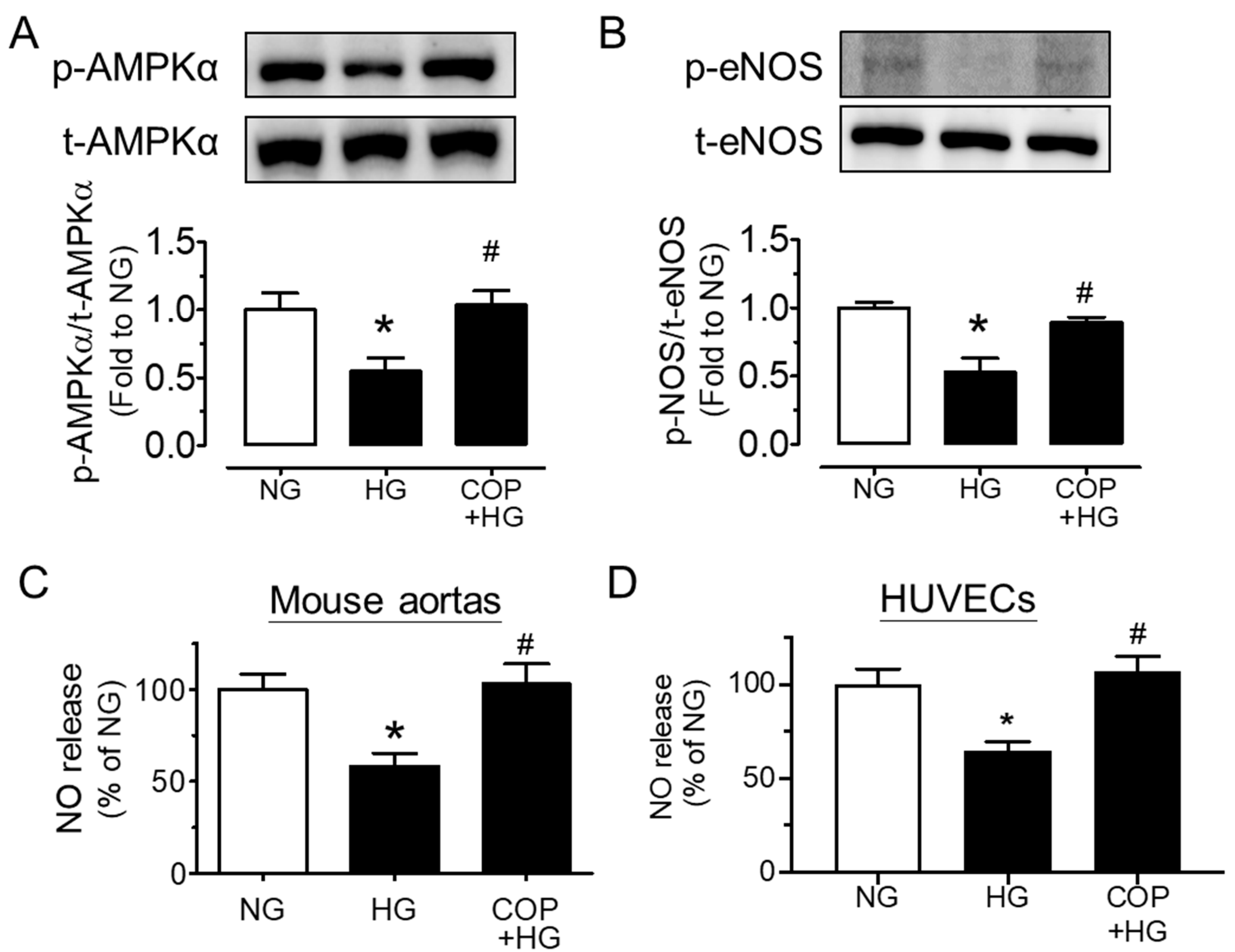

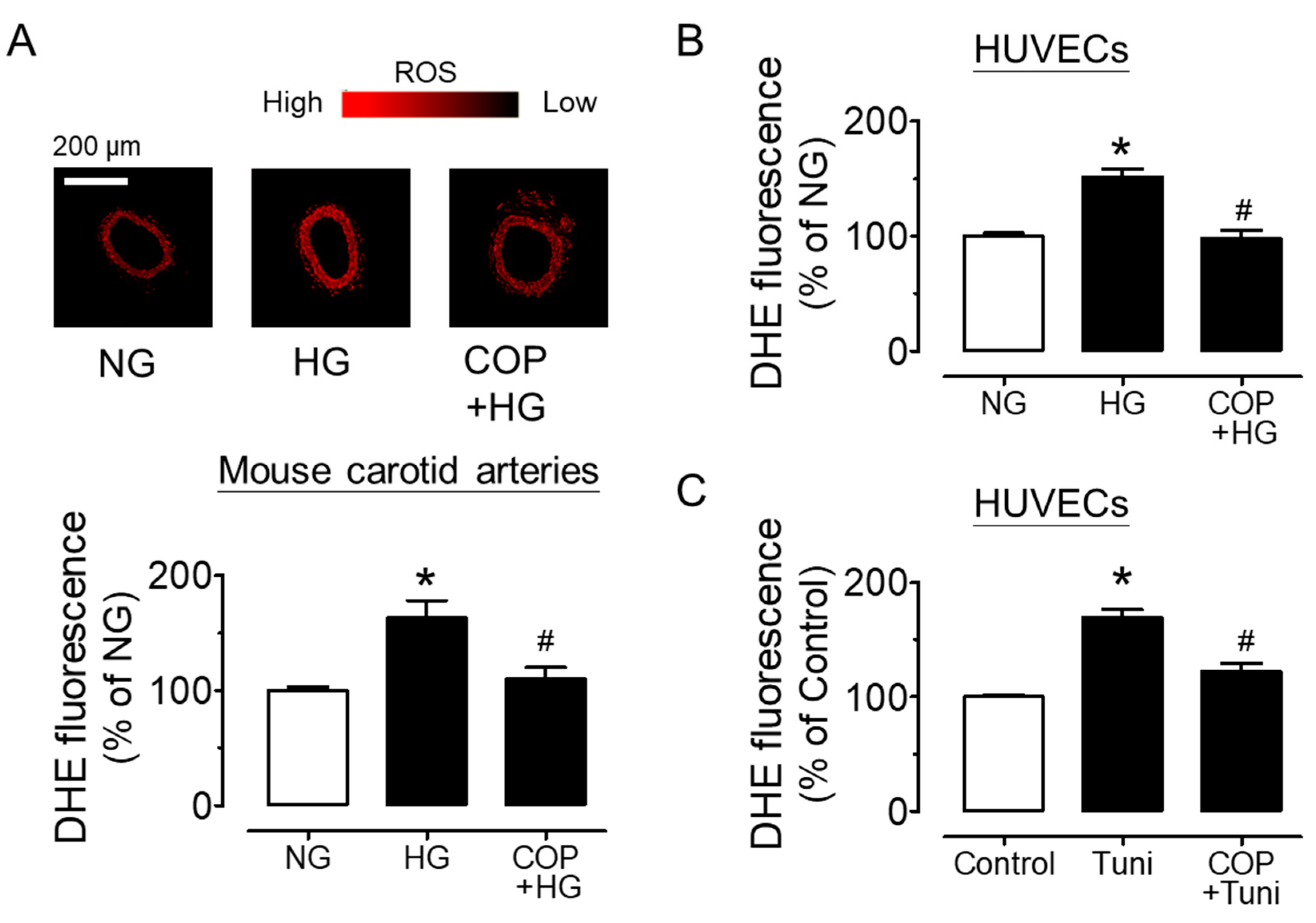
| Treatment | pD2 | Emax (%) |
|---|---|---|
| NG | 7.32 ± 0.10 | 96.23 ± 2.80 |
| HG | 7.08 ± 0.13 | 58.26 ± 2.70 * |
| 0.1 µM COP + HG | 7.18 ± 0.15 | 79.49 ± 4.12 # |
| 1 µM COP + HG | 7.45 ± 0.12 | 93.92 ± 3.12 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhou, C.; Zhang, X.; Vong, C.T.; Wang, Y.; Cheang, W.S. Coptisine Attenuates Diabetes—Associated Endothelial Dysfunction through Inhibition of Endoplasmic Reticulum Stress and Oxidative Stress. Molecules 2021, 26, 4210. https://doi.org/10.3390/molecules26144210
Zhou Y, Zhou C, Zhang X, Vong CT, Wang Y, Cheang WS. Coptisine Attenuates Diabetes—Associated Endothelial Dysfunction through Inhibition of Endoplasmic Reticulum Stress and Oxidative Stress. Molecules. 2021; 26(14):4210. https://doi.org/10.3390/molecules26144210
Chicago/Turabian StyleZhou, Yan, Chunxiu Zhou, Xutao Zhang, Chi Teng Vong, Yitao Wang, and Wai San Cheang. 2021. "Coptisine Attenuates Diabetes—Associated Endothelial Dysfunction through Inhibition of Endoplasmic Reticulum Stress and Oxidative Stress" Molecules 26, no. 14: 4210. https://doi.org/10.3390/molecules26144210








