Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency: A Strategy for Skin Cancer Prevention
Abstract
:1. Introduction
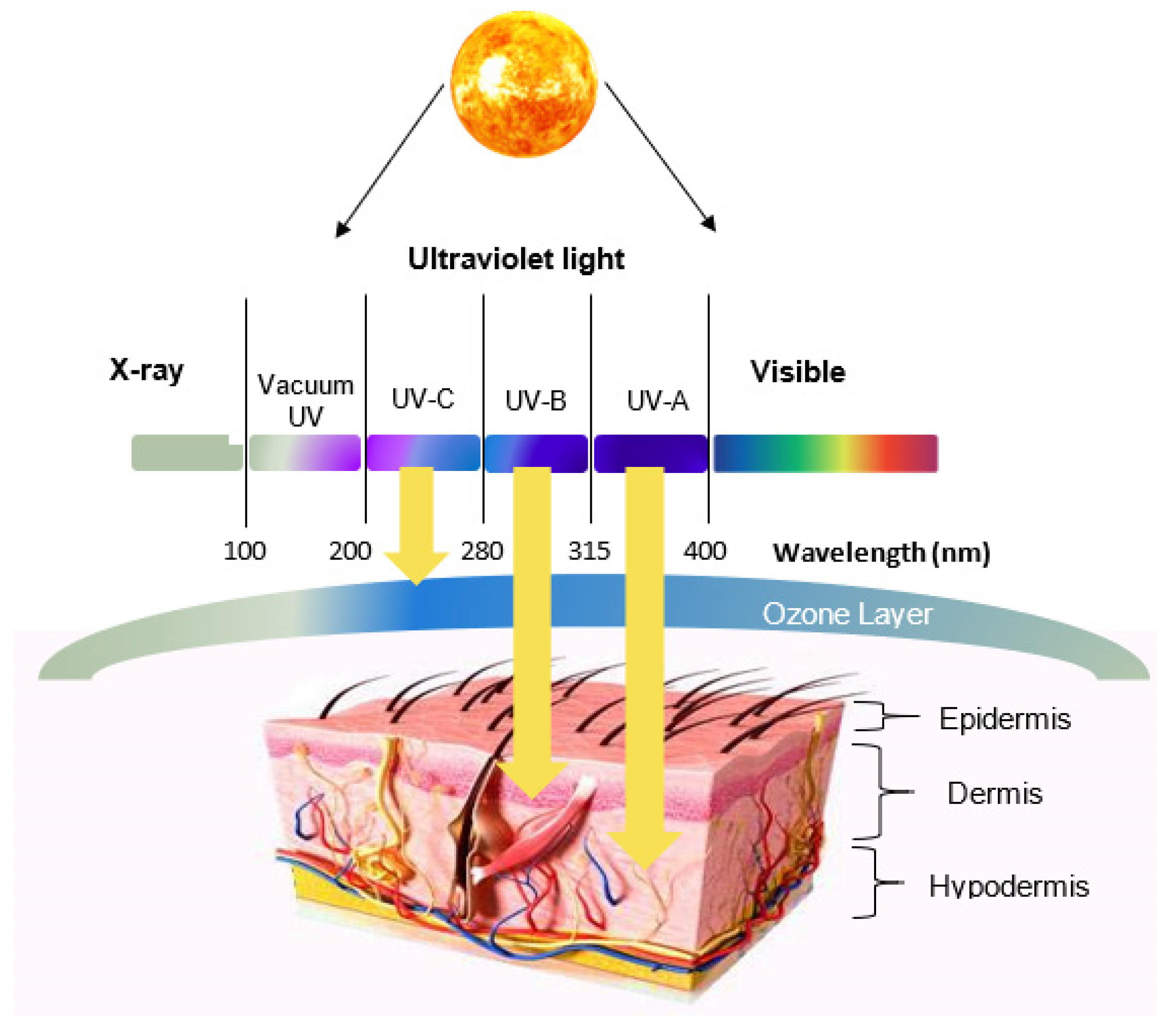
2. Skin Structure and UV Radiation
3. Sunscreens
3.1. UV Filters
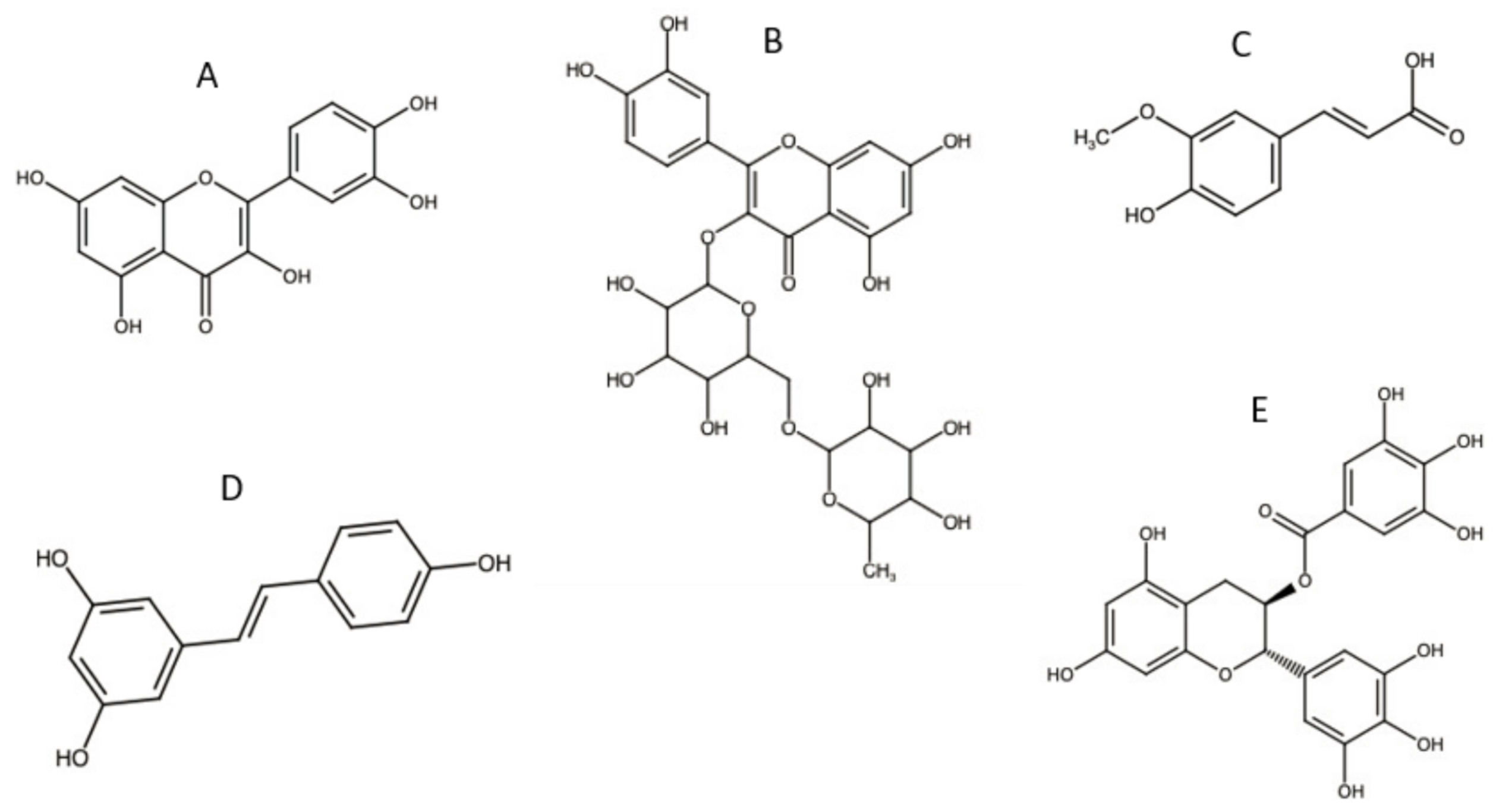
3.2. Antioxidants
4. Cyclodextrins (CDs)
4.1. General Concept
4.2. UV Filters/CDs Inclusion Complex
4.3. Natural Antioxidant/CDs Inclusion Complex
4.3.1. Antioxidant Solubility Enhancement
4.3.2. Antioxidant Stability Enhancement
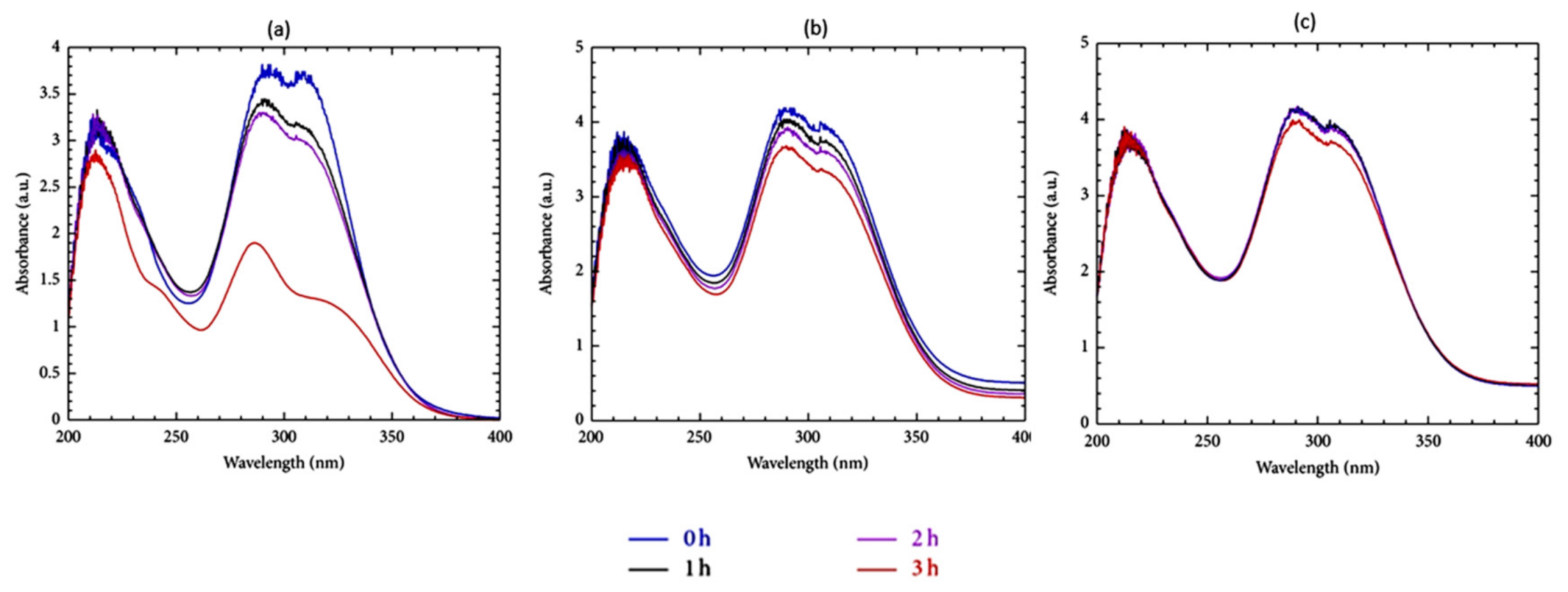
4.3.3. Antioxidants Activity Enhancement
4.3.4. Characterisation and Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Abid, A.R.; Marciniak, B.; Pędziński, T.; Shahid, M. Photo-Stability and Photo-Sensitizing Characterization of Selected Sunscreens’ Ingredients. J. Photochem. Photobiol. A Chem. 2017, 332, 241–250. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharm. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M.; Saeedi, M.; Yazdani Charati, J. Correlation between Sun Protection Factor and Antioxidant Activity, Phenol and Flavonoid Contents of Some Medicinal Plants. Iran. J. Pharm. Res. 2014, 13, 1041–1047. [Google Scholar] [PubMed]
- Jung, K.; Seifert, M. The Role of Antioxidants in Cosmetic Products to Prevent Free Radical Damage in Skin-an Efficacy Study. EURO COSMETICS 2007, 15, 16. [Google Scholar]
- Morabito, K.; Shapley, N.C.; Steeley, K.G.; Tripathi, A. Review of Sunscreen and the Emergence of Non-conventional Absorbers and Their Applications in Ultraviolet Protection. Int. J. Cosmet. Sci. 2011, 33, 385–390. [Google Scholar] [CrossRef]
- Osterwalder, U.; Herzog, B. Sun Protection Factors: World Wide Confusion. Br. J. Dermatol. 2009, 161 (Suppl. 3), 13–24. [Google Scholar] [CrossRef]
- Freitas, J.V.; Lopes, N.P.; Gaspar, L.R. Photostability Evaluation of Five UV-Filters, Trans-Resveratrol and Beta-Carotene in Sunscreens. Eur. J. Pharm. Sci. 2015, 78, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Gabard, B. Photostabilization of Butyl Methoxydibenzoylmethane (Avobenzone) and Ethylhexyl Methoxycinnamate by Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine (Tinosorb S), a New UV Broadband Filter. Photochem. Photobiol. 2001, 74, 401–406. [Google Scholar] [CrossRef]
- Graf, R.; Beck, J.; Rudolph, T.; Jung, K.; Herrling, T.; Pflücker, F. Antioxidative Power of Formulations Over Life Time: Unique Active Superior than Vitamins. SOFW J. 2008, 134, 52–60. [Google Scholar]
- Lefort, M.; Jung, K.; Pflücker, F.; Herrling, T. Create Lasting, Highly Effective Antioxidative Defence for the Skin. Househ. Pers. Care Today 2016, 11, 8–11. [Google Scholar]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.D.S.; Quintans-Júnior, L.J.; da Veiga Júnior, V.F.; Neves de Lima, Á.A. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and β-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matta, M.K.; Florian, J.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Yang, Y.; Oh, L.; Bashaw, E.; Zineh, I. Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 2020, 323, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, C.-P.; Chen, D.; Liu, C.-F.; Zhuo, L.-H.; Li, H.; Wang, C.; Bu, H.-T.; Tian, W. β-Cyclodextrin-Modified Hyaluronic Acid-Based Supramolecular Self-Assemblies for PH- and Esterase- Dual-Responsive Drug Delivery. Carbohydr. Polym. 2020, 246, 116654. [Google Scholar] [CrossRef] [PubMed]
- Physicochemical Basis of Pharmaceuticals: Amazon.Co.Uk: Moynihan, Humphrey, Crean, Abina: 9780199232840: Books. Available online: https://www.amazon.co.uk/Physicochemical-Basis-Pharmaceuticals-Humphrey-Moynihan/dp/0199232849 (accessed on 3 February 2021).
- Defraeye, T.; Bahrami, F.; Ding, L.; Malini, R.I.; Terrier, A.; Rossi, R.M. Predicting Transdermal Fentanyl Delivery Using Mechanistic Simulations for Tailored Therapy. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Jullian, C.; Moyano, L.; Yañez, C.; Olea-Azar, C. Complexation of Quercetin with Three Kinds of Cyclodextrins: An Antioxidant Study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 67, 230–234. [Google Scholar] [CrossRef]
- Types|Skin Cancer|Cancer Research UK. Available online: https://www.cancerresearchuk.org/about-cancer/skin-cancer/types (accessed on 17 February 2021).
- Kayaci, F.; Uyar, T. Encapsulation of Vanillin/Cyclodextrin Inclusion Complex in Electrospun Polyvinyl Alcohol (PVA) Nanowebs: Prolonged Shelf-Life and High Temperature Stability of Vanillin. Food Chem. 2012, 133, 641–649. [Google Scholar] [CrossRef]
- Pyo, S.M.; Maibach, H.I. Skin Metabolism: Relevance of Skin Enzymes for Rational Drug Design. Ski. Pharm. Physiol. 2019, 32, 283–294. [Google Scholar] [CrossRef]
- Diffey, B.L. Sources and Measurement of Ultraviolet Radiation. Methods 2002, 28, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Gabros, S.; Nessel, T.A.; Zito, P.M. Sunscreens and Photoprotection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Gruber, F.; Peharda, V.; Kastelan, M.; Brajac, I. Occupational Skin Diseases Caused by UV Radiation. Acta Dermatovenerol. Croat. 2007, 15, 191–198. [Google Scholar]
- Wang, S.Q.; Osterwalder, U.; Jung, K. Ex Vivo Evaluation of Radical Sun Protection Factor in Popular Sunscreens with Antioxidants. J. Am. Acad Dermatol. 2011, 65, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Staples, M.; Marks, R.; Giles, G. Trends in the Incidence of Non-Melanocytic Skin Cancer (NMSC) Treated in Australia 1985–1995: Are Primary Prevention Programs Starting to Have an Effect? Int. J. Cancer 1998, 78, 144–148. [Google Scholar] [CrossRef]
- Green, A.C.; Wallingford, S.C.; McBride, P. Childhood Exposure to Ultraviolet Radiation and Harmful Skin Effects: Epidemiological Evidence. Prog. Biophys. Mol. Biol. 2011, 107, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kricker, A.; Weber, M.; Sitas, F.; Banks, E.; Rahman, B.; Goumas, C.; Kabir, A.; Hodgkinson, V.S.; van Kemenade, C.H.; Waterboer, T. Early Life UV and Risk of Basal and Squamous Cell Carcinoma in New South Wales, Australia. Photochem. Photobiol. 2017, 93, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Bharath, A.K.; Turner, R.J. Impact of Climate Change on Skin Cancer. J. R. Soc. Med. 2009, 102, 215–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statistics by Cancer Type. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/melanoma-skin-cancer (accessed on 17 February 2021).
- Kolbe, L.; Pissavini, M.; Tricaud, C.; Trullás Cabanas, C.; Dietrich, E.; Matts, P.J. Anti-Inflammatory/Anti-Oxidant Activity of Ingredients of Sunscreen Products? Implications for SPF. Int. J. Cosmet. Sci. 2019, 41, 320–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syring, F.; Weigmann, H.-J.; Schanzer, S.; Meinke, M.C.; Knorr, F.; Lademann, J. Investigation of Model Sunscreen Formulations Comparing the Sun Protection Factor, the Universal Sun Protection Factor and the Radical Formation Ratio. Ski. Pharm. Physiol. 2016, 29, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Klimová, Z. Current Problems in the Use of Organic UV Filters to Protect Skin from Excessive Sun Exposure. Acta Chim. Slovaca 2013, 6, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Manaia, E.B.; Kaminski, R.C.K.; Corrêa, M.A.; Chiavacci, L.A. Inorganic UV Filters. Braz. J. Pharm. Sci. 2013, 49, 201–209. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Tran, V.V.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Suozzi, K.; Turban, J.; Girardi, M. Cutaneous Photoprotection: A Review of the Current Status and Evolving Strategies. Yale J. Biol. Med. 2020, 93, 55–67. [Google Scholar]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Influence of Titanium Dioxide Particle Size on the Photostability of the Chemical UV-Filters Butyl Methoxy Dibenzoylmethane and Ctocrylene in a Microemulsion. Cosmetics 2014, 1, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.K.; Gowder, S.J.T. Members of Antioxidant Machinery and Their Functions. In Basic Principal Clinical Significance Oxidative Stress; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.; Bisht, S.S. Antioxidants and Their Characterization. J. Pharm. Res 2011, 4, 2744–2746. [Google Scholar]
- Carlotti, M.E.; Sapino, S.; Ugazio, E.; Caron, G. On the Complexation of Quercetin with Methyl-β-Cyclodextrin: Photostability and Antioxidant Studies. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 81–90. [Google Scholar] [CrossRef]
- Souza, C.; Campos, P.M.; Schanzer, S.; Albrecht, S.; Lohan, S.B.; Lademann, J.; Darvin, M.E.; Meinke, M.C. Radical-Scavenging Activity of a Sunscreen Enriched by Antioxidants Providing Protection in the Whole Solar Spectral Range. Ski. Pharmacol. Physiol. 2017, 30, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Antioxidant Vitamin E/Cyclodextrin Inclusion Complex Electrospun Nanofibers: Enhanced Water Solubility, Prolonged Shelf Life, and Photostability of Vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Afonso, S.; Horita, K.; Sousa e Silva, J.P.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; Esteves da Silva, J.C.G.; Sousa Lobo, J.M. Photodegradation of Avobenzone: Stabilization Effect of Antioxidants. J. Photochem. Photobiol. B 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Di Mambro, V.M.; Fonseca, M.J.V. Assays of Physical Stability and Antioxidant Activity of a Topical Formulation Added with Different Plant Extracts. J. Pharm. Biomed. Anal. 2005, 37, 287–295. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrão, E. Antioxidants as Stabilizers of UV Filters: An Example for the UV-B Filter Octylmethoxycinnamate. Biomed. Dermatol. 2019, 3. [Google Scholar] [CrossRef]
- Abbaszadeh, F.; Fakhri, S.; Khan, H. Targeting Apoptosis and Autophagy Following Spinal Cord Injury: Therapeutic Approaches to Polyphenols and Candidate Phytochemicals. Pharmacol. Res. 2020, 160, 105069. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Tsuchida, E.; Uehara, M.; Ohhama, N.; Ohmine, W.; Ogi, T. The Leaf Extract of Mallotus Japonicus and Its Major Active Constituent, Rutin, Suppressed on Melanin Production in Murine B16F1 Melanoma. Asian Pac. J. Trop. Biomed. 2015, 5, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Corina, D.; Florina, B.; Iulia, P.; Cristina, D.; Rita, A.; Alexandra, P.; Virgil, P.; Hancianu, M.; Daliana, M.; Codruta, S. Rutin and Its Cyclodextrin Inclusion Complexes: Physico-Chemical Evaluation and in Vitro Activity on B164A5 Murine Melanoma Cell Line. Curr. Pharm. Biotechnol. 2017, 18, 1067–1077. [Google Scholar] [CrossRef]
- Anselmi, C.; Centini, M.; Maggiore, M.; Gaggelli, N.; Andreassi, M.; Buonocore, A.; Beretta, G.; Facino, R.M. Non-Covalent Inclusion of Ferulic Acid with α-Cyclodextrin Improves Photo-Stability and Delivery: NMR and Modeling Studies. J. Pharm. Biomed. Anal. 2008, 46, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Inoue, Y.; Ogata, Y.; Murata, I.; Meiyan, X.; Takayama, J.; Sakamoto, T.; Okazaki, M.; Kanamoto, I. Improvement of the Solubility and Evaluation of the Physical Properties of an Inclusion Complex Formed by a New Ferulic Acid Derivative and γ-Cyclodextrin. ACS Omega 2020, 5, 12073–12080. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, R.; Fu, R.; Xiong, J.; Hu, Y. Cytotoxicity and Inhibition of Lipid Peroxidation Activity of Resveratrol/Cyclodextrin Inclusion Complexes. J. Incl. Phenom. Macrocycl. Chem. 2011, 73. [Google Scholar] [CrossRef]
- Stojanović, S.; Sprinz, H.; Brede, O. Efficiency and Mechanism of the Antioxidant Action of Trans-Resveratrol and Its Analogues in the Radical Liposome Oxidation. Arch. Biochem. Biophys. 2001, 391, 79–89. [Google Scholar] [CrossRef]
- OyetakinWhite, P.; Tribout, H.; Baron, E. Protective Mechanisms of Green Tea Polyphenols in Skin. Oxid. Med. Cell. Longev. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.A.; Liu, B.; Zhao, J.; Thomas, D.S.; Hook, J.M. An Investigation into the Supramolecular Structure, Solubility, Stability and Antioxidant Activity of Rutin/Cyclodextrin Inclusion Complex. Food Chem 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Duchêne, D. Cyclodextrins and Their Inclusion Complexes. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–18. ISBN 978-0-470-92681-9. [Google Scholar]
- Said Suliman, A.; Khoder, M.; Tolaymat, I.; Webster, M.; Alany, R.G.; Wang, W.; Elhissi, A.; Najlah, M. Cyclodextrin Diethyldithiocarbamate Copper II Inclusion Complexes: A Promising Chemotherapeutic Delivery System against Chemoresistant Triple Negative Breast Cancer Cell Lines. Pharmaceutics 2021, 13, 84. [Google Scholar] [CrossRef]
- Mercader-Ros, M.T.; Lucas-Abellán, C.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Effect of HP-β-Cyclodextrins Complexation on the Antioxidant Activity of Flavonols. Food Chem. 2010, 118, 769–773. [Google Scholar] [CrossRef]
- Mamenzigou, U.; Ikeda, T.; Morohoshi, T.; Nakayama, M.; Yui, K. The Inclusion Complex Formation of Epigallocatechin Gallate in γ-Cyclodextrin and Its Effect on the Antioxidant Activity. Trans. Mater. Res. Soc. Jpn. 2013, 38, 681–685. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Effects on Drug Permeation through Biological Membranes. J. Pharm. Pharm. 2011, 63, 1119–1135. [Google Scholar] [CrossRef]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Characterisation of Inclusion Complex of Trans-Ferulic Acid and Hydroxypropyl-β-Cyclodextrin. Food Chem. 2011, 124, 1069–1075. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Yu, S.-C.; Tsai, F.-J.; Tsai, Y. Characterization of in Vitro and in Vivo Bioactivity of a Ferulic Acid-2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex. Colloids Surf. B Biointerfaces 2019, 180, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, J.; Memon, A.H.; Liang, H. Molecular Model and in Vitro Antioxidant Activity of a Water-Soluble and Stable Phloretin/Hydroxypropyl-β-Cyclodextrin Inclusion Complex. J. Mol. Liq. 2017, 236, 68–75. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Rakmai, J. Inclusion Complex Formation of Cyclodextrin with Its Guest and Their Applications. Biol. Eng. Med. 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhou, Z.; Han, L.; Li, S.; Zhou, W. Preparation and Characterization of Phloretin by Complexation with Cyclodextrins. New J. Chem. 2020, 44, 5218–5223. [Google Scholar] [CrossRef]
- Trollope, L.; Cruickshank, D.L.; Noonan, T.; Bourne, S.A.; Sorrenti, M.; Catenacci, L.; Caira, M.R. Inclusion of Trans-Resveratrol in Methylated Cyclodextrins: Synthesis and Solid-State Structures. Beilstein J. Org. Chem. 2014, 10, 3136–3151. [Google Scholar] [CrossRef]
- Yan, T.; Ji, M.; Sun, Y.; Yan, T.; Zhao, J.; Zhang, H.; Wang, Z. Preparation and Characterization of Baicalein/Hydroxypropyl-β-Cyclodextrin Inclusion Complex for Enhancement of Solubility, Antioxidant Activity and Antibacterial Activity Using Supercritical Antisolvent Technology. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 285–295. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery Systems for Cosmetics—From Manufacturing to the Skin of Natural Antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Buedenbender, S.; Schulz, G.E. Structural Base for Enzymatic Cyclodextrin Hydrolysis. J. Mol. Biol. 2009, 385, 606–617. [Google Scholar] [CrossRef]
- Perassinoto, N.L.; Raponi, M.R.B.; Shitara, J.L.Y.; Kumayama, T.M. Composition Comprising Cyclodextrin as Uv- and Ir-Radiation Screen Agent. U.S. Patent 10,485,748, 9 October 2014. [Google Scholar]
- De Oliveira, C.A.; Peres, D.D.; Graziola, F.; Chacra, N.A.B.; de Araújo, G.L.B.; Flórido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M.; et al. Cutaneous Biocompatible Rutin-Loaded Gelatin-Based Nanoparticles Increase the SPF of the Association of UVA and UVB Filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins. 1. Drug Solubilization and Stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Rajewski, R.A.; Stella, V.J. Pharmaceutical Applications of Cyclodextrins. 2. In Vivo Drug Delivery. J. Pharm. Sci. 1996, 85, 1142–1169. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawashdeh, N.A.F.; Al-Sadeh, K.S.; Al-Bitar, M.B. Inclusion Complexes of Sunscreen Agents with β-Cyclodextrin: Spectroscopic and Molecular Modeling Studies. Available online: https://www.hindawi.com/journals/jspec/2013/841409/ (accessed on 5 February 2021).
- Gaspar, L.R.; Maia Campos, P.M.B.G. Evaluation of the Photostability of Different UV Filter Combinations in a Sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Tursilli, R.; Iannuccelli, V. Complexation of the Sunscreen Agent, 4-Methylbenzylidene Camphor with Cyclodextrins: Effect on Photostability and Human Stratum Corneum Penetration. J. Pharm. Biomed. Anal. 2007, 44, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Coppi, G.; Iannuccelli, V. Microencapsulation of a Cyclodextrin Complex of the UV Filter, Butyl Methoxydibenzoylmethane: In Vivo Skin Penetration Studies. J. Pharm. Biomed. Anal. 2011, 54, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Masson, M. Cyclodextrins in Topical Drug Formulations: Theory and Practice. Int. J. Pharm. 2001, 225, 15–30. [Google Scholar] [CrossRef]
- Simeoni, S.; Scalia, S.; Tursilli, R.; Benson, H. Influence of Cyclodextrin Complexation on the in Vitro Human Skin Penetration and Retention of the Sunscreen Agent, Oxybenzone. J. Incl. Phenom. Macrocycl. Chem. 2005, 54, 275. [Google Scholar] [CrossRef]
- Shokri, J.; Hasanzadeh, D.; Ghanbarzadeh, S.; Dizadji-Ilkhchi, M.; Adibkia, K. The Effect of Beta-Cyclodextrin on Percutaneous Absorption of Commonly Used Eusolex® Sunscreens. Drug Res. 2013, 63, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Berbicz, F.; Nogueira, A.C.; Medina Neto, A.; Natali, M.R.M.; Baesso, M.L.; Matioli, G. Use of Photoacoustic Spectroscopy in the Characterization of Inclusion Complexes of Benzophenone-3-Hydroxypropyl-β-Cyclodextrin and Ex Vivo Evaluation of the Percutaneous Penetration of Sunscreen. Eur. J. Pharm. Biopharm. 2011, 79, 449–457. [Google Scholar] [CrossRef]
- Monteiro, M.S.D.S.D.B.; Ozzetti, R.A.; Vergnanini, A.L.; de Brito-Gitirana, L.; Volpato, N.M.; de Freitas, Z.M.F.; Ricci-Júnior, E.; dos Santos, E.P. Evaluation of Octyl P-Methoxycinnamate Included in Liposomes and Cyclodextrins in Anti-Solar Preparations: Preparations, Characterizations and in Vitro Penetration Studies. Int. J. Nanomed. 2012, 7, 3045–3058. [Google Scholar] [CrossRef] [Green Version]
- Sarveiya, V.; Templeton, J.F.; Benson, H.A.E. Inclusion Complexation of the Sunscreen 2-Hydroxy-4-Methoxy Benzophenone (Oxybenzone) with Hydroxypropyl-β-Cyclodextrin: Effect on Membrane Diffusion. J. Incl. Phenom. 2004, 49, 275–281. [Google Scholar] [CrossRef]
- Srinivasan, K.; Radhakrishnan, S.; Stalin, T. Inclusion Complexes of β-Cyclodextrin-Dinitrocompounds as UV Absorber for Ballpoint Pen Ink. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 551–564. [Google Scholar] [CrossRef]
- Felton, L.A.; Wiley, C.J.; Godwin, D.A. Influence of Cyclodextrin Complexation on the In Vivo Photoprotective Effects of Oxybenzone. Drug Dev. Ind. Pharm. 2004, 30, 95–102. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as Encapsulation Agents for Plant Bioactive Compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, A.; Martinho, A.; Luís, Â.; Figueiras, A.; Oleastro, M.; Domingues, F.C.; Silva, F. Resveratrol Encapsulation with Methyl-β-Cyclodextrin for Antibacterial and Antioxidant Delivery Applications. LWT Food Sci. Technol. 2015, 63, 1254–1260. [Google Scholar] [CrossRef]
- D’Aria, F.; Serri, C.; Niccoli, M.; Mayol, L.; Quagliariello, V.; Iaffaioli, R.V.; Biondi, M.; Giancola, C. Host–Guest Inclusion Complex of Quercetin and Hydroxypropyl-β-Cyclodextrin. J. Anal. Calorim. 2017, 130, 451–456. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Luo, Y.; Liu, Y.; Wang, X.-T.; Liu, F.; Guo, M.-Z.; Wang, Z.; Liu, A.-J.; Zhang, Y.-M. Inclusion of Chrysin in β-Cyclodextrin and Its Biological Activities. J. Drug Deliv. Sci. Technol. 2016, 31, 176–186. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, Z.; Xu, X.; Zhuang, H.; Shen, W. Preparation and Stability of the Inclusion Complex of Astaxanthin with Hydroxypropyl-β-Cyclodextrin. Food Chem. 2008, 109, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Fir, M.M.; Smidovnik, A.; Milivojevic, L.; Zmitek, J.; Prosek, M. Studies of CoQ10 and Cyclodextrin Complexes: Solubility, Thermo- and Photo-Stability. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 225–232. [Google Scholar] [CrossRef]
- Iohara, D.; Umezaki, Y.; Anraku, M.; Uekama, K.; Hirayama, F. In Vitro and In Vivo Evaluation of Hydrophilic C60(OH)10/2-Hydroxypropyl-β-Cyclodextrin Nanoparticles as an Antioxidant. J. Pharm. Sci. 2016, 105, 2959–2965. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.C.-S.; Lai, Y.-C.; Lin, C.-L.; Tzeng, W.-S.; Yen, F.-L. Inclusion Complex of Saikosaponin-d with Hydroxypropyl-β-Cyclodextrin: Improved Physicochemical Properties and Anti-Skin Cancer Activity. Phytomedicine 2019, 57, 174–182. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hu, S.C.-S.; Huang, P.-H.; Lin, T.-C.; Yen, F.-L. Electrospun Resveratrol-Loaded Polyvinylpyrrolidone/Cyclodextrin Nanofibers and Their Biomedical Applications. Pharmaceutics 2020, 12, 552. [Google Scholar] [CrossRef]
- Centini, M.; Maggiore, M.; Casolaro, M.; Andreassi, M.; Facino, R.; Anselmi, C. Cyclodextrins as Cosmetic Delivery Systems. J. Incl. Phenom. 2007, 57, 109–112. [Google Scholar] [CrossRef]
- Yang, S.-L.; Zhao, L.-J.; Chi, S.-M.; Du, J.-J.; Ruan, Q.; Xiao, P.-L.; Zhao, Y. Inclusion Complexes of Flavonoids with Propylenediamine Modified β-Cyclodextrin:Preparation, Characterization and Antioxidant. J. Mol. Struct. 2019, 1183, 118–125. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic-Gajic, I.M.; Nikolic, V.D.; Nikolic, L.B.; Radovanovic, B.C.; Milenkovic-Andjelkovic, A. Enhencemnet of Solubility and Photostability of Rutin by Complexation with β-Cyclodextrin and (2-Hydroxypropyl)-β-Cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 33–43. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anselmi, C.; Centini, M.; Ricci, M.; Buonocore, A.; Granata, P.; Tsuno, T.; Facino, R.M. Analytical Characterization of a Ferulic Acid/γ-Cyclodextrin Inclusion Complex. J. Pharm. Biomed. Anal. 2006, 40, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Basu, S.; Lahiri, A.; Basak, S. Inclusion of Chrysin in β-Cyclodextrin Nanocavity and Its Effect on Antioxidant Potential of Chrysin: A Spectroscopic and Molecular Modeling Approach. J. Mol. Struct. 2010, 977, 180–188. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.; Xuan, G. Preparation of PH-Sensitive β-Cyclodextrin Derivatives and Evaluation of Their Drug-Loading Propertie. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 774, p. 012009. [Google Scholar] [CrossRef]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of Rutin with β-Cyclodextrin as Potential Delivery System. PLoS ONE 2015, 10, e0120858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and Release Characterisation of Garlic Oil-β-Cyclodextrin Inclusion Complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- HIGUCHI, T. A Phase Solubility Technique. Adv. Anal. Chem. Instrum. 1965, 4, 117–211. [Google Scholar]
- Oliva, E.; Mathiron, D.; Bertaut, E.; Landy, D.; Cailleu, D.; Pilard, S.; Clément, C.; Courot, E.; Bonnet, V.; Djedaïni-Pilard, F. Physico-Chemical Studies of Resveratrol, Methyl-Jasmonate and Cyclodextrin Interactions: An Approach to Resveratrol Bioproduction Optimization. RSC Adv. 2018, 8, 1528–1538. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The Complexation Efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Gabelica, V.; Galic, N.; Rosu, F.; Houssier, C.; Pauw, E.D. Influence of Response Factors on Determining Equilibrium Association Constants of Non-Covalent Complexes by Electrospray Ionization Mass Spectrometry. J. Mass Spectrom. 2003, 38, 491–501. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Effect of Cyclodextrin Complexation on Phenylpropanoids’ Solubility and Antioxidant Activity. Beilstein J. Org. Chem. 2014, 10, 2322–2331. [Google Scholar] [CrossRef] [Green Version]
- Vian, M.A.; Tomao, V.; Gallet, S.; Coulomb, P.O.; Lacombe, J.M. Simple and Rapid Method for Cis- and Trans-Resveratrol and Piceid Isomers Determination in Wine by High-Performance Liquid Chromatography Using Chromolith Columns. J. Chromatogr. A 2005, 1085, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Tampucci, S.; Chetoni, P.; Burgalassi, S.; Saino, V.; Centini, M.; Staltari, L.; Anselmi, C. Permeation and Distribution of Ferulic Acid and Its α-Cyclodextrin Complex from Different Formulations in Hairless Rat Skin. Aaps Pharmscitech 2011, 12, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in the Solid State: A Review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Pessine, F.B.T.; Calderini, A.; Alexandrino, G.L. Review: Cyclodextrin Inclusion Complexes Probed by NMR Techniques. Magn. Reson. Spectrosc. 2012. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Sandström, C.; Zhang, H.; Tan, T. NMR Study on the Inclusion Complexes of β-Cyclodextrin with Isoflavones. Molecules 2016, 21, 372. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Zeb, A.; Arif, S.T.; Malik, M.; Shah, F.A.; Din, F.U.; Qureshi, O.S.; Lee, E.-S.; Lee, G.-Y.; Kim, J.-K. Potential of Nanoparticulate Carriers for Improved Drug Delivery via Skin. J. Pharm. Investig. 2019, 49, 485–517. [Google Scholar] [CrossRef] [Green Version]
- Spada, G.; Gavini, E.; Cossu, M.; Rassu, G.; Carta, A.; Giunchedi, P. Evaluation of the Effect of Hydroxypropyl-β-Cyclodextrin on Topical Administration of Milk Thistle Extract. Carbohydr. Polym. 2013, 92, 40–47. [Google Scholar] [CrossRef]







| Chemical UV Filters | Physical UV Filters |
|---|---|
| Benzophenones (UVA) Oxybenzone (UVA and UVB) Dioxybenzone (UVA and UVB) Avobenzone (UVA) Octocrylene (UVB) Homosalate (UVB) Ensulizole (UVB) Bemotrizinol (UVA and UVB) Octinoxate (UVB) Enzacamene (UVB) | Titanium dioxide Zinc oxide Iron oxide Kaolin Talc |
| Antioxidants | CDs | Inclusion Complex Preparation Method, Stoichiometry, and Main Outcome | Reference |
|---|---|---|---|
| Rutin | β-CD, HP–α-CD, HP–β-CD, HP–γ-CD | Inclusion complex prepared by mixing in solution with (1:1) stoichiometry with greater stability obtained using HP-β-CD and HP-γ-CD. Moderate protection of rutin against thermal and UVR degradation and significant enhancement of antioxidant capacity. | [56] |
| Astaxanthin | HP–β-CD | Inclusion complex obtained by freeze drying. Significant enhancement in antioxidant stability against light and oxygen, allowing controlled release. | [92] |
| Coenzyme Q10 | β-CD, γ-CD | Inclusion complex prepared by co-precipitation method with (1:1) stoichiometry. Significant enhancement in antioxidant solubility and thermo- and photostability. | [93] |
| C60(OH)10 | HP–β-CD | Inclusion complex/nanoparticles obtained by dry grinding using automated magnetic mortar. Significant enhancement in antioxidant scavenging ability, allowing better cell protection against oxidative stress. The IV administration of nanoparticles suppressed liver injury induced by oxidative stress. | [94] |
| Vitamin E | HP–β-CD | Inclusion complex prepared by mixing and electrospinning. Enhancement of vitamin E solubility, antioxidant activity, and light and shelf stability. | [43] |
| Phloretin | Me–β-CD, HP–β-CD | Inclusion complex prepared by freeze drying with (1:1) stoichiometry. Improved water solubility and antioxidant activities of phloretin. | [67] |
| Baicalein | HP–β-CD | Inclusion complex prepared by co-precipitation with compressed antisolvents method. In vitro and in vivo enhancement in Baicalein solubility, antioxidant activities, and bioavailability. | [69] |
| Lycopene | β-CD | Inclusion complex obtained by co-precipitation with (1:1) stoichiometry. Increased thermal and irradiant stabilities of lycopene. | [12] |
| Saikosaponin-d | HP–β-CD | Inclusion complex obtained by the freeze-drying method. Enhancement of water solubility allowing anticancer effects against cutaneous SCC cells. | [95] |
| Resveratrol | HP–β-CD | Inclusion complex obtained by mixing and electrospinning in the presence of polyvinylpyrrolidone. Resveratrol converted to amorphous form with intermolecular bonds with PVP and HP-β-CD. Good antioxidant activity and skin penetration and suppressed particulate matter-induced expression of inflammatory proteins. | [96] |
| Ferulic Acid | α-CD, β-CD, γ-CD, HP–β-CD, HP–γ-CD | Inclusion complex obtained by co-precipitation with (1:1) stoichiometry. α-CD was best in term of association constant, degree of photostability, and FA release. | [97] |
| QCT | Me–β-CD | Inclusion complex obtained by freeze drying. Enhanced QCT solubility and photostability, without significantly affecting the antioxidant activity or skin accumulation. | [41] |
| Taxifolin, QCT morin hydrate | DP–β-CD | Inclusion complex prepared by saturated aqueous solution method with (1:1) stoichiometry. Water solubility increased by 70 to 102 times with improved antioxidant activity. | [98] |
| Rutin | β-CD, HP–β-CD | Inclusion complex obtained by co-precipitation with (1:1) stoichiometry. Enhanced antioxidant activity, solubility, and photostability. | [99] |
| Curcumin | β-CD crosslinked polymer | Inclusion complex obtained by pressure distillation and oven drying resulting in (1:1) stoichiometry. Improved physicochemical characteristics, novel AO activity, with higher antiproliferative activity on A375 cell, and A375 cell apoptosis. | [100] |
| Ferulic Acid | γ-CD | Inclusion complex obtained by co-precipitation. Good encapsulation/release of ferulic acid for pharmaceutical application and biological activity. | [101] |
| Ferulic Acid | α-CD | Inclusion complex obtained by co-precipitation with (1:1) stoichiometry. Slower release, with improved photostability and bioavailability. | [51] |
| Ferulic Acid | HP–β-CD | Inclusion complex obtained by freeze drying and improved water solubility and bioactivity. | [64] |
| Chrysin | β-CD | (1:1) stoichiometry. The inclusion complex formed via A-ring of chrysin. Increased solubility and antioxidant potential. | [102] |
| Epigallocatechin Gallate | γ-CD | (1:1) stoichiometry. Slight enhancement in antioxidant activity. | [61] |
| Different flavanols | HP–β-CD | Increased antioxidant activity was attributed to CD capacity of protecting flavanols against rapid oxidation by free radicals. | [60] |
| Vitamin E | HP–β-CD EN–HP–β-CD | Inclusion complex prepared by mixing in solution with stoichiometry of (1:1). EN-HP-β-CD improved vitamin E solubility by 25 times. Higher release in pH 4.5 (pH-sensitive properties). | [103] |
| Ferulic acid 012 | γ-CD | Inclusion complex obtained by co-grinding using a three-dimensional ball mill with a stoichiometry of (1:1). Five-fold enhancement in solubility. | [52] |
| Rutin | β-CD HP–β-CD | Inclusion complex obtained by co-kneading method. Improved antioxidant activity with antiproliferative and pro-apoptotic activities against B164A5 cells. | [50] |
| Rutin | β-CD | Inclusion complex obtained by co-grinding method with (1:1) stoichiometry. Greater stability and solubility, but antibacterial effect was slightly decreased. Prolonged released was observed with twice as much rutin skin permeation. | [104] |
| QCT | β-CD, HP–β-CD, SBE–β-CD | (1:1) stoichiometry. Inclusion ability of investigated CDs was in the following order: SBE-βCD > HP-βCD > βCD. All obtained complexes showed enhanced scavenging capability. | [17] |
| Phloretin | HP–β-CD | Inclusion complex prepared by simple co-evaporation method with (1:1) stoichiometry. The aromatic ring of phloretin was included into the HP-β-CD cavity from the narrow side. Enhanced solubility (by 5808 times) and stability, with preserved radical-scavenging capacity. | [65] |
| Chrysin | β-CD | Inclusion complex obtained by mixing in solution with (1:3) stoichiometry and inclusion rate of 90%. Increased solubility, antioxidant, antimicrobial, and antitumour activities. | [91] |
| Resveratrol | Me–β-CD | (1:1) stoichiometry. Enhanced solubility (400-fold) and good antibacterial and antioxidant activities with no haemolytic effect. | [89] |
| Garlic Oil | β-CD | Inclusion complex obtained by co-precipitation with (1:1) stoichiometry. Improved solubility with controlled release profile. | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahabra, L.; Broadberry, G.; Le Gresley, A.; Najlah, M.; Khoder, M. Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency: A Strategy for Skin Cancer Prevention. Molecules 2021, 26, 1698. https://doi.org/10.3390/molecules26061698
Dahabra L, Broadberry G, Le Gresley A, Najlah M, Khoder M. Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency: A Strategy for Skin Cancer Prevention. Molecules. 2021; 26(6):1698. https://doi.org/10.3390/molecules26061698
Chicago/Turabian StyleDahabra, Layan, Grace Broadberry, Adam Le Gresley, Mohammad Najlah, and Mouhamad Khoder. 2021. "Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency: A Strategy for Skin Cancer Prevention" Molecules 26, no. 6: 1698. https://doi.org/10.3390/molecules26061698
APA StyleDahabra, L., Broadberry, G., Le Gresley, A., Najlah, M., & Khoder, M. (2021). Sunscreens Containing Cyclodextrin Inclusion Complexes for Enhanced Efficiency: A Strategy for Skin Cancer Prevention. Molecules, 26(6), 1698. https://doi.org/10.3390/molecules26061698








