Influence of the Storage in Bottle on the Antioxidant Activities and Related Chemical Characteristics of Wine Spirits Aged with Chestnut Staves and Micro-Oxygenation
Abstract
:1. Introduction
2. Results and Discussion
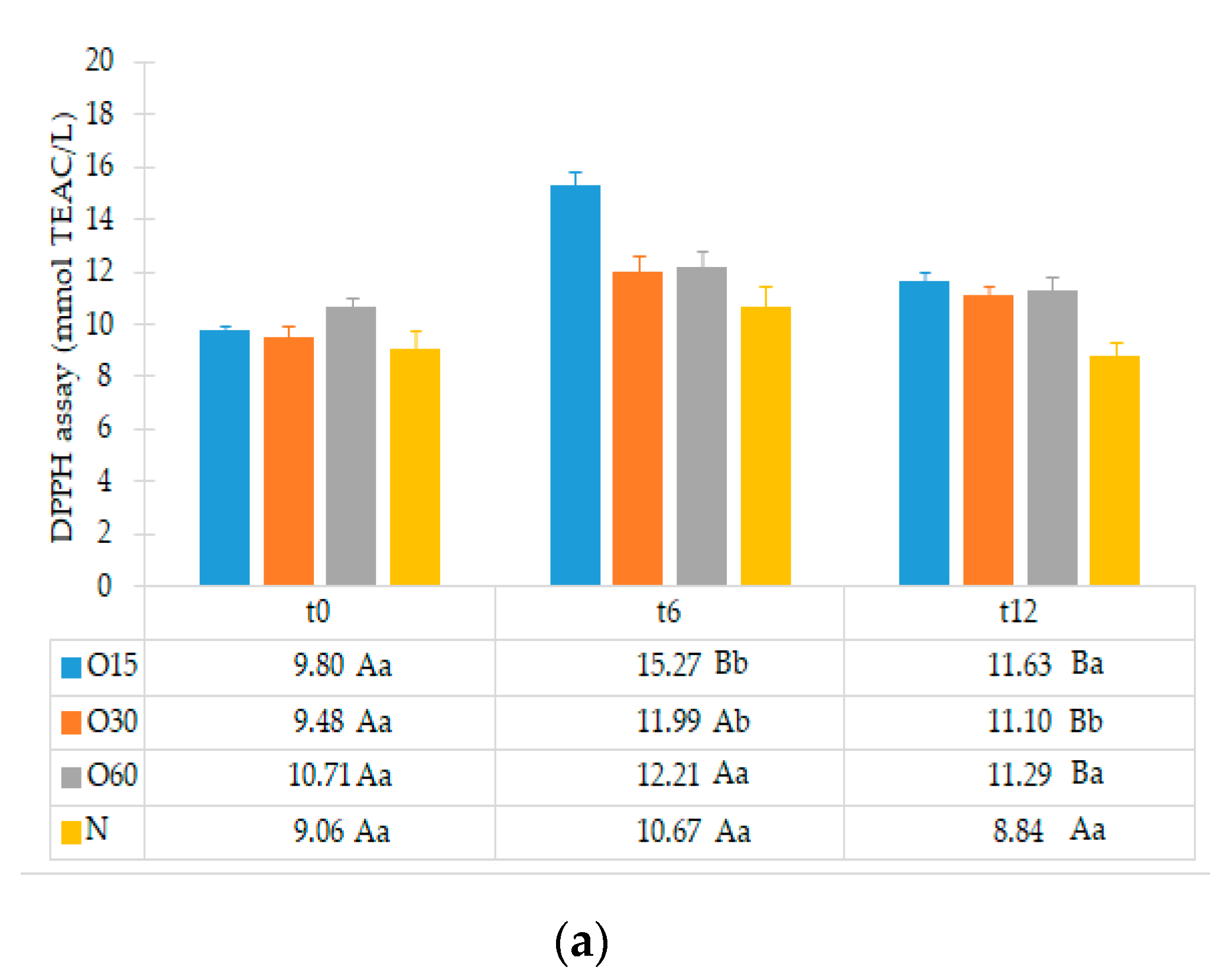
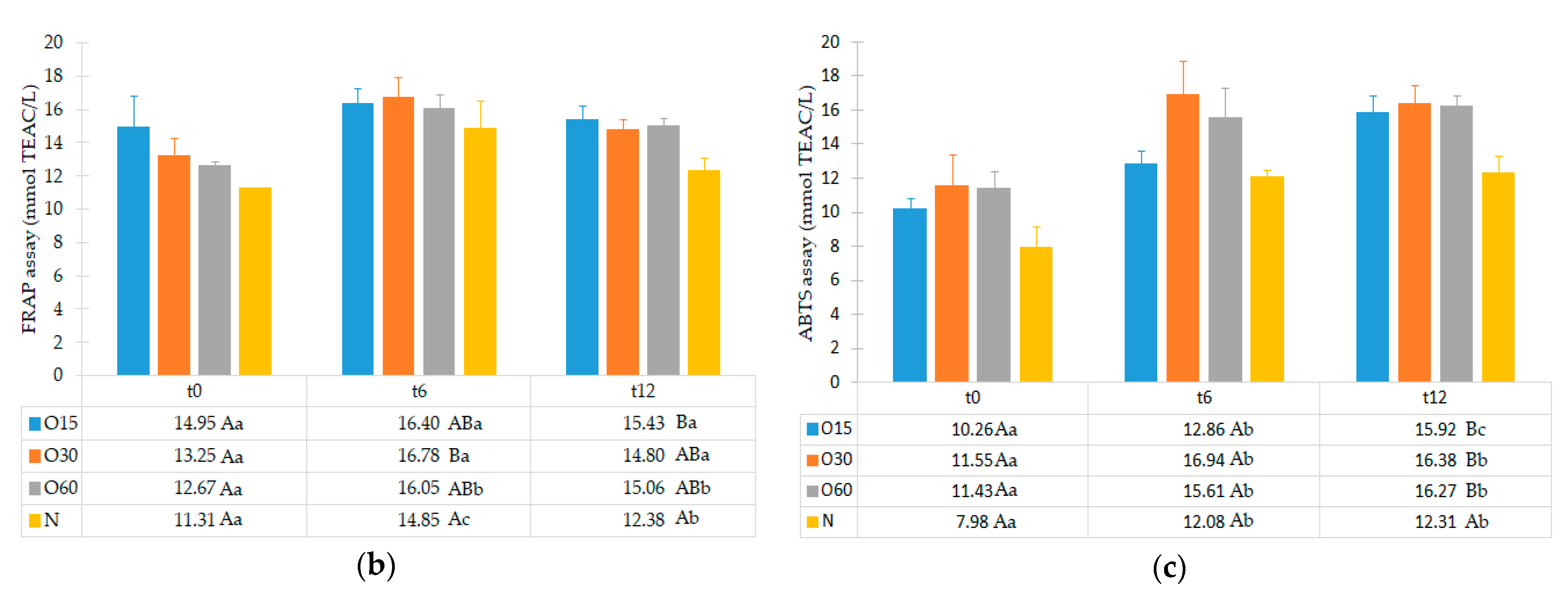
2.1. Effect of the Storage Time in Bottle on the Antioxidant Activities of the Aged WS
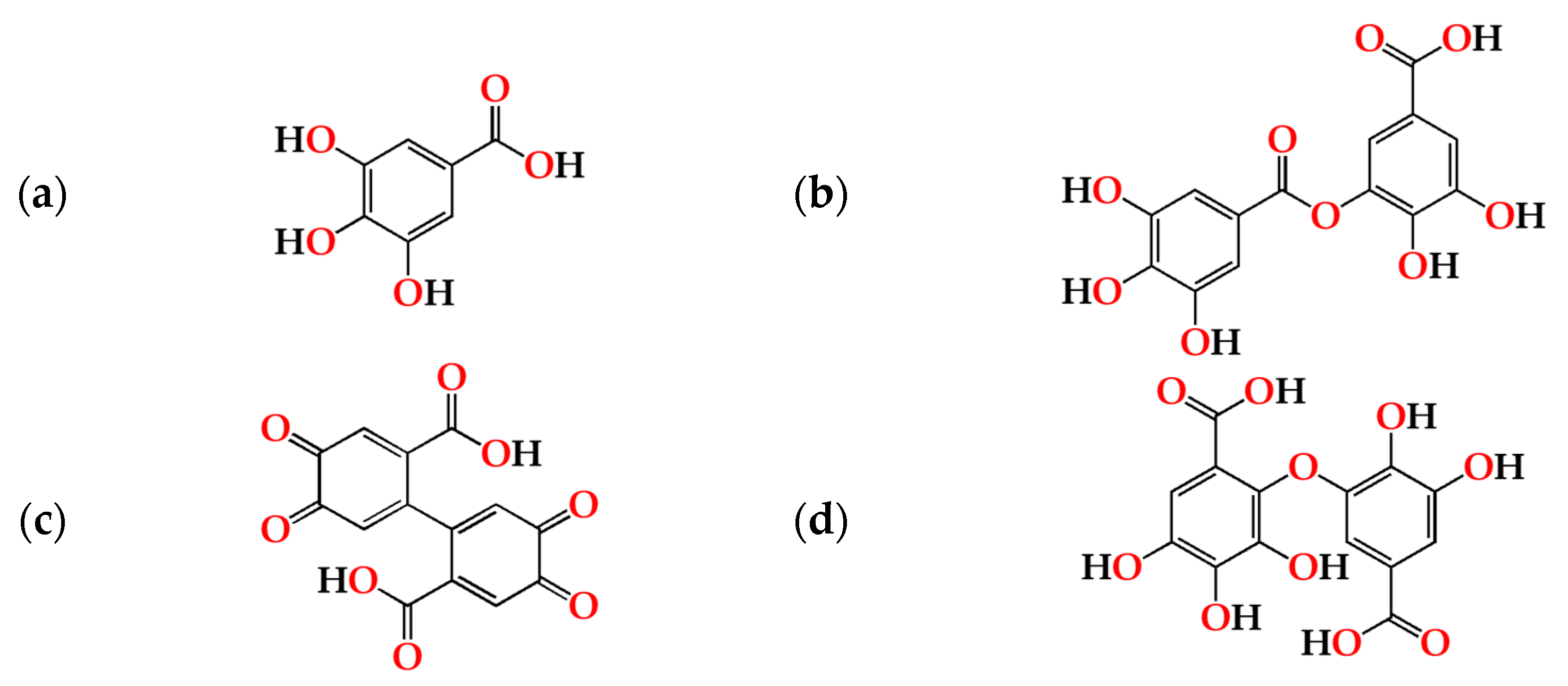
2.2. Relationship between the Antioxidant Activity and Phenolic Content of the Aged WS
2.3. Multivariate Analysis.
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Experimental Design and Aged WSs Sampling
3.3. In Vitro Antioxidant Activity Analyses
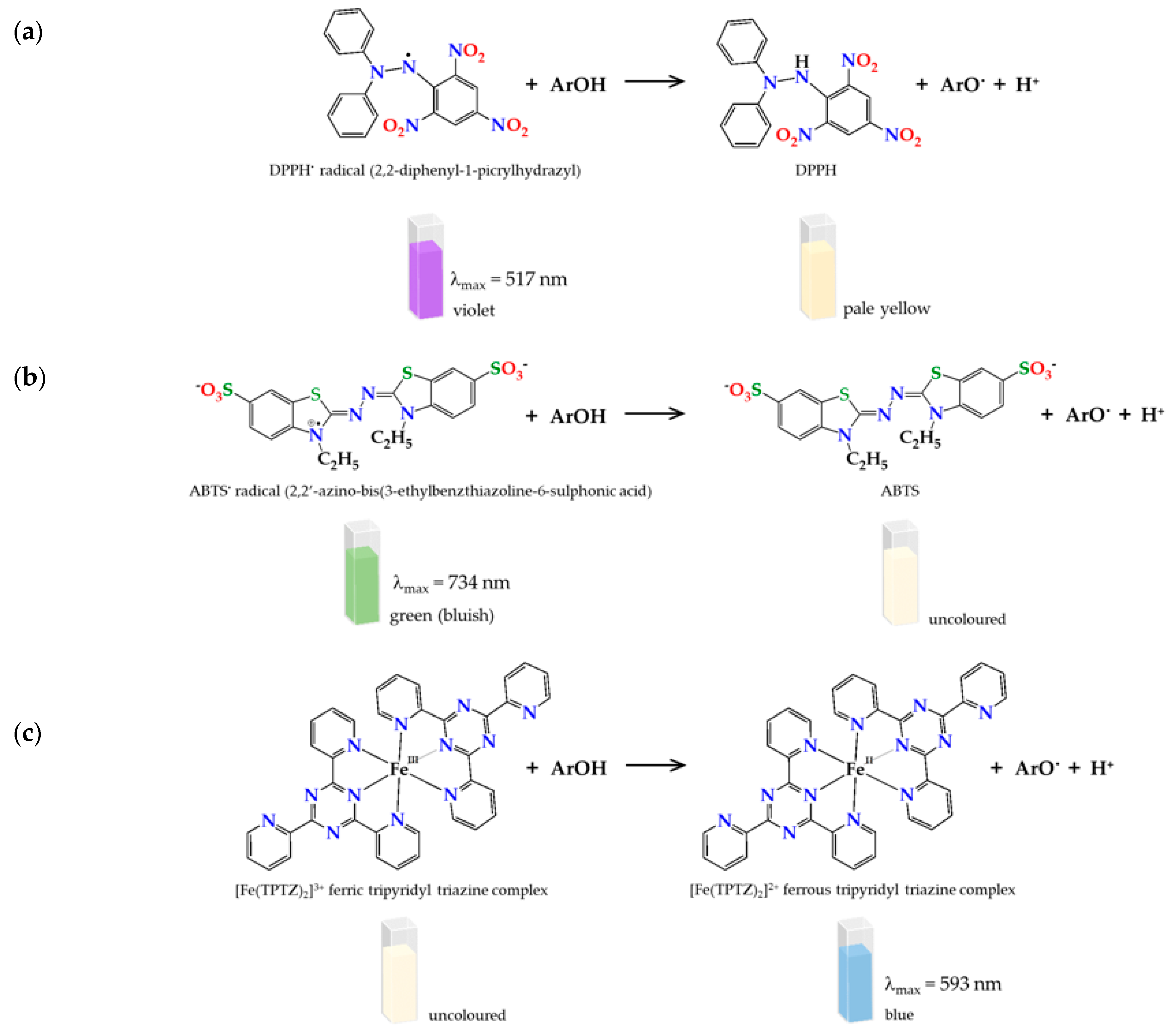
3.3.1. DPPH Assay
3.3.2. ABTS Assay
3.3.3. FRAP Assay
3.4. Total Phenolic Index Determination
3.5. Low Molecular Weight Composition Determination
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parish, M.; Wollan, D.; Paul, R. Micro-Oxygenation—A review. The Australian and New Zealand Grapegrower and Wine-maker, 30th Annual New York Wine Industry Workshop, New York, USA. 2000. Available online: https://www.infowine.com/intranet/libretti/libretto1112-01-1.pdf (accessed on 1 December 2021).
- Canas, S.; Caldeira, I.; Anjos, O.; Belchior, A.P. Phenolic profile and colour acquired by the wine spirit in the beginning of ageing: Alternative technology using micro-oxygenation vs. traditional technology. LWT 2019, 111, 260–269. [Google Scholar] [CrossRef]
- Anjos, O.; Caldeira, I.; Roque, R.; Pedro, S.; Lourenço, S.; Canas, S. Screening of Different Ageing Technologies of Wine Spirit by Application of Near-Infrared (NIR) Spectroscopy and Volatile Quantification. Process 2020, 8, 736. [Google Scholar] [CrossRef]
- Caldeira, I.; Vitória, C.; Anjos, O.; Fernandes, T.; Gallardo, E.; Fargeton, L.; Boissier, B.; Catarino, S.; Canas, S. Wine Spirit Ageing with Chestnut Staves under Different Micro-Oxygenation Strategies: Effects on the Volatile Compounds and Sensory Profile. Appl. Sci. 2021, 11, 3991. [Google Scholar] [CrossRef]
- Anli, R.E.; Cavuldak, Özge A. A review of microoxygenation application in wine. J. Inst. Brew. 2012, 118, 368–385. [Google Scholar] [CrossRef]
- Canas, S.; Danalache, F.; Anjos, O.; Fernandes, T.A.; Caldeira, I.; Santos, N.; Fargeton, L.; Boissier, B.; Catarino, S. Behaviour of Low Molecular Weight Compounds, Iron and Copper of Wine Spirit Aged with Chestnut Staves under Different Levels of Micro-Oxygenation. Molecules 2020, 25, 5266. [Google Scholar] [CrossRef]
- Anjos, O.; Comesaña, M.M.; Caldeira, I.; Pedro, S.I.; Oller, P.E.; Canas, S. Application of Functional Data Analysis and FTIR-ATR Spectroscopy to Discriminate Wine Spirits Ageing Technologies. Mathematics 2020, 8, 896. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A. Healthy Drinks with Lovely Colors: Phenolic Compounds as Constituents of Functional Beverages. Beverages 2021, 7, 12. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Litescu, S.C.; Eremia, S.A.V.; Tache, A.; Vasilescu, I.; Radu, G.-L. Chapter 25—The use of Oxygen Radical Absorbance Capacity (ORAC) and Trolox Equivalent Antioxidant Capacity (TEAC) assays in the assessment of beverages’ antioxidant properties. In Processing and Impact on Antioxidants in Beverages; Elsevier: London, UK, 2014; pp. 245–251. [Google Scholar]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otręba, M.; Kośmider, L.; Rzepecka-Stojko, A. Polyphenols’ Cardioprotective Potential: Review of Rat Fibroblasts as Well as Rat and Human Cardiomyocyte Cell Lines Research. Molecules 2021, 26, 774. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Liu, Y.; Wang, S.; Zhang, H.; Fang, X.; Zhang, J.; Fan, L.; Zheng, B.; Roman, R.J.; Wang, Z.; et al. Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 2074. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2020, 1–48. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Serra, A.T.; Bronze, M.R. Chapter 7—Food matrices that improve the oral bioavailability of pharma-ceuticals and nutraceuticals. In Nutraceuticals and Natural Product Pharmaceuticals; Academic Press: London, UK, 2019; pp. 197–233. [Google Scholar]
- Ketnawa, S.; Reginio, F.C., Jr.; Thuengtung, S.; Ogawa, Y. Changes in bioactive compounds and antioxidant activity of plant-based foods by gastrointestinal digestion: A review. Crit. Rev. Food Sci. Nutr. 2021, 29, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, T.O.; Saraste, A.; O Toikka, J.; Saraste, M.; Raitakari, O.T.; Pärkkä, J.P.; Lehtimäki, T.; Hartiala, J.J.; Viikari, J.; Koskenvuo, J.W. Effects of cognac on coronary flow reserve and plasma antioxidant status in healthy young men. Cardiovasc. Ultrasound 2008, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Canas, S. Phenolic Composition and Related Properties of Aged Wine Spirits: Influence of Barrel Characteristics. A Review. Beverages 2017, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Canas, S.; Caldeira, I.; Belchior, A.P.; Spranger, M.I.; Clímaco, M.C.; de Sousa, R.B. A.P.; Spranger, M.I.; Clímaco, M.C.; Bruno de Sousa, R. A sustainable alternative for the aging of wine brandies. In Food Quality: Control, Analysis and Consumer Concerns 2011; Medina, D.A., Laine, A.M., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 181–228. [Google Scholar]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; de Simón, B.F.; Hernández, T.; Estrella, I. Phenolic Compounds in Chestnut (Castanea sativa Mill.) Heartwood. Effect of Toasting at Cooperage. J. Agric. Food Chem. 2010, 58, 9631–9640. [Google Scholar] [CrossRef]
- Canas, S.; Leandro, M.C.; Spranger, M.I.; Belchior, A.P. Low molecular weight organic compounds of chestnut wood (Castanea sativa L.) and corresponding aged brandies. J. Agric. Food Chem. 1999, 47, 5023–5030. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Casanova, V.; Belchior, A.P. Antioxidant activity and phenolic content of Portuguese wine aged brandies. J. Food Compos. Anal. 2008, 21, 626–633. [Google Scholar] [CrossRef]
- Nocera, A.; Ricardo-Da-Silva, J.M.; Canas, S. Antioxidant activity and phenolic composition of wine spirit resulting from an alternative ageing technology using micro-oxygenation: A preliminary study. Oeno One 2020, 54, 485–496. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Burin, V.M.; Costa, L.L.F.; Rosier, J.P.; Bordignon-Luiz, M.T. Cabernet Sauvignon wines from two different clones, characterization and evolution during bottle ageing. LWT 2011, 44, 1931–1938. [Google Scholar] [CrossRef]
- Pastor, F.T.; Šegan, D.M.; Gorjanović, S.Ž.; Kalušević, A.M.; Sužnjević, D.Ž. Development of voltammetric methods for antioxidant activity determination based on Fe(III) reduction. Microchem. J. 2020, 155, 104721. [Google Scholar] [CrossRef]
- Canas, S.; Anjos, O.; Caldeira, I.; Fernandes, T.A.; Santos, N.; Lourenço, S.; Granja-Soares, J.; Fargeton, L.; Boissier, B.; Catarino, S. Micro-oxygenation level as a key to explain the variation in the colour and chemical composition of wine spirits aged with chestnut wood staves. LWT 2021, 154, 112658. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of Antioxidant Activity by Using Different In Vitro Methods. Free. Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef]
- Rivero-Pérez, M.D.; Muñiz, P.; González-Sanjosé, M.L. Antioxidant Profile of Red Wines Evaluated by Total Antioxidant Capacity, Scavenger Activity, and Biomarkers of Oxidative Stress Methodologies. J. Agric. Food Chem. 2007, 55, 5476–5483. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric re-ducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Moharram, H.A.; Youssef, M.M. Methods for determining the antioxidant activity: A review. Alex. J. Fd. Sci. Technol. 2014, 11, 31–42. [Google Scholar]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Elolution of the phenolic content of red wines from Vitis viníferas L. during ageing in bottle. Food Chem. 2006, 95, 405–412. [Google Scholar] [CrossRef]
- Roginsky, V.; De Beer, D.; Harbertson, J.F.; Kilmartin, P.A.; Barsukova, T.; Adams, D.O. The antioxidant activity of Californian red wines does not correlate with wine age. J. Sci. Food Agric. 2006, 86, 834–840. [Google Scholar] [CrossRef]
- Bañuelos, G.G.; Buica, A.; Du Toit, W. Phenolic and Sensorial Evolution during Bottle Ageing of South African Shiraz Wines with Different Initial Phenolic Profiles. South. Afr. J. Enol. Vitic. 2020, 41, 17–32. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kefalas, P.; Moutounet, M. Extraction of phenolics in liquid model matrices containing oak chips: Kinetics, liquid chromatography–mass spectroscopy characterisation and association with in vitro antiradical activity. Food Chem. 2008, 110, 263–272. [Google Scholar] [CrossRef]
- Mrvčić, J.; Posavec, S.; Kazazic, S.; Stanzer, D.; Peša, A.; Stehlik-Tomas, V. Spirit drinks: A source of dietary polyphenols. Croat. J. Food Sci. Technol. 2012, 4, 102–111. [Google Scholar]
- Badarinath, A.V.; Mallikarjuna RAo, K.; Chetty, C.M.S.; Ramkanth, S.; Rajan, T.V.S.; Gnanaprakash, K. A Review on in-vitro antioxidant methods: Comparisons, correlations and considerations. Int. J. Pharm. Tech. Res. 2010, 2, 1276–1285. [Google Scholar]
- Kallithraka, S.; Salacha, M.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity using the DPPH. Free Radical Method. LWT 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Schwarz, M.; Rodríguez, M.S.; Martínez, C.; Bosquet, V.; Guillén, D.; Barroso, C.G. Antioxidant activity of Brandy de Jerez and other aged distillates, and correlation with their polyphenolic content. Food Chem. 2009, 116, 29–33. [Google Scholar] [CrossRef]
- Ariga, T.; Hamano, M. Radical Scavenging Action and Its Mode in Procyanidins B-1 and B-3 from Azuki Beans to Peroxyl Radicals. Agric. Biol. Chem. 1990, 54, 2499–2504. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Harzfeld, P.W.; Riechel, T.L. High molecular weight plant poly-phenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1992. [Google Scholar] [CrossRef]
- Bakkalbaşi, E.; Menteş, Ö.; Artik, N. Food Ellagitannins–Occurrence, Effects of Processing and Storage. Crit. Rev. Food Sci. Nutr. 2008, 49, 283–298. [Google Scholar] [CrossRef]
- Tulyathan, V.; Boulton, R.B.; Singleton, V.L. Oxygen uptake by gallic acid as a model for similar reactions in wines. J. Agric. Food Chem. 1989, 37, 844–849. [Google Scholar] [CrossRef]
- Pan, J.; Yin, D.; Ma, L.; Zhao, Y.; Zhao, J.; Guo, L. Dimer and Tetramer of Gallic Acid: Facile Synthesis, Antioxidant and Antiproliferative Activities. Lett. Drug Des. Discov. 2013, 11, 27–32. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Ferric Reducing and Radical Scavenging Activities of Selected Important Polyphenols Present In Foods. Int. J. Food Prop. 2012, 15, 702–708. [Google Scholar] [CrossRef]
- Yıldırım, H.K.; Altındışli, A. Changes of phenolic acids during aging of organic wines. Int. J. Food Prop. 2015, 18, 1038–1045. [Google Scholar] [CrossRef] [Green Version]
- Gagic, T.; Knez, Z.; Skerget, M. Hydrothermal hydrolysis of sweet chestnut (Castanea sativa) tannins. J. Serbian Chem. Soc. 2020, 85, 869–883. [Google Scholar] [CrossRef] [Green Version]
- Hanousek-Cica, K.; Pezer, M.; Mrvcic, J.; Stanzer, D.; Cacic, J.; Jurak, V.; Krajnovic, M.; Gajdos-Kljusuric, J. Identification of phenolic and alcoholic compounds in wine spirits and their classification by use of multivariate analysis. J. Serbian Chem. Soc. 2019, 84, 663–677. [Google Scholar] [CrossRef] [Green Version]
- Canas, S.; Caldeira, I.; Belchior, A.P. Extraction/oxidation kinetics of low molecular weight compounds in wine brandy resulting from different ageing technologies. Food Chem. 2013, 138, 2460–2467. [Google Scholar] [CrossRef]
- García-Estévez, I.; Alcalde-Eon, C.; Martínez-Gil, A.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Nevares, I.; del Alamo-Sanza, M. An Approach to the Study of the Interactions between Ellagitannins and Oxygen during Oak Wood Aging. J. Agric. Food Chem. 2017, 65, 6369–6378. [Google Scholar] [CrossRef] [Green Version]
- Solana, R.R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. First Approach to the Analytical Characterization of Barrel-Aged Grape Marc Distillates Using Phenolic Compounds and Colour Parameters. Food Technol. Biotechnol. 2014, 52, 391–402. [Google Scholar] [CrossRef]
- Revilla, I.; González-SanJosé, M.L. Compositional changes during the storage of red wines treated with pectolytic enzymes: Low molecular-weight phenols and flavan-3-ol derivative levels. Food Chem. 2003, 80, 205–214. [Google Scholar] [CrossRef]
- Cadahía, E.; Muñoz, L.; de Simón, B.F.; García-Vallejo, M.C. Changes in Low Molecular Weight Phenolic Compounds in Spanish, French, and American Oak Woods during Natural Seasoning and Toasting. J. Agric. Food Chem. 2001, 49, 1790–1798. [Google Scholar] [CrossRef]
- Delgado-González, M.; Sánchez-Guillén, M.; García-Moreno, M.; Rodríguez-Dodero, M.; García-Barroso, C.; Sánchez, D.A.G. Study of a laboratory-scaled new method for the accelerated continuous ageing of wine spirits by applying ultrasound energy. Ultrason. Sonochemistry 2017, 36, 226–235. [Google Scholar] [CrossRef]
- Le Floch, A.; Jourdes, M.; Teissedre, P.-L. Polysaccharides and lignin from oak wood used in cooperage: Composition, interest, assays: A review. Carbohydr. Res. 2015, 417, 94–102. [Google Scholar] [CrossRef]
- Gupta, N.K.; Fukuoka, A.; Nakajima, K. Metal-Free and Selective Oxidation of Furfural to Furoic Acid with an N-Heterocyclic Carbene Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 3434–3442. [Google Scholar] [CrossRef]
- Iroegbu, A.O.; Sadiku, E.R.; Ray, S.S.; Hamam, Y. Sustainable Chemicals: A Brief Survey of the Furans. Chem. Afr. 2020, 3, 481–496. [Google Scholar] [CrossRef] [Green Version]
- Karbowiak, T.; Crouvisier-Urion, K.; Lagorce, A.; Ballester, J.; Geoffroy, A.; Roullier-Gall, C.; Chanut, J.; Gougeon, R.D.; Schmitt-Kopplin, P.; Bellat, J.-P. Wine aging: A bottleneck story. NPJ Sci. Food 2019, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rufino, M.D.S.M.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Cetó, X.; Gutiérrez, J.M.; Gutiérrez, M.; Céspedes, F.; Capdevila, J.; Mínguez, S.; Jiménez-Jorquera, C.; del Valle, M. Determination of total polyphenol index in wines employing a voltammetric electronic tongue. Anal. Chim. Acta 2012, 732, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Canas, S.; Belchior, A.P.; Spranger, M.I.; Bruno-De-Sousa, R. High-performance liquid chromatography method for analysis of phenolic acids, phenolic aldehydes, and furanic derivatives in brandies. Development and validation. J. Sep. Sci. 2003, 26, 496–502. [Google Scholar] [CrossRef]


| Time (Months) | |||||
|---|---|---|---|---|---|
| Phenolic Acids (mg/L) | MOX | 0 | 6 | 12 | |
| Gall | O15 | 123.13 ± 16.62 Ab | 81.64 ± 13.06 Ba | 81.86 ± 10.36 Ba |  |
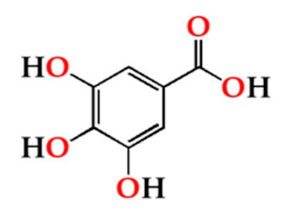 | O30 | 105.52 ± 14.81 Ab | 64.21 ± 8.15 ABa | 62.58 ± 10.60 Aa | |
| O60 | 94.89 ± 12.99 Ab | 57.28 ± 5.22 Aa | 54.10 ± 5.45 Aa | ||
| N | 85.69 ± 20.08 Ab | 51.56 ± 10.14 Aa | 50.01 ± 9.25 Aa | ||
| Van | O15 | 17.53 ± 7.18 Aa | 15.71 ± 4.99 Aa | 17.42 ± 5.90 Aa | 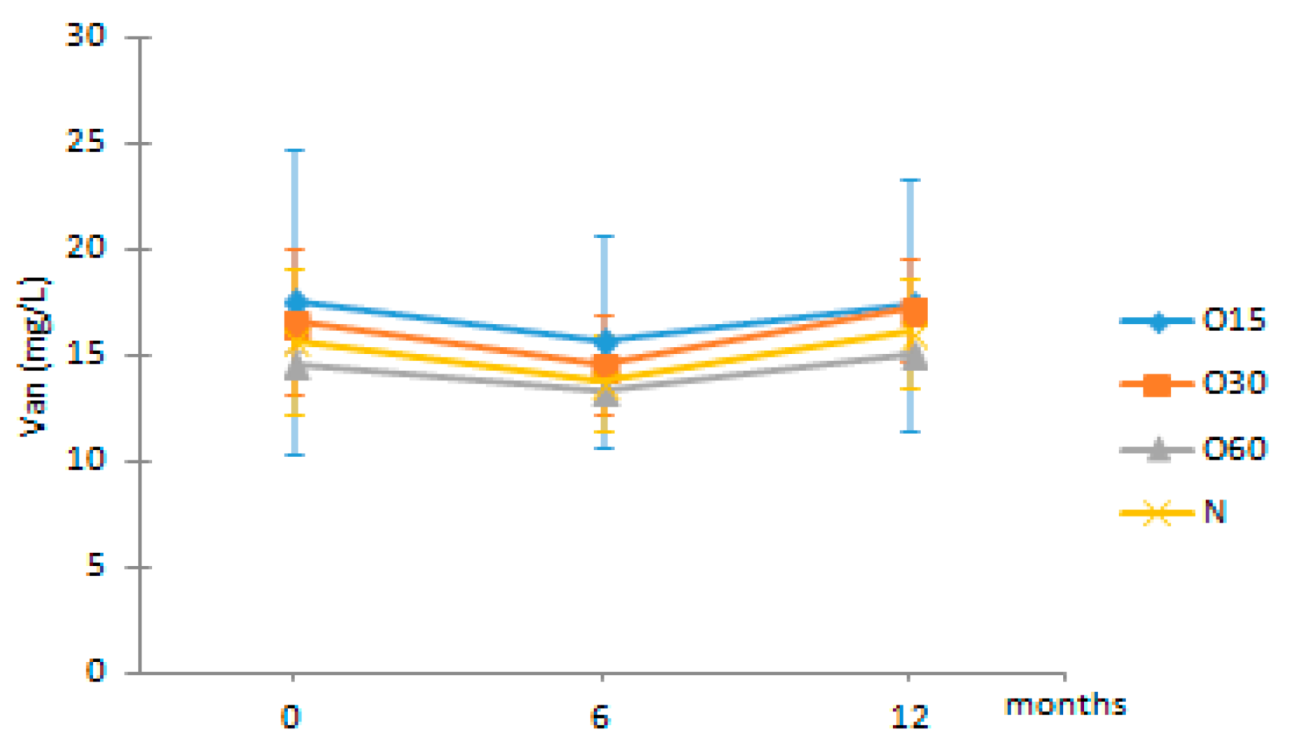 |
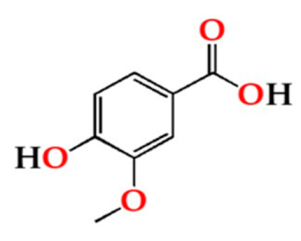 | O30 | 16.63 ± 3.46 Aa | 14.55 ± 2.38 Aa | 17.18 ± 2.41 Aa | |
| O60 | 14.59 ± 0.61 AAb | 13.34 ± 0.06 Aa | 15.01 ± 0.14 Ab | ||
| N | 15.66 ± 3.42 Aa | 13.75 ± 2.25 Aa | 16.07 ± 2.58 Aa | ||
| Ellag | O15 | 22.71 ± 2.45 Aa | 21.71 ± 1.71 ABa | 21.73 ± 2.29 ABa | 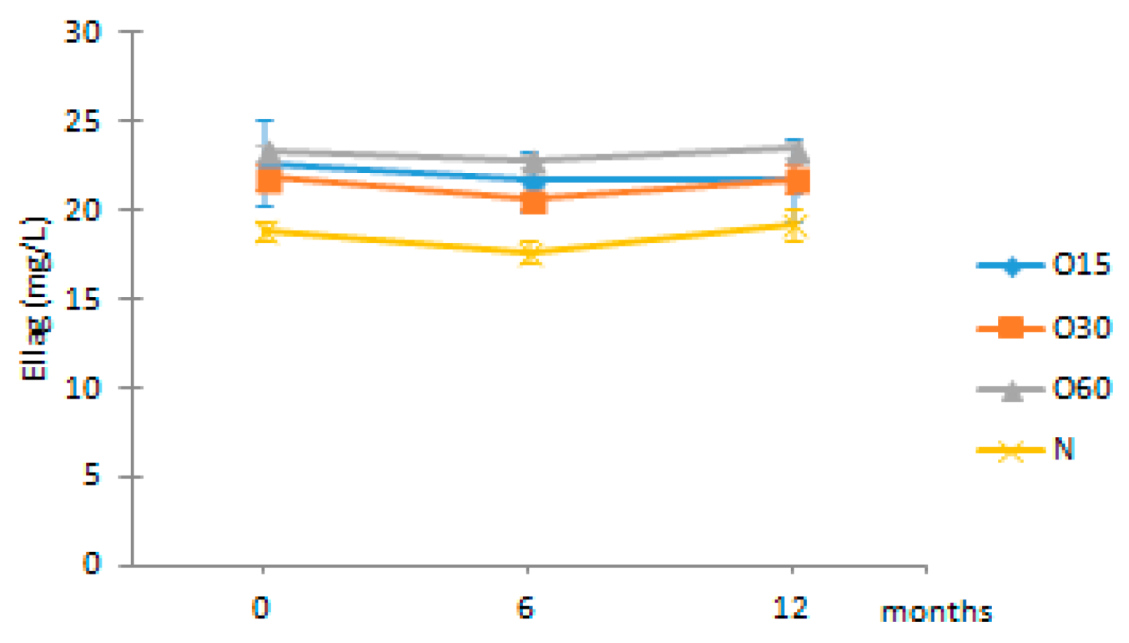 |
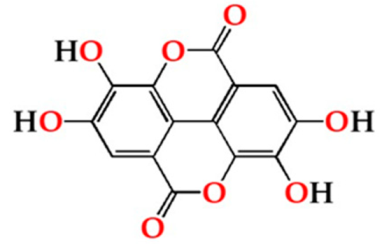 | O30 | 21.89 ± 0.67 Aa | 20.63 ± 0.42 Ba | 21.82 ± 0.86 ABa | |
| O60 | 23.29 ± 0.48 Aa | 22.81 ± 0.37 Ba | 23.44 ± 0.44 Ba | ||
| N | 18.93 ± 0.53 AAb | 17.70 ± 0.62 Aa | 19.30 ± 0.89 Ab | ||
| Fer | O15 | 1.53 ± 0.79 Ab | 1.66 ± 0.67 Ab | 0.37 ± 0.11 Aa | 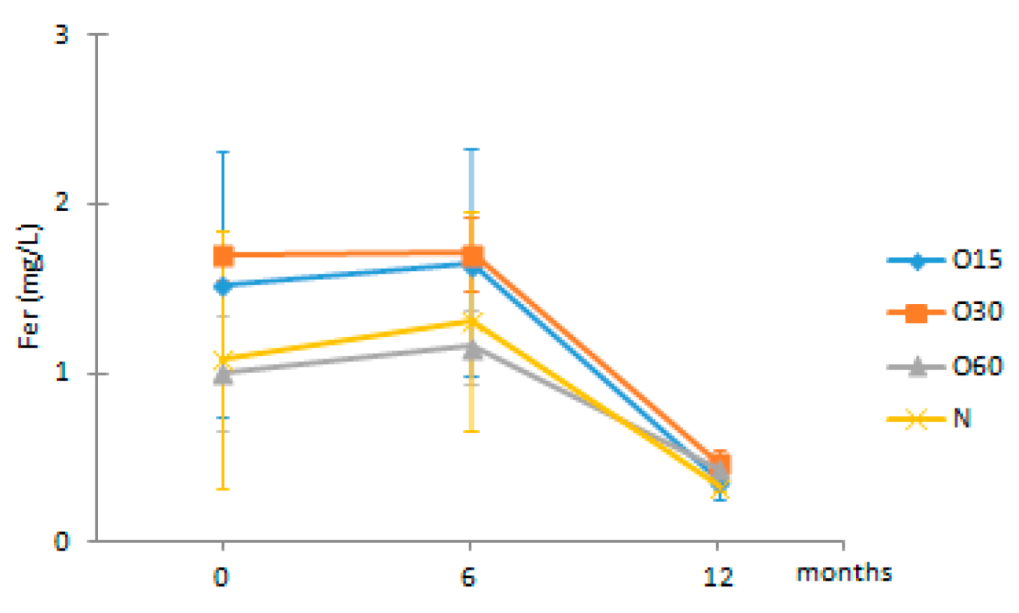 |
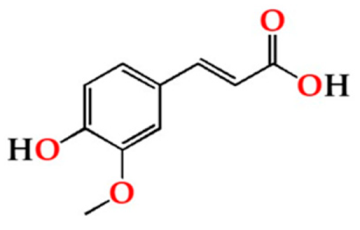 | O30 | 1.70 ± 0.15 Ab | 1.71 ± 0.21 Ab | 0.46 ± 0.08 Aa | |
| O60 | 1.01 ± 0.35 Ab | 1.16 ± 0.23 Ab | 0.43 ± 0.04 Aa | ||
| N | 1.08 ± 0.76 Aa | 1.31 ± 0.65 Aa | 0.33 ± 0.02 Aa | ||
| Syrg | O15 | 12.66 ± 0.90 Ba | 12.78 ± 0.55 Ca | 13.12 ± 1.49 Aa |  |
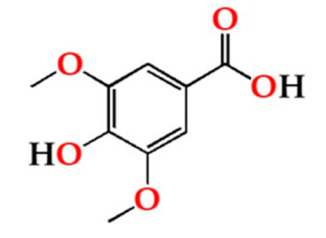 | O30 | 11.50 ± 0.35 ABa | 10.77 ± 0.52 Ba | 12.17 ± 0.92 Aa | |
| O60 | 11.58 ± 0.13 ABa | 11.29 ± 0.37 Ba | 12.97 ± 0.85 Aa | ||
| N | 9.38 ± 0.83 Aa | 9.31 ± 0.95 Aa | 11.27 ± 0.72 Aa | ||
| Time (Months) | |||||
|---|---|---|---|---|---|
| Phenolic Aldehydes (mg/L) | MOX | 0 | 6 | 12 | |
| Vanil | O15 | 6.05 ± 0.27 Aa | 6.45 ± 0.64 Ba | 6.52 ± 0.20 Ba | 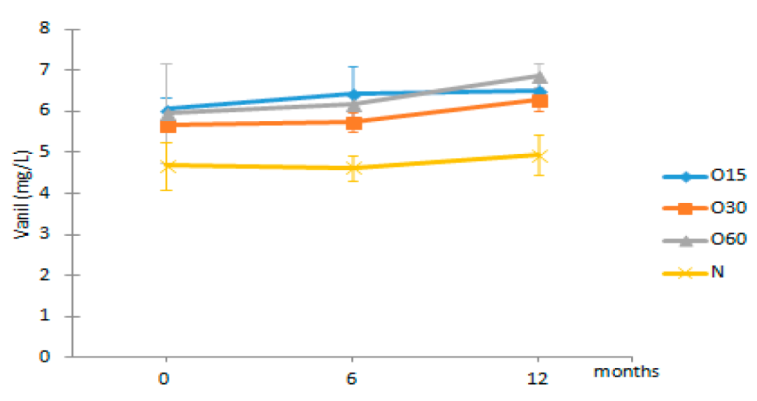 |
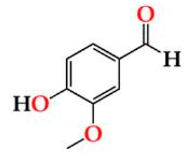 | O30 | 5.66 ± 0.03 Aa | 5.74 ± 0.25 Ba | 6.29 ± 0.28 Ba | |
| O60 | 5.97 ± 1.21 Aa | 6.19 ± 0.17 Ba | 6.88 ± 0.29 Ba | ||
| N | 4.68 ± 0.59 Aa | 4.62 ± 0.31 Aa | 4.93 ± 0.50 Aa | ||
| Syrde | O15 | 16.01 ± 0.17 Aa | 16.80 ± 1.32 Ca | 17.20 ± 0.11 Ba | 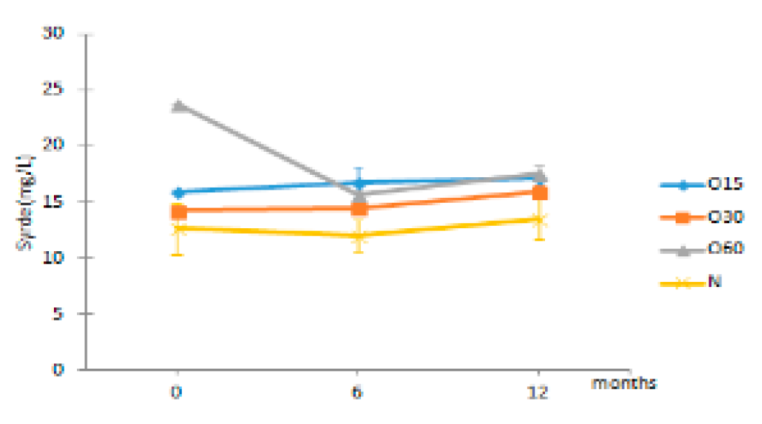 |
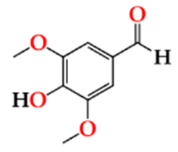 | O30 | 14.32 ± 0.35 Aa | 14.49 ± 0.26 Ba | 16.01 ± 0.49 Bb | |
| O60 | 23.71 ± 11.25 Aa | 15.67 ± 0.26 BCa | 17.50 ± 0.38 Ba | ||
| N | 12.65 ± 2.30 Aa | 12.05 ± 1.38 Aa | 13.52 ± 1.89 Aa | ||
| Cofde | O15 | 5.74 ± 0.47 Aa | 5.30 ± 0.66 Aa | 5.81 ± 0.23 Aa | 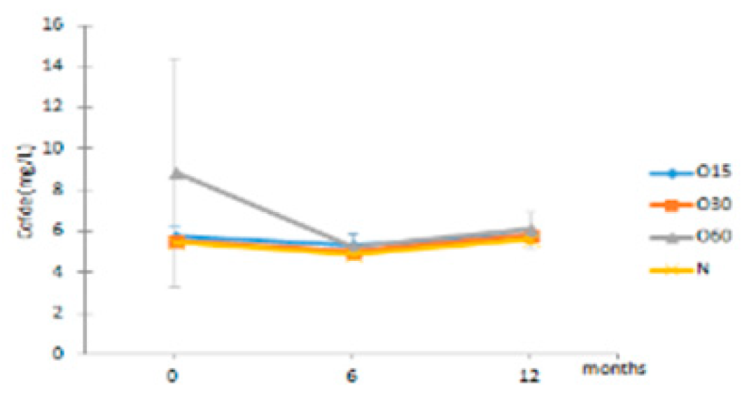 |
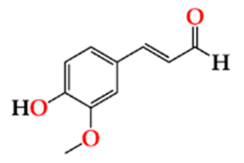 | O30 | 5.54 ± 0.25 Aa | 5.01 ± 0.29 Aa | 5.81 ± 0.31 Aa | |
| O60 | 8.87 ± 5.58 Aa | 5.25 ± 0.63 Aa | 6.09 ± 0.89 Aa | ||
| N | 5.43 ± 0.13 Ab | 4.88 ± 0.28 Aa | 5.62 ± 0.26 Ab | ||
| Sipde | O15 | 27.48 ± 0.78 Aa | 24.59 ± 2.13 Ba | 25.45 ± 0.39 Aa | 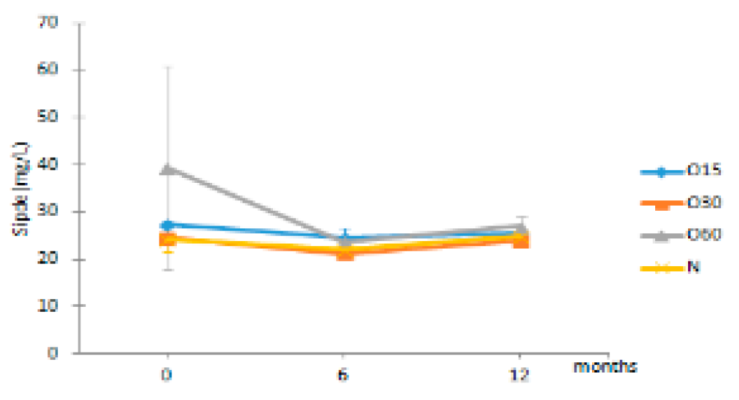 |
 | O30 | 24.61 ± 0.17 Ab | 21.31 ± 0.54 Aa | 23.98 ± 0.85 Ab | |
| O60 | 39.53 ± 21.54 Aa | 23.69 ± 1.30 ABa | 27.06 ± 2.25 Aa | ||
| N | 24.43 ± 2.65 Aa | 22.08 ± 1.29 ABa | 24.96 ± 1.40 Aa | ||
| Time (Months) | |||||
|---|---|---|---|---|---|
| Furanic Aldehydes (mg/L) | MOX | 0 | 6 | 12 | |
| Furf | O15 | 74.32 ± 7.40 Aa | 72.36 ± 4.87 Ba | 72.80 ± 5.83 Ba | 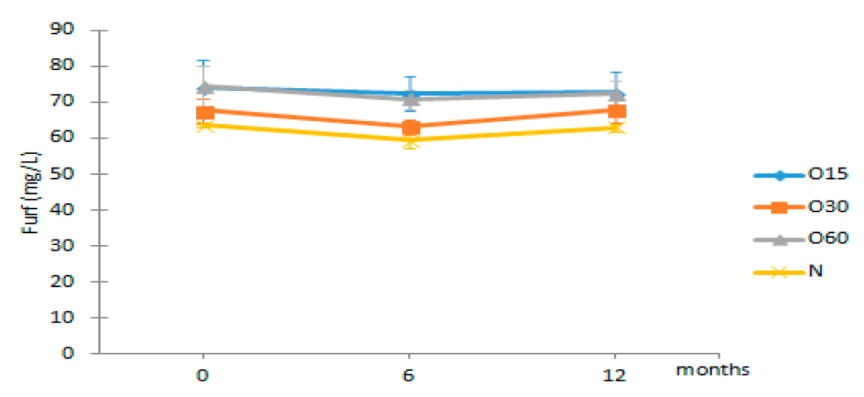 |
 | O30 | 67.70 ± 3.30 Aa | 63.21 ± 2.06 Aa | 67.94 ± 3.72 ABa | |
| O60 | 74.34 ± 5.64 Aa | 70.89 ± 2.68 Ba | 72.42 ± 3.56 Ba | ||
| N | 63.63 ± 0.35 Ab | 59.42 ± 2.21 Aa | 62.95 ± 0.97 Ab | ||
| HMF | O15 | 33.55 ± 14.11 Aa | 31.60 ± 10.16 Aa | 33.38 ± 11.36 Aa | 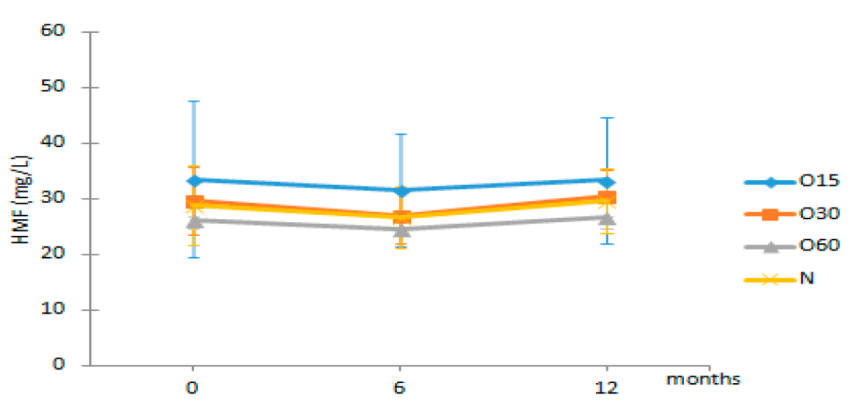 |
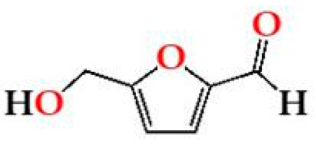 | O30 | 29.64 ± 6.00 Aa | 26.95 ± 5.02 Aa | 30.41 ± 4.68 Aa | |
| O60 | 26.08 ± 2.69 Aa | 24.53 ± 1.12 Aa | 26.63 ± 1.63 Aa | ||
| N | 28.91 ± 7.13 Aa | 26.71 ± 5.58 Aa | 29.72 ± 5.80 Aa | ||
| 5Mfurf | O15 | 1.96 ± 0.31 Ab | 0.74 ± 0.28 Aa | 0.82 ± 0.23 Aa |  |
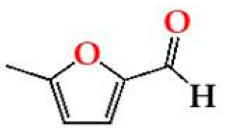 | O30 | 1.97 ± 0.07 Ab | 0.60 ± 0.08 Aa | 0.71 ± 0.10 Aa | |
| O60 | 1.71 ± 0.49 Ab | 0.51 ± 0.13 Aa | 0.60 ± 0.15 Aa | ||
| N | 1.44 ± 0.48 Ab | 0.40 ± 0.16 Aa | 0.48 ± 0.17 Aa | ||
| Ageing Trial | Storage in Bottle | |||
|---|---|---|---|---|
| Ageing Technology | MOX Flow Rate | N2 Flow Rate | Storage Time | Samples Code |
| O15 | 2 mL/L/month–0 to15th day 0.6 mL/L/month–15 to 365th day | − | 0 months 6 months 12 months | O151G0a, O151G0b; O152G0a, O152G0b O151G6a, O151G6b; O152G6a, O152G6b O151G12a, O151G12b; O152G12a, O152G12b |
| O30 | 2 mL/L/month–0 to 30th day 0.6 mL/L/month–30 to 365th day | − | 0 months 6 months 12 months | O301G0a, O301G0b; O302G0a, O302G0b O301G6a, O301G6b; O302G6a, O302G6b O301G12a, O301G12b; O302G12a, O302G12b |
| O60 | 2 mL/L/month–0 to 60th day 0.6 mL/L/month–60 to 365th day | − | 0 months 6 months 12 months | O601G0a, O601G0b; O602G0a, O602G0b O601G6a, O601G6b; O602G6a, O602G6b O601G12a, O601G12b; O602G12a, O602G12b |
| N | − | 20 mL/L/month 0 to 365th day | 0 months 6 months 12 months | N1G0a, N1G0b; N2G0a, N2G0b N1G6a, N1G6b; N2G6a, N2G6b N1G12a, N1G12b; N2G12a, N2G12b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Alves, S.; Lourenço, S.; Anjos, O.; Fernandes, T.A.; Caldeira, I.; Catarino, S.; Canas, S. Influence of the Storage in Bottle on the Antioxidant Activities and Related Chemical Characteristics of Wine Spirits Aged with Chestnut Staves and Micro-Oxygenation. Molecules 2022, 27, 106. https://doi.org/10.3390/molecules27010106
Oliveira-Alves S, Lourenço S, Anjos O, Fernandes TA, Caldeira I, Catarino S, Canas S. Influence of the Storage in Bottle on the Antioxidant Activities and Related Chemical Characteristics of Wine Spirits Aged with Chestnut Staves and Micro-Oxygenation. Molecules. 2022; 27(1):106. https://doi.org/10.3390/molecules27010106
Chicago/Turabian StyleOliveira-Alves, Sheila, Sílvia Lourenço, Ofélia Anjos, Tiago A. Fernandes, Ilda Caldeira, Sofia Catarino, and Sara Canas. 2022. "Influence of the Storage in Bottle on the Antioxidant Activities and Related Chemical Characteristics of Wine Spirits Aged with Chestnut Staves and Micro-Oxygenation" Molecules 27, no. 1: 106. https://doi.org/10.3390/molecules27010106
APA StyleOliveira-Alves, S., Lourenço, S., Anjos, O., Fernandes, T. A., Caldeira, I., Catarino, S., & Canas, S. (2022). Influence of the Storage in Bottle on the Antioxidant Activities and Related Chemical Characteristics of Wine Spirits Aged with Chestnut Staves and Micro-Oxygenation. Molecules, 27(1), 106. https://doi.org/10.3390/molecules27010106










