Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles
Abstract
:1. Introduction
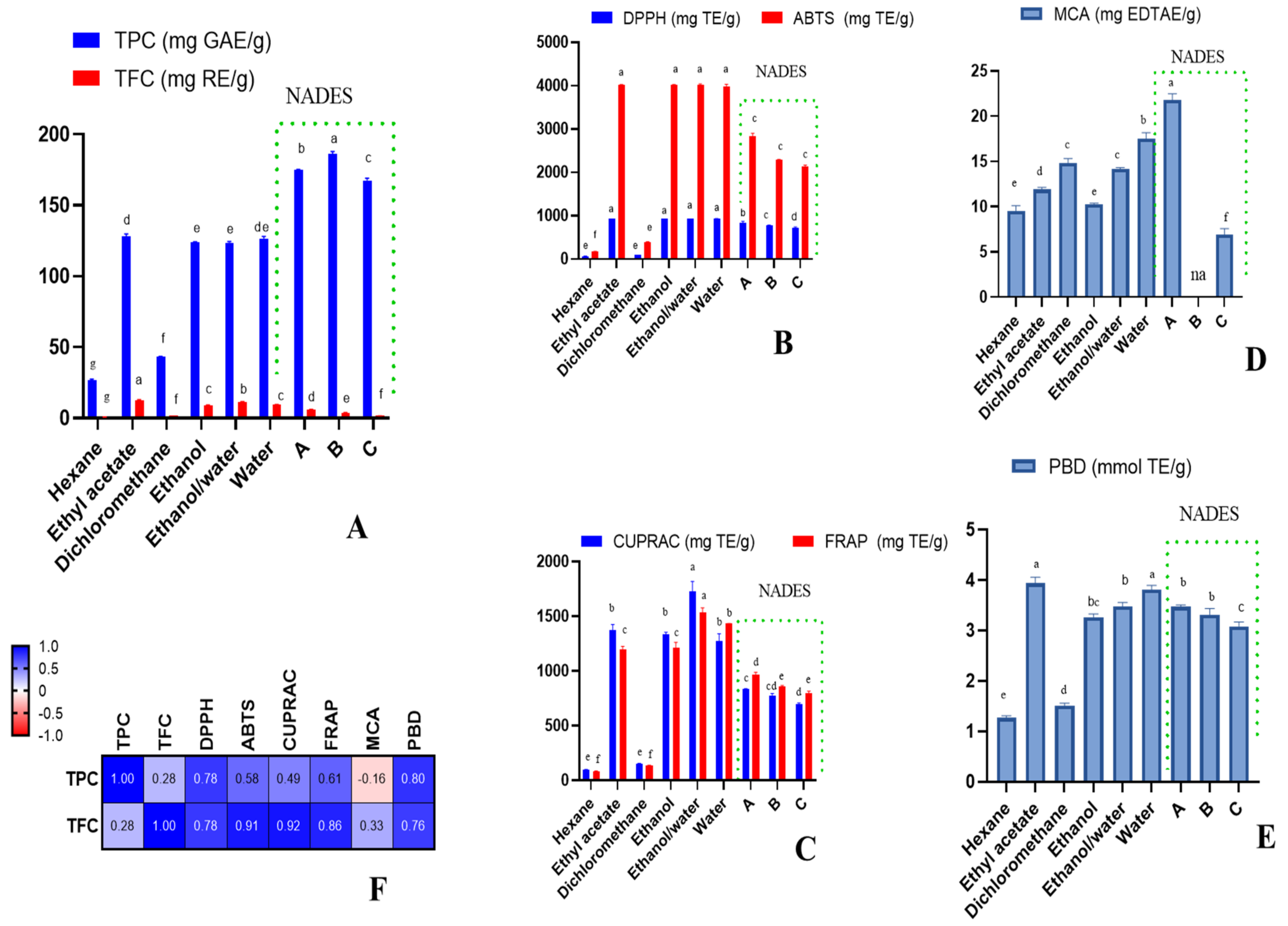
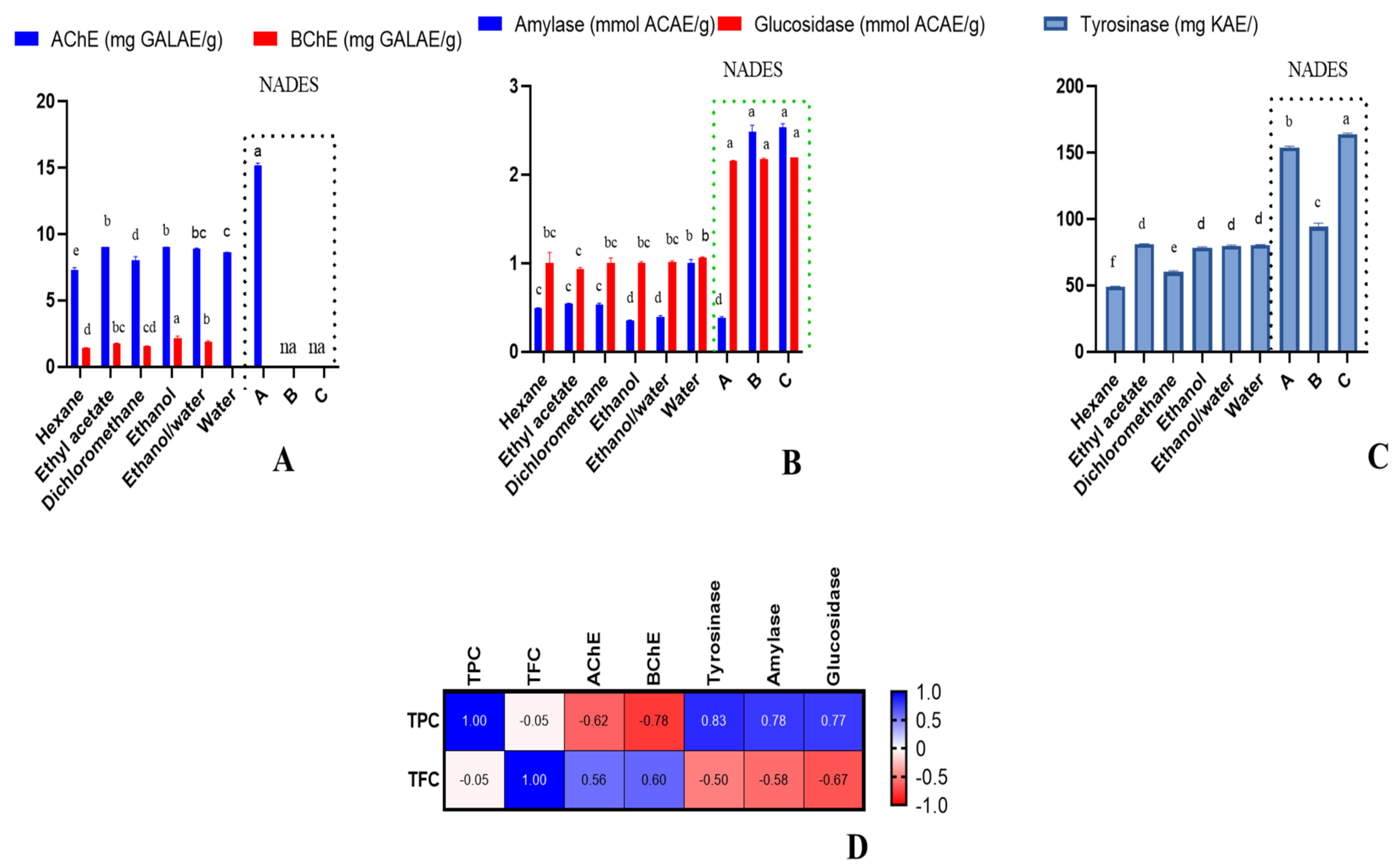
2. Results and Discussion
2.1. Phytochemical Profiles
2.2. Characterization of Polar Bioactive Compounds from C. hypocistis Extracts by UPLC-ESI-QTOF-MS
2.3. Antioxidant Effects
2.4. Enzyme Inhibitory Effects
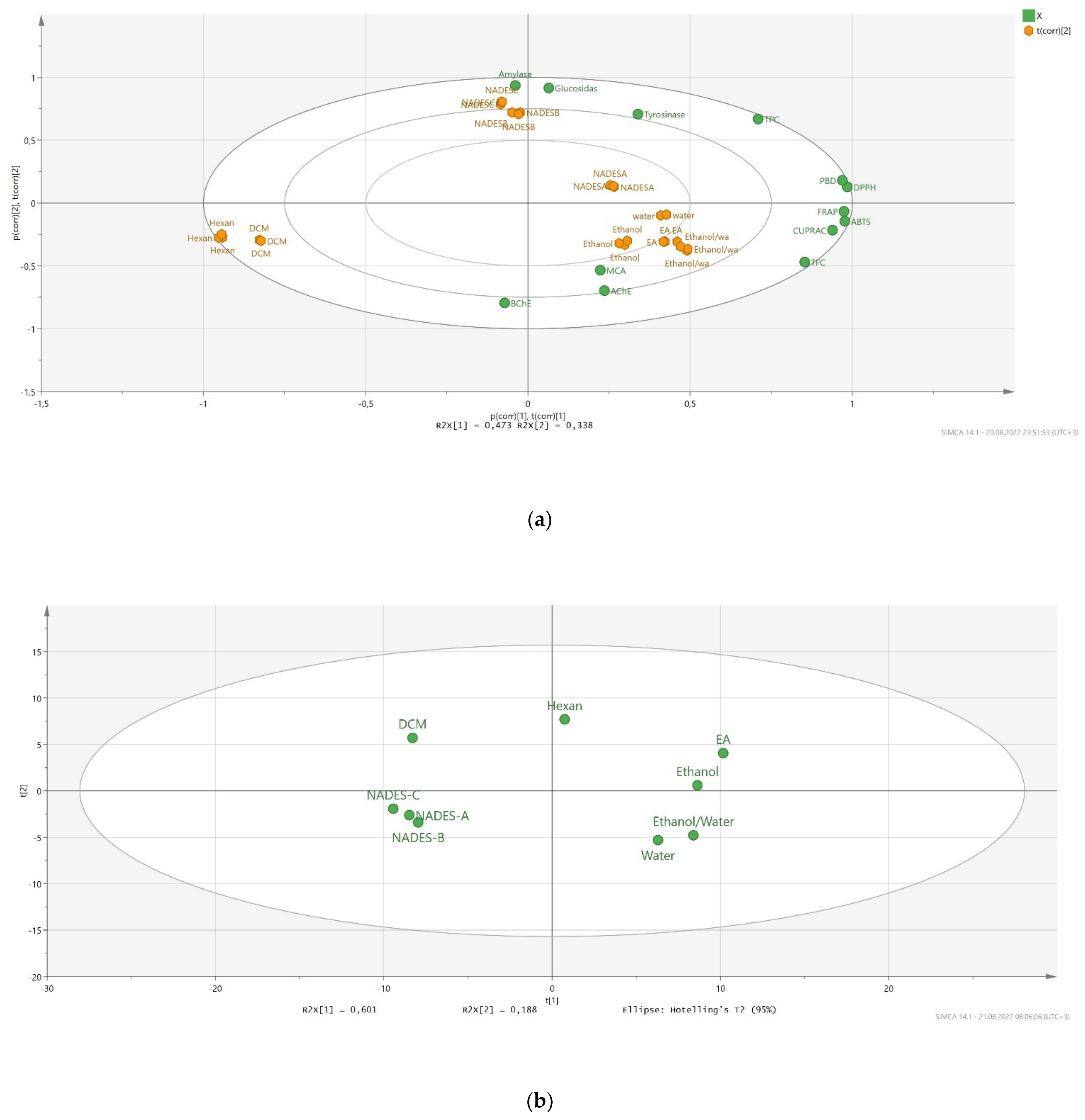
2.5. Data Mining
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Preparation of NADESs
3.4. Plant Material and Preparation of Extracts
3.5. Chemical Reagents
3.6. UPLC-ESI-QTOF-MS Conditions
3.7. UPLC-ESI-QTOF-MS Data Processing
3.8. Determination of Total Polyphenol and Flavonoids Contents
3.9. Antioxidant and Enzyme Inhibitory Assays
3.10. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanjust, E.; Rinaldi, A.C. Cytinus under the Microscope: Disclosing the Secrets of a Parasitic Plant. Plants 2021, 10, 146. [Google Scholar] [CrossRef]
- Zucca, P.; Pintus, M.; Manzo, G.; Nieddu, M.; Steri, D.; Rinaldi, A.C. Antimicrobial, antioxidant and anti-tyrosinase properties of extracts of the Mediterranean parasitic plant Cytinus hypocistis. BMC Res. Notes 2015, 8, 562. [Google Scholar] [CrossRef]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complementary Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Mandrone, M.; Bonvicini, F.; Lianza, M.; Sanna, C.; Maxia, A.; Gentilomi, G.; Poli, F. Sardinian plants with antimicrobial potential. Biological screening with multivariate data treatment of thirty-six extracts. Ind. Crops Prod. 2019, 137, 557–565. [Google Scholar] [CrossRef]
- Silva, A.R.; Pinela, J.; García, P.A.; Ferreira, I.C.; Barros, L. Cytinus hypocistis (L.) L.: Optimised heat/ultrasound-assisted extraction of tannins by response surface methodology. Sep. Purif. Technol. 2021, 276, 119358. [Google Scholar] [CrossRef]
- Magiatis, P.; Pratsinis, H.; Kalpoutzakis, E.; Konstantinidou, A.; Davaris, P.; Skaltsounis, A.-L. Hydrolyzable tannins, the active constituents of three Greek Cytinus taxa against several tumor cell lines. Biol. Pharm. Bull. 2001, 24, 707–709. [Google Scholar] [CrossRef]
- Silva, A.R.; Ayuso, M.; Pereira, C.; Dias, M.I.; Kostić, M.; Calhelha, R.C.; Soković, M.; García, P.A.; Ferreira, I.C.; Barros, L. Evaluation of parasite and host phenolic composition and bioactivities—The Practical Case of Cytinus hypocistis (L.) L. and Halimium lasianthum (Lam.) Greuter. Ind. Crops Prod. 2022, 176, 114343. [Google Scholar] [CrossRef]
- Koçak, E.; Pazır, F. Effect of Extraction Methods on Bioactive Compounds of Plant Origin. Turk. J. Agric. Food Sci. Technol. 2018, 6, 663–675. [Google Scholar]
- Momotko, M.; Łuczak, J.; Przyjazny, A.; Boczkaj, G. A natural deep eutectic solvent-protonated L-proline-xylitol-based stationary phase for gas chromatography. J. Chromatogr. A 2022, 1676, 463238. [Google Scholar] [CrossRef]
- Momotko, M.; Łuczak, J.; Przyjazny, A.; Boczkaj, G. First deep eutectic solvent-based (DES) stationary phase for gas chromatography and future perspectives for DES application in separation techniques. J. Chromatogr. A 2021, 1635, 461701. [Google Scholar] [CrossRef]
- Faraz, N.; Haq, H.U.; Balal Arain, M.; Castro-Muñoz, R.; Boczkaj, G.; Khan, A. Deep eutectic solvent based method for analysis of Niclosamide in pharmaceutical and wastewater samples—A green analytical chemistry approach. J. Mol. Liq. 2021, 335, 116142. [Google Scholar] [CrossRef]
- Serna-Vázquez, J.; Ahmad, M.Z.; Boczkaj, G.; Castro-Muñoz, R. Latest Insights on Novel Deep Eutectic Solvents (DES) for Sustainable Extraction of Phenolic Compounds from Natural Sources. Molecules 2021, 26, 5037. [Google Scholar] [CrossRef] [PubMed]
- Khajavian, M.; Vatanpour, V.; Castro-Muñoz, R.; Boczkaj, G. Chitin and derivative chitosan-based structures—Preparation strategies aided by deep eutectic solvents: A review. Carbohydr. Polym. 2022, 275, 118702. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Msahel, A.; Galiano, F.; Serocki, M.; Ryl, J.; Hamouda, S.B.; Hafiane, A.; Boczkaj, G.; Figoli, A. Towards azeotropic MeOH-MTBE separation using pervaporation chitosan-based deep eutectic solvent membranes. Sep. Purif. Technol. 2022, 281, 119979. [Google Scholar] [CrossRef]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural deep eutectic solvents (Nades): Phytochemical extraction performance enhancer for pharmaceutical and nutraceutical product development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Kljakić, A.C.; Pojić, M.; Mandić, A.; Stupar, A.; Santos, F.; Duarte, A.R.C.; Mišan, A. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules 2022, 27, 1508. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewi, S.R.; Stevens, L.A.; Pearson, A.E.; Ferrari, R.; Irvine, D.J.; Binner, E.R. Investigating the role of solvent type and microwave selective heating on the extraction of phenolic compounds from cacao (Theobroma cacao L.) pod husk. Food Bioprod. Process. 2022, 134, 210–222. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Silva, A.R.; Pinela, J.; Dias, M.I.; Calhelha, R.C.; Alves, M.J.; Mocan, A.; García, P.A.; Barros, L.; Ferreira, I.C. Exploring the phytochemical profile of Cytinus hypocistis (L.) L. as a source of health-promoting biomolecules behind its in vitro bioactive and enzyme inhibitory properties. Food Chem. Toxicol. 2020, 136, 111071. [Google Scholar] [CrossRef]
- Luo, F.; Fu, Y.; Xiang, Y.; Yan, S.; Hu, G.; Huang, X.; Huang, G.; Sun, C.; Li, X.; Chen, K. Identification and quantification of gallotannins in mango (Mangifera indica L.) kernel and peel and their antiproliferative activities. J. Funct. Foods 2014, 8, 282–291. [Google Scholar] [CrossRef]
- Namngam, C.; Pinsirodom, P.; Boonyuen, S. Fractionation, antioxidant and inhibitory activity of Thai mango seed kernel extracts. Czech J. Food Sci. 2018, 36, 8–15. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS profile, gastrointestinal and gut microbiota stability and antioxidant activity of Rhodiola rosea herb metabolites: A comparative study with subterranean organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef]
- Tong, N.-N.; Zhou, X.-Y.; Peng, L.-P.; Liu, Z.-A.; Shu, Q.-Y. A comprehensive study of three species of Paeonia stem and leaf phytochemicals, and their antioxidant activities. J. Ethnopharmacol. 2021, 273, 113985. [Google Scholar] [CrossRef] [PubMed]
- Gok, H.N.; Pekacar, S.; Orhan, D.D. Investigation of Enzyme Inhibitory Activities, Antioxidant Activities, and Chemical Properties of Pistacia vera Leaves Using LC-QTOF-MS and RP-HPLC. Iran. J. Pharm. Res. 2022, 21, e127033. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Kong, K.-W.; Li, H.-B.; Wu, K.; Ge, Y.-Y.; Chan, C.-L.; Shi, X.-M.; Corke, H. Separation, identification, and bioactivities of the main gallotannins of red sword bean (Canavalia gladiata) coats. Front. Chem. 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.-V.; Roy, A.; Foote, S.; Vo, P.H.; Lall, N.; Lin, C.-H. Profiling anticancer and antioxidant activities of phenolic compounds present in black walnuts (Juglans nigra) using a high-throughput screening approach. Molecules 2020, 25, 4516. [Google Scholar] [CrossRef] [PubMed]
- Quintana, S.E.; Salas, S.; García-Zapateiro, L.A. Bioactive compounds of mango (Mangifera indica): A review of extraction technologies and chemical constituents. J. Sci. Food Agric. 2021, 101, 6186–6192. [Google Scholar] [CrossRef]
- Wang, H.; Fowler, M.I.; Messenger, D.J.; Ordaz-Ortiz, J.J.; Gu, X.; Shi, S.; Terry, L.A.; Berry, M.J.; Lian, G.; Wang, S. Inhibition of the intestinal postprandial glucose transport by gallic acid and gallic acid derivatives. Food Funct. 2021, 12, 5399–5406. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Jiang, Y.; Guo, Z.; Fang, S. Separation, UPLC-QTOF-MS/MS analysis, and antioxidant activity of hydrolyzable tannins from water caltrop (Trapa quadrispinosa) pericarps. LWT 2020, 133, 110010. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, Q.; Liang, W.; Zhou, K.; Jian, P.; She, G.; Zhang, L. A Comprehensive Review of the Structure Elucidation of Tannins from Terminalia Linn. Evid.-Based Complement. Altern. Med. 2019, 2019, 8623909. [Google Scholar] [CrossRef]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B. Fruit seeds as sources of bioactive compounds: Sustainable production of high value-added ingredients from by-products within circular economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef]
- Tameye, N.S.J.; Akak, C.M.; Happi, G.M.; Frese, M.; Stammler, H.-G.; Neumann, B.; Lenta, B.N.; Sewald, N.; Nkengfack, A.E. Antioxidant norbergenin derivatives from the leaves of Diospyros gilletii De Wild (Ebenaceae). Phytochem. Lett. 2020, 36, 63–67. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [PubMed]
- Szwajgier, D.; Baranowska-Wójcik, E.; Winiarska-Mieczan, A.; Gajowniczek-Ałasa, D. Honeys as Possible Sources of Cholinesterase Inhibitors. Nutrients 2022, 14, 2969. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.; Karanth, S.; Liu, J. Pharmacology and toxicology of cholinesterase inhibitors: Uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005, 19, 433–446. [Google Scholar]
- Yu, Z.-Y.; Xu, K.; Wang, X.; Wen, Y.-T.; Wang, L.-J.; Huang, D.-Q.; Chen, X.-X.; Chai, W.-M. Punicalagin as a novel tyrosinase and melanin inhibitor: Inhibitory activity and mechanism. LWT 2022, 161, 113318. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.; Menichini, F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules 2019, 10, 61. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Janicka, P.; Przyjazny, A.; Boczkaj, G. Novel “acid tuned” deep eutectic solvents based on protonated L-proline. J. Mol. Liq. 2021, 333, 115965. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of antioxidant potentials of solvent extracts from different anatomical parts of Asphodeline anatolica E. Tuzlaci: An endemic plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
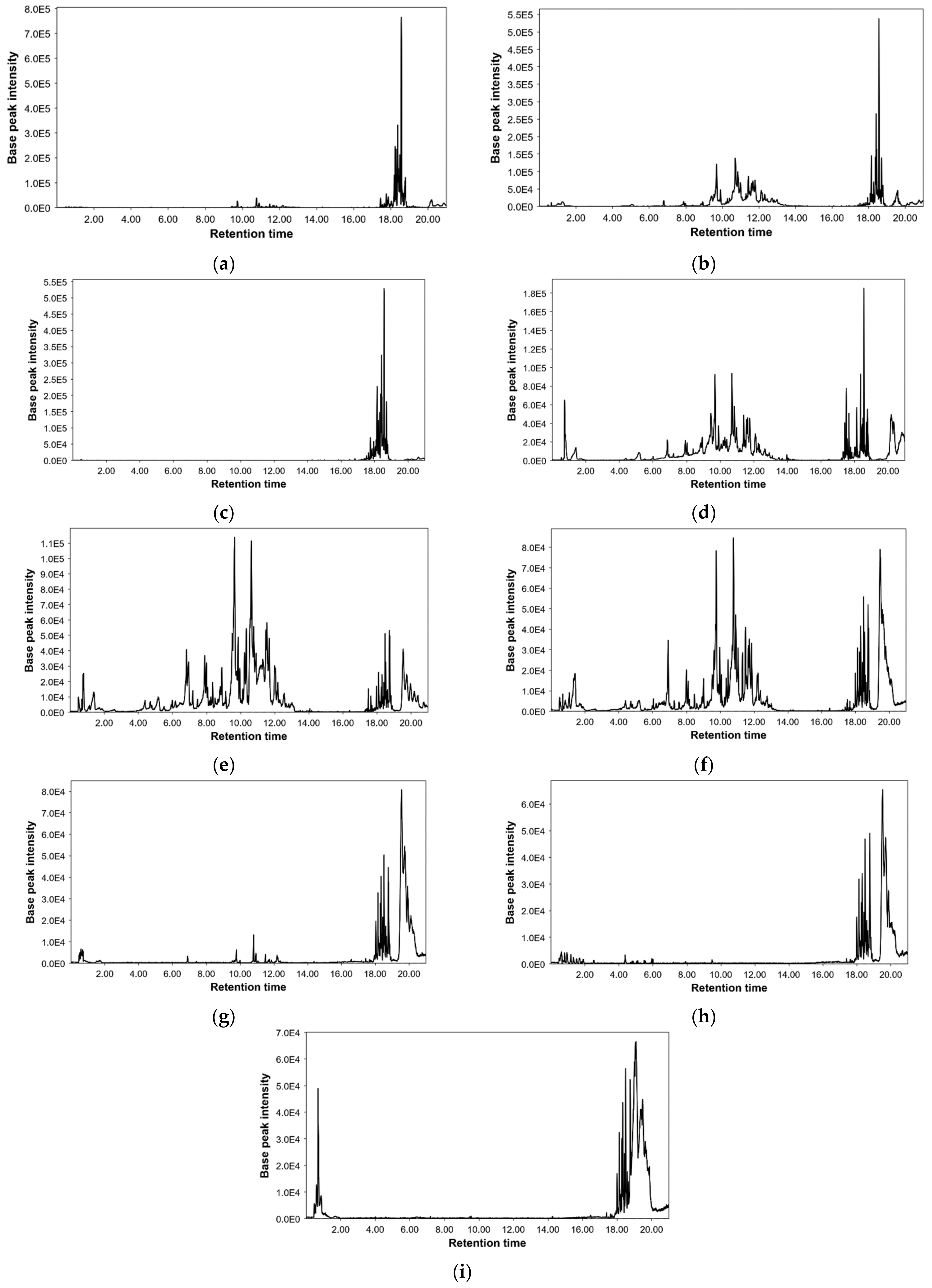
| Peak | Compound | Hexane | Ethyl Acetate | Dichloromethane | Ethanol | Ethanol/Water | Water | NADES-A | NADES-B | NADES-C |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hydroxy-pseudouric acid | + | + | + | ND | + | + | ND | + | ND |
| 2 | Galloyl-galactarolactone | + | + | ND | ND | + | + | ND | ND | ND |
| 3 | Unknown 1 | + | + | + | + | + | + | + | + | + |
| 4 | Unknown 2 | ND | ND | ND | ND | ND | ND | ND | + | ND |
| 5 | Proline | ND | ND | ND | ND | ND | ND | + | ND | + |
| 6 | Disaccharide | + | + | ND | + | + | + | ND | ND | ND |
| 7 | Unknown 3 | ND | ND | ND | ND | ND | ND | ND | ND | + |
| 8 | Glucose | ND | ND | ND | + | + | ND | ND | ND | ND |
| 9 | Unknown 4 | ND | ND | ND | ND | ND | ND | ND | ND | + |
| 10 | Quinic acid | ND | + | ND | + | + | + | ND | ND | ND |
| 11 | Galloyl-diglucose | ND | ND | ND | + | + | ND | ND | ND | ND |
| 12 | Galloylglucose isomer 1 | + | + | ND | + | + | + | ND | + | ND |
| 13 | Galloylglucose isomer 2 | + | + | ND | + | + | + | ND | + | ND |
| 14 | Galloylglucose isomer 3 | + | + | ND | + | + | + | ND | + | ND |
| 15 | Pyrogallol | ND | + | ND | + | + | + | + | + | + |
| 16 | Gallic acid | ND | + | ND | + | + | + | + | + | + |
| 17 | Galloylglucose isomer 4 | ND | + | ND | + | + | + | ND | + | ND |
| 18 | Fukiic acid | ND | + | ND | ND | ND | + | ND | + | ND |
| 19 | Digalloylglucose isomer 1 | ND | + | ND | + | + | + | ND | + | ND |
| 20 | Digalloylglucose isomer 2 | ND | ND | ND | + | + | + | ND | + | ND |
| 21 | Digalloylglucose isomer 3 | ND | + | ND | + | + | + | ND | + | ND |
| 22 | Digalloylglucose isomer 4 | ND | + | ND | + | + | + | ND | + | ND |
| 23 | Brevifolin carboxylic acid | ND | ND | ND | ND | + | + | ND | ND | ND |
| 24 | Digallate isomer 1 | ND | ND | ND | + | + | + | ND | ND | ND |
| 25 | Unknown 5 | + | + | ND | ND | ND | ND | ND | ND | ND |
| 26 | Trigalloyl-glucoside isomer 1 | ND | + | ND | + | + | + | + | ND | ND |
| 27 | HHDP-galloylglucose isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 28 | Galloylnorbergenin isomer 1 | + | + | ND | + | + | + | + | ND | ND |
| 29 | Brevifolin | ND | ND | ND | + | + | + | + | ND | ND |
| 30 | Galloylnorbergenin isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 31 | Digalloyl-HHDP-glucose isomer 1 | ND | ND | ND | + | + | + | ND | ND | ND |
| 32 | Galloylnorbergenin isomer 3 | + | + | ND | + | + | + | ND | ND | ND |
| 33 | Trigalloyl-glucoside isomer 2 | ND | + | ND | + | + | + | ND | + | ND |
| 34 | HHDP-galloylglucose isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 35 | Trigalloyl-glucoside isomer 3 | ND | + | ND | + | + | + | ND | + | ND |
| 36 | Digalloyl-HHDP-glucose isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 37 | HHDP-galloylglucose isomer 3 | ND | + | ND | + | + | + | ND | ND | ND |
| 38 | Trigalloyl-glucoside isomer 4 | ND | + | ND | + | + | + | ND | ND | ND |
| 39 | Terflavin B isomer 1 | ND | + | ND | + | ND | ND | ND | ND | ND |
| 40 | Galloyl-HHDP-glucose isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 41 | Tetragalloyl-glucoside isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 42 | Balanophotannin E isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 43 | Trigalloyl-HHDP-glucose isomer 1 | ND | ND | ND | ND | + | ND | ND | ND | ND |
| 44 | Terflavin B isomer 2 | ND | + | ND | + | ND | ND | ND | ND | ND |
| 45 | Galloflavin | + | + | ND | ND | ND | ND | ND | ND | ND |
| 46 | Ellagic acid | + | + | ND | + | + | + | + | + | ND |
| 47 | Terflavin B isomer 3 | + | + | ND | + | ND | ND | ND | ND | ND |
| 48 | Trigalloyl-HHDP-glucose isomer 2 | + | + | ND | + | + | + | ND | ND | ND |
| 49 | Digalloyl-lactonised valoneoyl-d-glucose isomer 1 | + | + | ND | + | ND | ND | ND | ND | ND |
| 50 | Trigalloyl-brevifolincarboxyl-glucose isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 51 | Tetragalloyl-glucoside isomer 2 | + | + | ND | + | + | + | + | ND | + |
| 52 | Catechin | ND | + | ND | + | + | + | ND | ND | ND |
| 53 | Tetragalloyl-glucoside isomer 3 | + | + | ND | + | + | + | + | ND | + |
| 54 | Digallate isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 55 | Tetragalloyl-glucoside isomer 4 | + | + | ND | + | + | + | + | ND | ND |
| 56 | Digallate isomer 3 | ND | + | ND | + | + | + | ND | ND | ND |
| 57 | Terflavin B isomer 4 | + | + | ND | + | + | ND | ND | ND | ND |
| 58 | Galloyl-HHDP-glucose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 59 | Tetragalloyl-glucoside isomer 5 | + | + | ND | + | + | + | + | ND | ND |
| 60 | Neochebulagic acid isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 61 | Neochebulagic acid isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 62 | Isorhamnetin glucoside isomer 1 | + | + | ND | + | + | + | ND | ND | ND |
| 63 | Epicatechin | ND | + | ND | + | + | + | ND | ND | ND |
| 64 | Quercetin | ND | + | ND | ND | + | ND | ND | ND | ND |
| 65 | Balanophotannin E isomer 2 | ND | + | ND | + | + | + | ND | ND | ND |
| 66 | Unknown 6 | + | + | ND | + | + | + | ND | ND | ND |
| 67 | Digalloyl-lactonised valoneoyl-d-glucose isomer 2 | ND | + | ND | + | ND | ND | ND | ND | ND |
| 68 | Trigalloyl-brevifolincarboxyl-glucose isomer 2 | + | + | ND | + | + | + | ND | ND | ND |
| 69 | Pentagalloyl-glucose isomer 1 | + | + | ND | + | + | + | ND | ND | ND |
| 70 | Neochebulagic acid isomer 3 | ND | ND | ND | ND | + | ND | ND | ND | ND |
| 71 | Trigalloyl-lactonised valoneoyl glucose isomer 1 | ND | + | ND | + | + | ND | ND | ND | ND |
| 72 | (Galloyl)galloyl-tetragalloylglucose isomer 1 | ND | + | ND | + | + | + | ND | ND | ND |
| 73 | Trigalloyl-brevifolincarboxyl-glucose isomer 3 | + | + | ND | + | + | + | ND | ND | ND |
| 74 | Trigalloyl-DHHDP-glucose isomer 1 | + | + | ND | ND | ND | ND | ND | ND | ND |
| 75 | Digalloyl-lactonised valoneoyl-d-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 76 | Castalagin | + | + | ND | ND | ND | ND | ND | ND | ND |
| 77 | Trigalloyl-HHDP-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 78 | Galloyl-penta-hydroxy-benzoic-brevifolincarboxyl-glucose isomer 1 | + | + | ND | + | + | + | + | ND | ND |
| 79 | Pentagalloyl-glucose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 80 | Trisgalloyl HHDP glucose isome isomer 1 | + | + | ND | ND | ND | ND | ND | ND | ND |
| 81 | Amurensisin | + | + | ND | + | + | + | ND | ND | ND |
| 82 | Trigalloyl-DHHDP-glucose isomer 2 | ND | + | ND | + | ND | + | ND | ND | ND |
| 83 | Digalloyl-lactonised valoneoyl-d-glucose isomer 4 | + | + | ND | + | ND | ND | ND | ND | ND |
| 84 | Pentagalloyl-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 85 | Galloyl-penta-hydroxy-benzoic-brevifolincarboxyl-glucose isomer 2 | + | + | ND | + | + | + | ND | ND | ND |
| 86 | Trigalloyl-brevifolincarboxyl-glucose isomer 4 | + | + | ND | + | + | ND | ND | ND | ND |
| 87 | Phyllanthusiin C isomer 1 | + | + | ND | + | + | + | ND | ND | ND |
| 88 | Trigalloyl-DHHDP-glucose isomer 3 | + | + | ND | ND | + | + | ND | ND | ND |
| 89 | Ethyl gallate | ND | ND | ND | + | + | + | ND | ND | ND |
| 90 | Trigalloyl-brevifolincarboxyl-glucose isomer 5 | + | + | ND | + | + | + | ND | ND | ND |
| 91 | Trisgalloyl HHDP glucose isome isomer 2 | ND | + | ND | ND | ND | + | ND | ND | ND |
| 92 | Trigalloyl-lactonised valoneoyl glucose isomer 2 | + | + | ND | + | ND | ND | ND | ND | ND |
| 93 | (Galloyl)galloyl-tetragalloylglucose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 94 | Digalloyl-HHDP-iso DHDG-glucose isomer 1 | + | + | ND | + | ND | ND | ND | ND | ND |
| 95 | Balanophotannin E isomer 3 | ND | + | ND | + | + | ND | ND | ND | ND |
| 96 | Isorhamnetin glucoside isomer 2 | ND | + | ND | + | + | ND | ND | ND | ND |
| 97 | Hexagalloyl-glucose isomer 1 | + | + | ND | + | + | + | + | ND | ND |
| 98 | Trigalloyl-lactonised valoneoyl glucose isomer 3 | + | + | ND | + | + | ND | ND | ND | ND |
| 99 | Ellagic acid derivative | + | + | ND | ND | ND | + | ND | ND | ND |
| 100 | (Galloyl)galloyl-tetragalloylglucose isomer 3 | + | + | ND | + | + | + | ND | ND | ND |
| 101 | (Galloyl)galloyl-tetragalloylglucose isomer 4 | + | + | ND | + | + | + | ND | ND | ND |
| 102 | Galloyl-penta-hydroxy-benzoic-brevifolincarboxyl-glucose isomer 3 | ND | + | ND | + | + | + | ND | ND | ND |
| 103 | Hexagalloyl-glucose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 104 | Galloyl-HHDP-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 105 | Hexagalloyl-glucose isomer 3 | + | + | ND | + | + | + | + | ND | ND |
| 106 | Digalloyl-HHDP-iso DHDG-glucose isomer 2 | ND | + | ND | ND | + | + | ND | ND | ND |
| 107 | Tetragalloyl-hydroxybenzoyl-glucopyranoside isomer 1 | ND | + | ND | + | + | ND | ND | ND | ND |
| 108 | Heptagalloyl hexose isomer 1 | + | + | ND | + | + | + | ND | ND | ND |
| 109 | Galloylmyricetin | + | + | ND | + | + | + | + | ND | ND |
| 110 | Heptagalloyl hexose isomer 2 | + | + | ND | + | + | + | + | ND | ND |
| 111 | Phyllanthusiin C isomer 2 | ND | + | ND | ND | + | + | ND | ND | ND |
| 112 | Digalloyl-HHDP-iso DHDG-glucose isomer 3 | ND | + | ND | ND | + | + | ND | ND | ND |
| 113 | Trigalloyl-brevifolincarboxyl-glucose isomer 6 | ND | + | ND | + | + | ND | ND | ND | ND |
| 114 | Heptagalloyl hexose isomer 3 | + | + | ND | + | + | + | ND | ND | ND |
| 115 | Tetragalloyl-hydroxybenzoyl-glucopyranoside isomer 2 | + | + | ND | + | + | + | ND | ND | ND |
| 116 | Tetragalloyl-hydroxybenzoyl-glucopyranoside isomer 3 | + | + | ND | + | ND | + | ND | ND | ND |
| 117 | Unknown 7 | ND | ND | ND | + | ND | ND | ND | ND | ND |
| 118 | Trihydroxy-octadecenoic acid | ND | + | + | ND | ND | ND | ND | ND | ND |
| 119 | Hydroxyretinoic acid | + | ND | + | ND | ND | ND | ND | ND | ND |
| 120 | Unknown 8 | + | + | + | ND | ND | ND | ND | ND | ND |
| 121 | Hexadecanedioic acid | + | + | + | ND | ND | ND | ND | ND | ND |
| 122 | Hydroxyeicosatrienoic acid | + | + | + | + | ND | ND | ND | ND | ND |
| 123 | Valerenic acid | + | + | + | + | ND | ND | ND | ND | ND |
| 124 | Hydroxylinoleic acid | + | + | + | ND | ND | ND | ND | ND | ND |
| 125 | Linoleic acid | ND | + | + | + | ND | ND | ND | ND | ND |
| 126 | Hydroxylinolenic acid | + | + | + | + | + | ND | ND | ND | ND |
| 127 | Dodecenyl-succinic anhydride | + | + | + | + | ND | ND | ND | ND | ND |
| 128 | Retinoic acid | + | + | + | ND | ND | ND | ND | ND | ND |
| 129 | Oleic acid | + | + | + | + | + | ND | ND | + | ND |
| 130 | Pentadecenoic acid | + | + | + | + | + | ND | ND | ND | ND |
| 131 | Oleiyl glucoside | ND | + | + | ND | ND | ND | ND | ND | ND |
| 132 | Linolenic acid | + | + | + | + | + | + | ND | ND | ND |
| 133 | Stearic acid | + | + | ND | + | ND | ND | ND | ND | ND |
| 134 | Unknown 9 | + | + | + | + | + | ND | ND | ND | ND |
| 135 | Unknown 10 | + | + | + | + | ND | ND | ND | ND | ND |
| 136 | Eicosapentaenoic acid | + | + | + | + | ND | + | ND | ND | ND |
| 137 | Palmitoleic acid | + | + | + | ND | + | + | + | ND | + |
| 138 | Linoleic acid | + | + | + | + | + | + | ND | ND | + |
| 139 | Oxodecanedioic acid | + | ND | ND | ND | ND | ND | ND | ND | ND |
| 140 | Methyl arachidonate | + | + | + | + | ND | ND | ND | ND | ND |
| 141 | Arjungenin | ND | ND | + | ND | ND | ND | ND | ND | ND |
| 142 | Heptadecenoic acid | + | ND | ND | ND | + | + | ND | ND | ND |
| 143 | Unknown 11 | ND | + | + | + | ND | ND | ND | ND | ND |
| 144 | Unknown 12 | + | + | + | + | + | + | ND | ND | ND |
| 145 | Glycerylmonooleate | + | ND | ND | ND | ND | ND | ND | ND | ND |
| 146 | Palmitic acid | + | ND | ND | + | + | + | + | + | + |
| 147 | Hydroxydocosanoic acid | + | + | + | + | + | ND | ND | ND | ND |
| 148 | Dodecenylsuccinic acid | ND | + | ND | ND | + | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Cádiz-Gurrea, M.d.l.L.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Carretero, A.S.; Momotko, M.; Yildiztugay, E.; Karatas, R.; Jugreet, S.; Mahomoodally, M.F.; et al. Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles. Molecules 2022, 27, 5788. https://doi.org/10.3390/molecules27185788
Zengin G, Cádiz-Gurrea MdlL, Fernández-Ochoa Á, Leyva-Jiménez FJ, Carretero AS, Momotko M, Yildiztugay E, Karatas R, Jugreet S, Mahomoodally MF, et al. Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles. Molecules. 2022; 27(18):5788. https://doi.org/10.3390/molecules27185788
Chicago/Turabian StyleZengin, Gokhan, María de la Luz Cádiz-Gurrea, Álvaro Fernández-Ochoa, Francisco Javier Leyva-Jiménez, Antonio Segura Carretero, Malwina Momotko, Evren Yildiztugay, Refik Karatas, Sharmeen Jugreet, Mohamad Fawzi Mahomoodally, and et al. 2022. "Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles" Molecules 27, no. 18: 5788. https://doi.org/10.3390/molecules27185788
APA StyleZengin, G., Cádiz-Gurrea, M. d. l. L., Fernández-Ochoa, Á., Leyva-Jiménez, F. J., Carretero, A. S., Momotko, M., Yildiztugay, E., Karatas, R., Jugreet, S., Mahomoodally, M. F., & Boczkaj, G. (2022). Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles. Molecules, 27(18), 5788. https://doi.org/10.3390/molecules27185788












