Formation of Lutein, β-Carotene and Astaxanthin in a Coelastrella sp. Isolate
Abstract
:1. Introduction
2. Results and Discussion
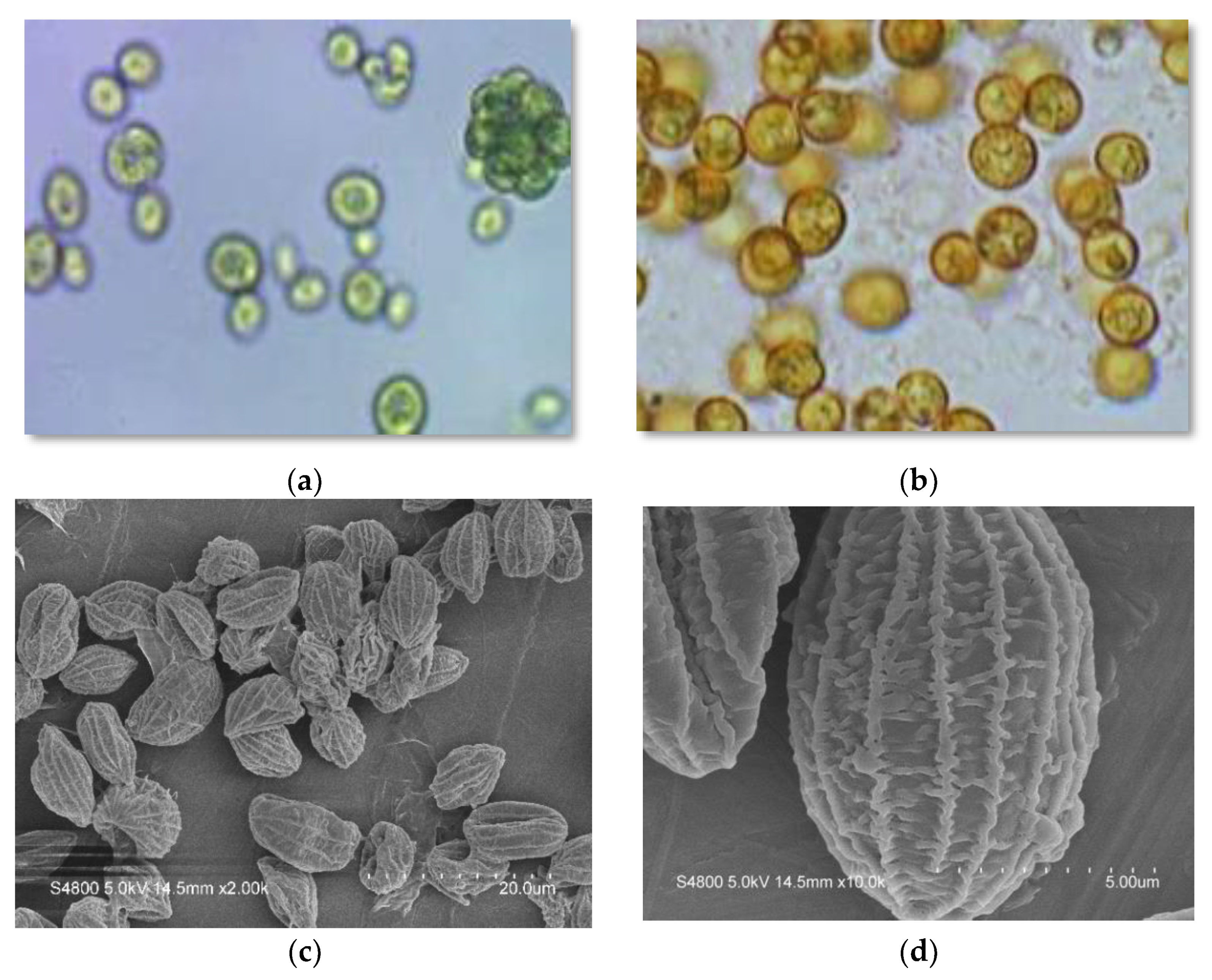
2.1. Taxonomic Putative Identification
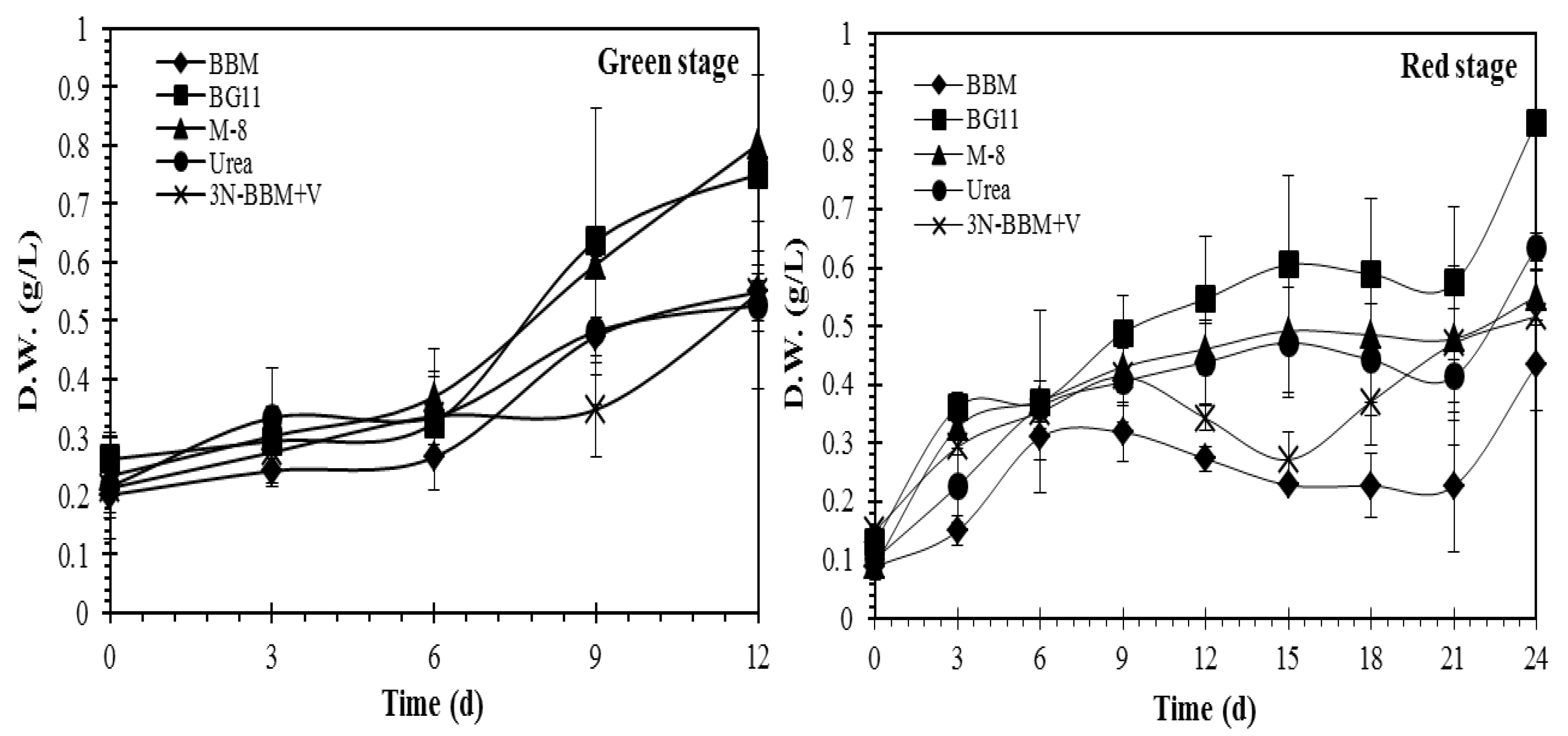
2.2. Media Screening
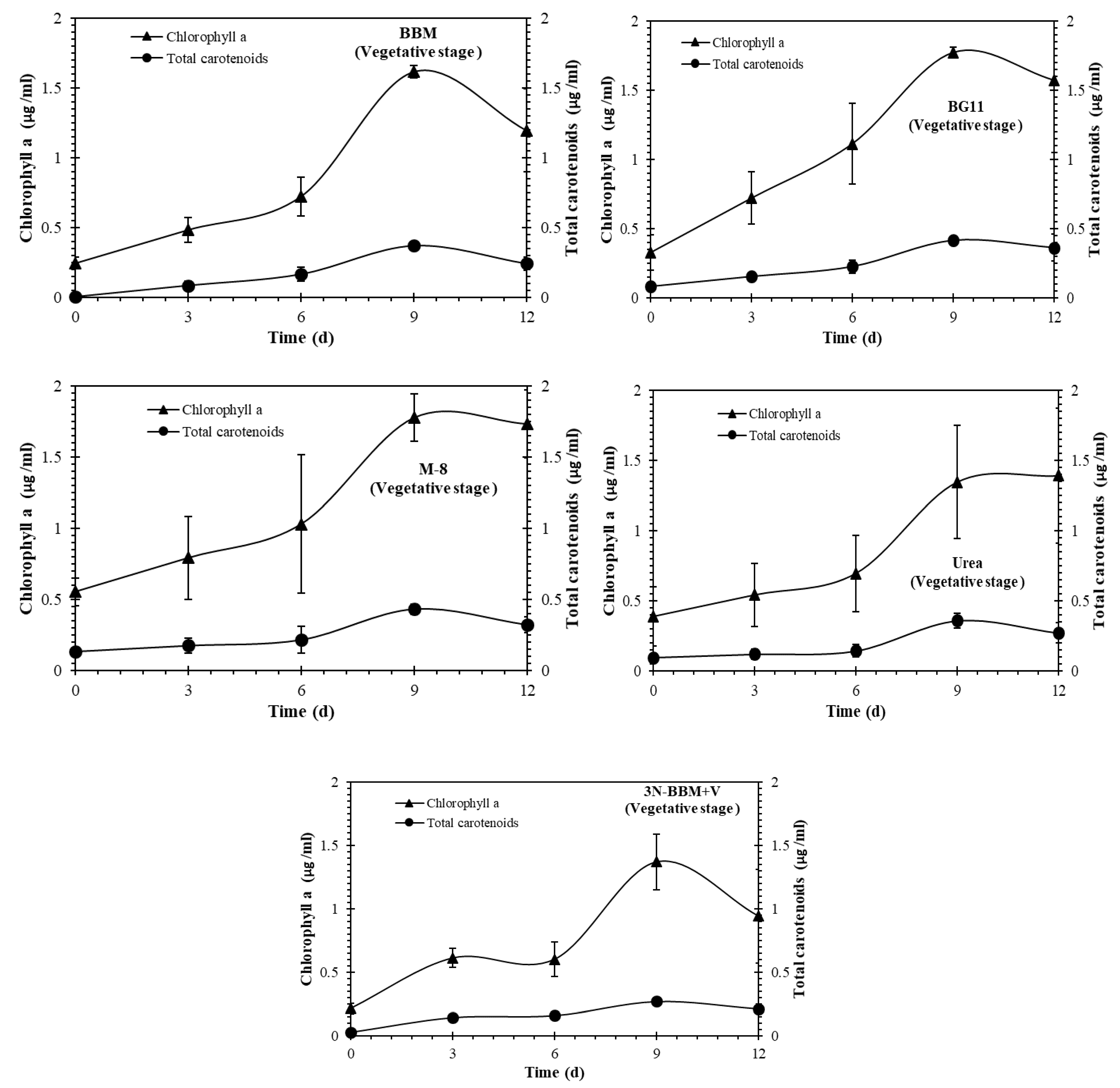
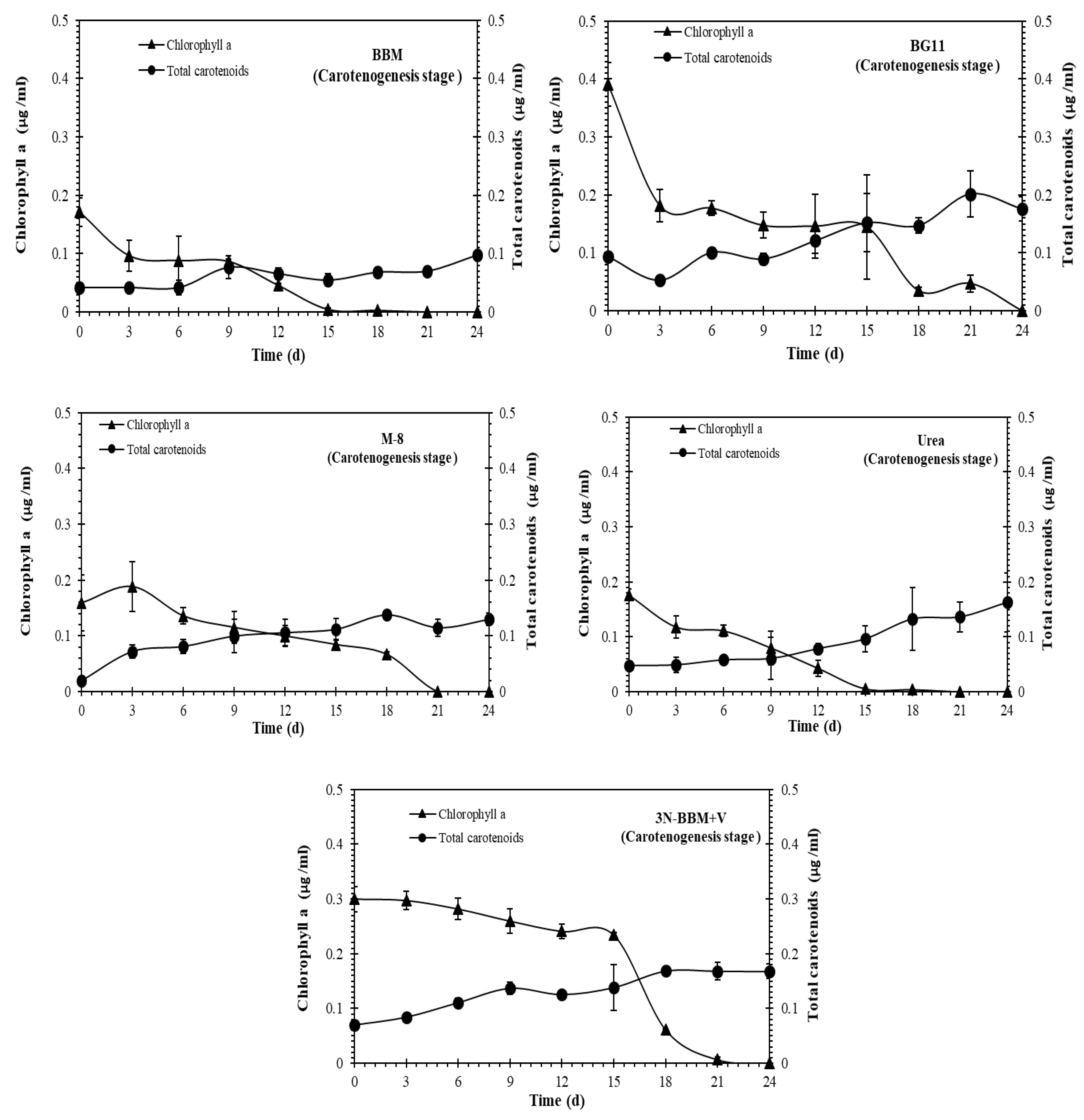
2.3. Effect of N and P Concentrations on Growth and Effect of Stressing Conditions on Carotenoid Production
2.4. Lipid Production
3. Materials and Methods
3.1. Reagents
3.2. Molecular Identification
3.3. Optical Microscopy and SEM Analysis
3.4. Protocol for Media Screening Experiments
3.5. Protocol to Test Effect of Nitrogen and Phosphorus Concentrations on Growth and Stressing Conditions on Carotenoid Production
3.6. Growth Measurements
3.6.1. Optical Density (OD)
3.6.2. Dry Weight (DW)
3.7. Pigment Extraction and Quantification
3.8. Pigment Analysis by High Performance Liquid Chromatography (HPLC)
3.9. Lipid, Protein and Total Carbohydrate Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- März, U. FOD025C-The Global Market for Carotenoids. BCC Res. 2008. [Google Scholar]
- Bazilian, M.; Davis, R.T.; Pienkos, P.; Arent, D. The energy-water-food nexus through the lens of algal systems. Ind. Biotechnol. 2013, 9, 158–162. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Durn, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraloni, C.; Torzillo, G. Synthesis of Antioxidant Carotenoids in Microlagae in Response to Physiological Stress. In Carotenoids; Cvetkovic, D., Nikolic, G., Eds.; IntechOpen: London, UK, 2017; Chapter 9; pp. 143–157. [Google Scholar]
- Céron, M.C.; Campos, I.; Sánchez, J.F.; Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M. Recovery of Lutein from Microalgae Biomass: Development of a Process for Scenedesmus almeriensis Biomass. J. Agric. Food Chem. 2008, 65, 11761–11766. [Google Scholar] [CrossRef]
- Mohler, D.; Wilson, M.H.; Kesner, S.; Schambach, J.Y.; Vaughan, D.; Frazar, M.; Stewart, J.; Groppo, J.; Pace, R.; Crocker, M. Beneficial re-use of CO2 emissions using microalgae: Demonstration assessment and biomass characterization. Bioresour. Technol. 2019, 293, 122014. [Google Scholar] [CrossRef]
- Wilson, M.H.; Mohler, D.T.; Groppo, J.G.; Grubbs, T.; Kesner, S.; Frazar, E.M.; Shea, A.; Crofcheck, C.; Crocker, M. Capture and recycle of industrial CO2 emissions using microalgae. Appl. Petrochem. Res. 2016, 6, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.H.; Groppo, J.; Placido, A.; Graham, S.; Morton, S.A., III; Santillan-Jimenez, E.; Shea, A.; Crocker, M.; Crofcheck, C.; Andrews, R. CO2 recycling using microalgae for the production of fuels. Appl. Petrochem. Res. 2014, 4, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chivkunova, O.; Chekanov, K.; Lobakova, E.; Sidorov, R. pH and CO2 effects on Coelastrella (Scotiellopsis) rubescens growth and metabolism. Russ. J. Plant Phys. 2016, 63, 566–574. [Google Scholar] [CrossRef]
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen Effect Exerted by Secondary Carotenoids and Mycosporine-like Amino Acids in the Aeroterrestrial Chlorophyte Coelastrella rubescens under High Light and UV-A Irradiation. Plants 2021, 10, 2601. [Google Scholar] [CrossRef]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Iyer, G.; Nagle, V.; Gupte, Y.V.; Desai, S.; Iyer, M.; Moramkar, N.; Sawant, V. Characterization of high carotenoid producing Coelastrella oocystiformis and its anti-cancer potential. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 527–536. [Google Scholar]
- Hu, C.-W.; Chuang, L.-T.; Yu, P.-C.; Chen, C.-N.N. Pigment production by a new thermotolerant microalga Coelastrella sp. F50. Food Chem. 2013, 138, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, S.; Sangeetha, R.; Ariraman, S.; Ashokkumar, B.; Varalakshmi, P. Photocatalyst (TiO2) as an enhancer: An attempt to enhance the production of carotenoids and lipids with the combined oxidative stresses in Coelastrella sp. M60. Clean Technol. Environ. Policy 2021, 23, 41–53. [Google Scholar] [CrossRef]
- Aburai, N.; Ohkubo, S.; Miyashita, H.; Abe, K. Composition of carotenoids and identification of aerial microalgae isolated from the surface of rocks in mountainous districts of Japan. Algal Res. 2013, 2, 237–243. [Google Scholar] [CrossRef]
- Doppler, P.; Kriechbaum, R.; Käfer, M.; Kopp, J.; Remias, D.; Spadiut, O. Coelastrella terrestris for Adonixanthin Production: Physiological Characterization and Evaluation of Secondary Carotenoid Productivity. Mar. Drugs 2022, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, S.; Yoshida, R.; Ohkoshi, K.; Toyoshima, H. Coelastrella astaxanthina sp. nov. (Sphaeropleales, Chlorophyceae), a novel microalga isolated from an asphalt surface in midsummer in Japan. Phycol. Res. 2019, 68, 107–114. [Google Scholar] [CrossRef]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Sashidhar, B.; Adholeya, A. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour. Technol. 2016, 211, 556–565. [Google Scholar] [CrossRef]
- Corato, A.; Le, T.T.; Baurain, D.; Jacques, P.; Remacle, C.; Franck, F. A Fast-Growing Oleaginous Strain of Coelastrella Capable of Astaxanthin and Canthaxanthin Accumulation in Phototrophy and Heterotrophy. Life 2022, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Goecke, F.; Noda, J.; Paliocha, M.; Gislerød, H.R. Revision of Coelastrella (Scenedesmaceae, Chlorophyta) and first register of this green coccoid microalga for continental Norway. World J. Microbiol. Biotechnol. 2020, 36, 149. [Google Scholar] [CrossRef]
- Chang, Y.; Chu, T.; Yu, P.; Wang, E.; Huang, C.; Hu, T.; Wen, Z.; Ko, C.; Chen, C.N.; Tai, M. Microalgal extract from thermotolerant Coelastrella sp. F50 retards the liver tumor progression by targeting hepatic cancer stem cells. Phytother. Res. 2021, 35, 3954–3967. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Liu, X.; Liu, B.; Hu, Z.; Liu, G. Morphology and molecular phylogeny of coccoid green algae Coelastrella sensu lato (Scenedesmaceae, Sphaeropeales), including the description of three new species and two new varieties. J. Phycol. 2019, 55, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, H.; Liu, X.; Zhu, H.; Hu, Z.; Liu, G. Deep genomic analysis of Coelastrella saipanensis (Scenedesmaceae, Chlorophyta): Comparative chloroplast genomics of Scenedesmaceae. Eur. J. Phycol. 2019, 54, 52–65. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin Accumulation in the Green Alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A Review on the Assessment of Stress Conditions for Simultaneous Production of Microalgal Lipids and Carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeki, K.; Aburai, N.; Aratani, S.; Miyashita, H.; Abe, K. Salt-stress and plant hormone-like responses for selective reactions of esterified xanthophylls in the aerial microalga Coelastrella sp. KGU-Y002. J. Appl. Phycol. 2017, 29, 115–122. [Google Scholar] [CrossRef]
- Yoshida, K.; Igarashi, E.; Wakatsuki, E.; Miyamoto, K.; Hirata, K. Mitigation of osmotic and salt stresses by abscisic acid through reduction of stress-derived oxidative damage in Chlamydomonas reinhardtii. Plant Sci. 2004, 167, 1335–1341. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Maurya, R.; Trivedi, K.; Patidar, S.K.; Ghosh, A.; Mishra, S. Salinity induced oxidative stress enhanced biofuel production potential of microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 189, 341–348. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotech. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Quinn, M.; Van Wychen, S.; Templeton, D.W.; Wolfrum, E.J. Accurate and reliable quantification of total microalgal fuel potential as fatty acid methyl esters by in situ transesterification. Anal. Bioanal. Chem. 2012, 403, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Crofcheck, C.; E, X.; Shea, A.; Montross, M.; Crocker, M.; Andrews, R. Influence of media composition on the growth rate of Chlorella vulgaris and Scenedesmus acutus utilized for CO2 mitigation. J. Biochem. Tech. 2012, 4, 589–594. [Google Scholar]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Mandalam, R.K.; Palsson, B. Elemental balancing of biomass and medium composition enhances growth capacity in high-density Chlorella vulgaris cultures. Biotechnol. Bioeng. 1998, 59, 605–611. [Google Scholar] [CrossRef]
- Gachon, C.M.; Day, J.G.; Campbell, C.N.; Pröschold, T.; Saxon, R.J.; Küpper, F.C. The Culture Collection of Algae and Protozoa (CCAP): A biological resource for protistan genomics. Gene 2007, 406, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.W.; Bold, H.C. Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas: Austin, TX, USA, 1963. [Google Scholar]
- Vidyashankar, S.; VenuGopal, K.S.; Swarnalatha, G.V.; Kavitha, M.D.; Chauhan, V.S.; Ravi, R.; Sarada, R. Characterization of fatty acids and hydrocarbons of chlorophycean microalgae towards their use as biofuel source. Biomass Bioenerg 2015, 77, 75–91. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Phys. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Jin, E.; Polle, J.E.; Melis, A. Involvement of zeaxanthin and of the Cbr protein in the repair of photosystem II from photoinhibition in the green alga Dunaliella salina. BBA-Bioenerg. 2001, 1506, 244–259. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-Y.; Min, B.-S.; Chang, C.-S.; Jin, E. Isolation and Characterization of a Xanthophyll Aberrant Mutant of the Green Alga Nannochloropsis oculata. Mar. Biotechnol. 2006, 8, 238–245. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Van Wychen, S.; McAllister, J.P.; Arrowsmith, S.; Dempster, T.A.; McGowen, J.; Pienkos, P.T. Strain, biochemistry, and cultivation-dependent measurement variability of algal biomass composition. Anal. Biochem. 2014, 452, 86–95. [Google Scholar] [CrossRef]
- Van Wychen, S.; Laurens, L.M.L. Determination of Total Carbohydrates in Algal Biomass: Laboratory Analytical Procedure (LAP). NREL/TP-5100-60957, 2016. Available online: https://www.osti.gov/biblio/1118073 (accessed on 1 September 2022).

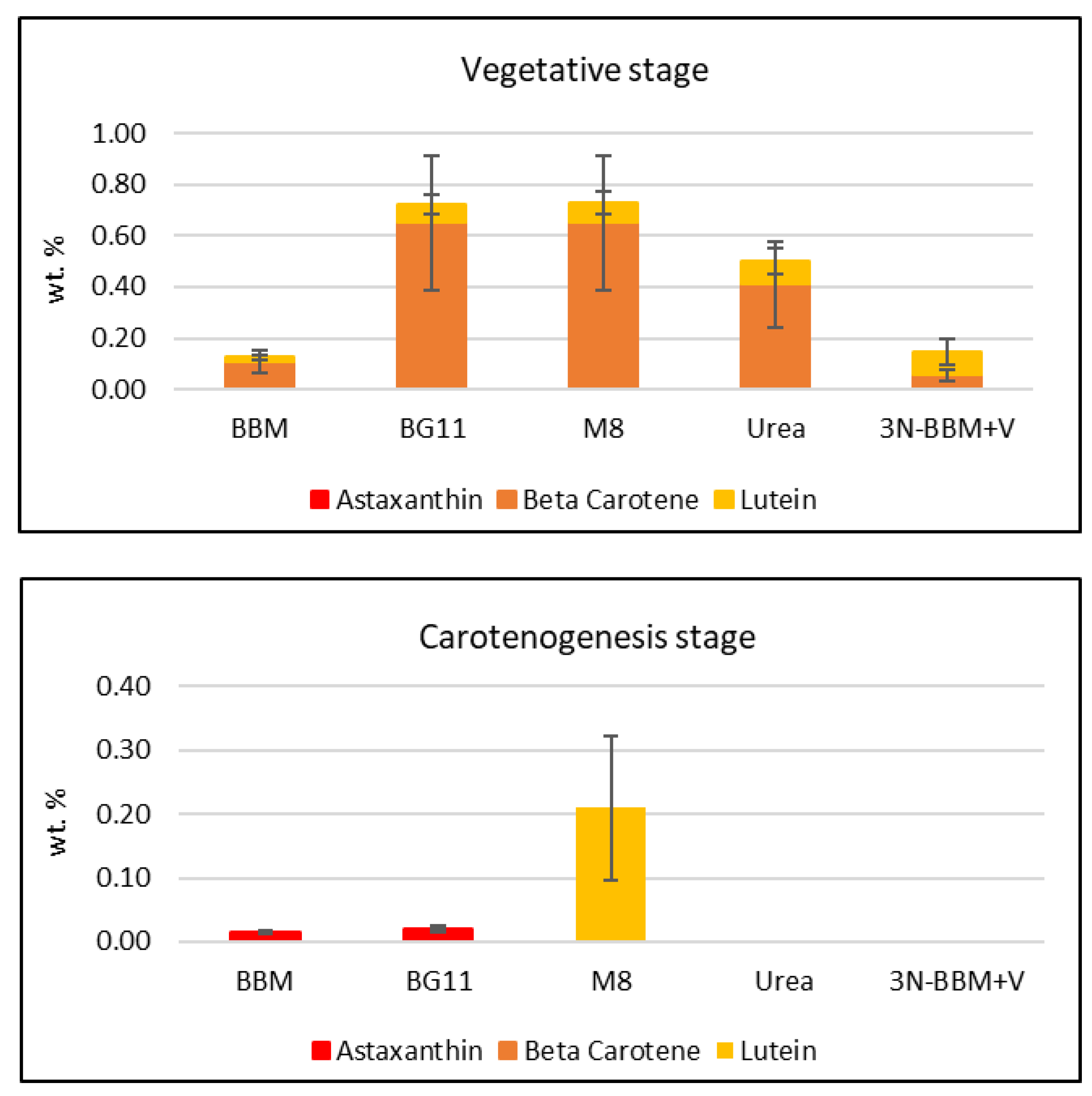
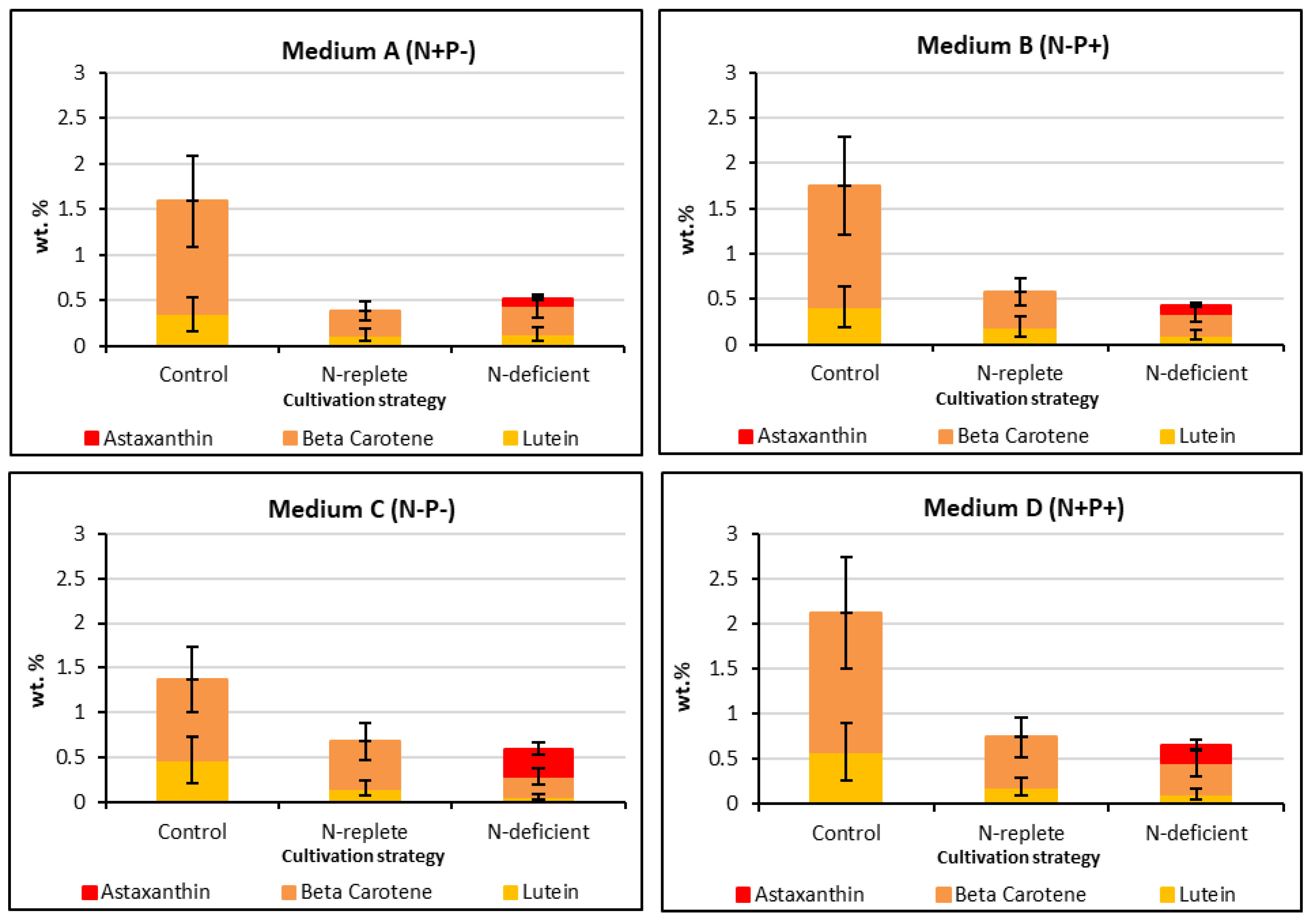
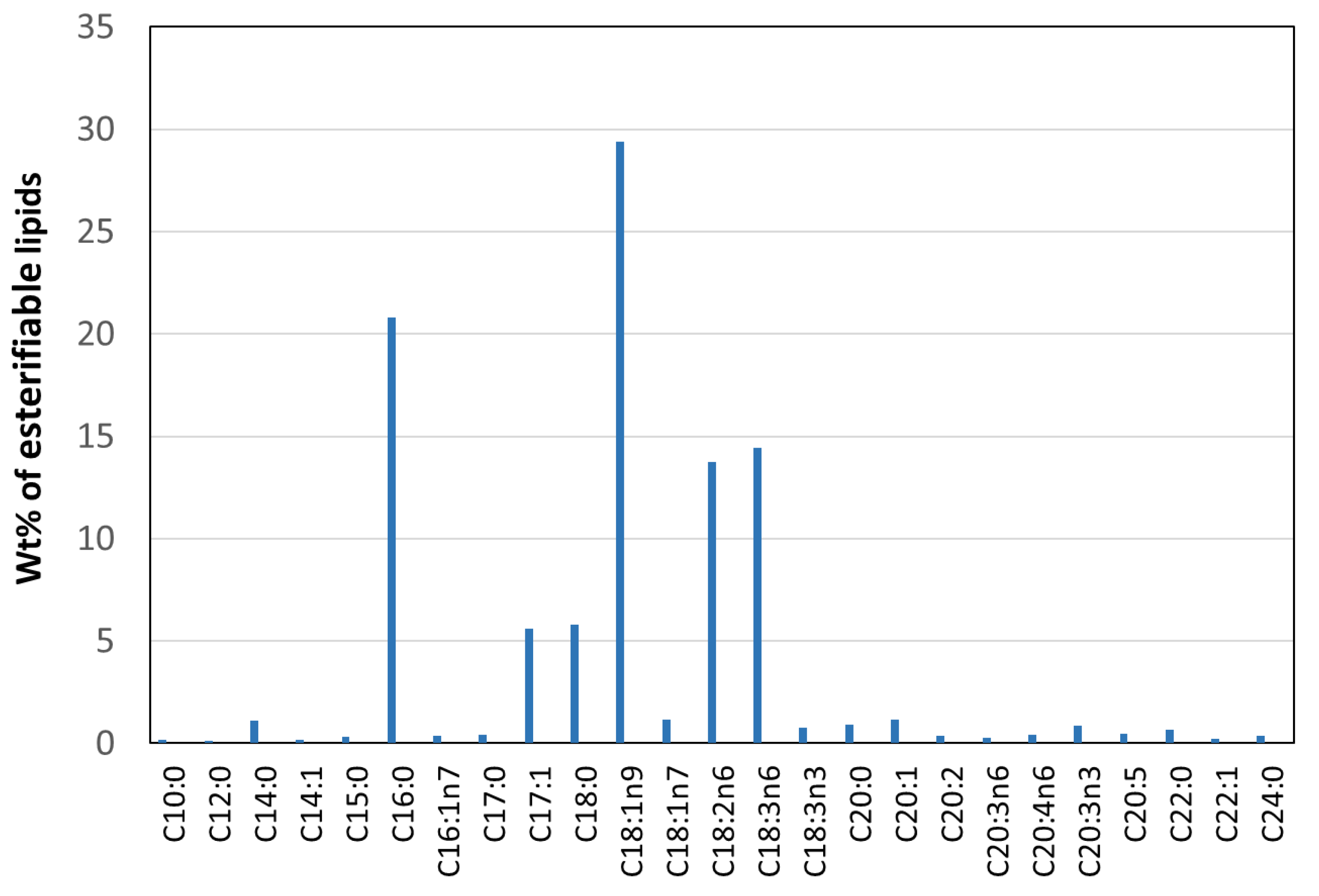
| Media * | Growth Stage | Biomass Productivity (mg L−1d−1) | Specific Growth Rate (µ) (d−1) | Doubling Time (td) (d) | Biomass Yield (g L−1) |
|---|---|---|---|---|---|
| BBM | vegetative stage | 29.00 ± 1.01 b | 0.083 ± 0.002 ab | 8.31 ± 0.17 ab | 0.35 ± 0.01 b |
| BG-11 | 39.34 ± 2.57 a | 0.087± 0.004 ab | 7.96 ± 0.41 ab | 0.49 ± 0.04 a | |
| M-8 | 47.25 ± 3.48 a | 0.102 ± 0.009 a | 6.80 ± 0.69 b | 0.57 ± 0.04 a | |
| Urea | 25.83 ± 4.58 b | 0.074 ± 0.010 b | 9.49 ± 1.21 a | 0.31 ± 0.06 b | |
| 3N-BBM+V | 27.72 ± 4.49 b | 0.078 ± 0.009 b | 8.99 ± 1.80 ab | 0.33 ± 0.05 b | |
| BBM/BG-11 | carotenogenesis stage | 14.37 ± 3.42 c | 0.066 ± 0.011 ab | 10.66 ± 1.96 b | 0.36 ± 0.08 c |
| BG-11/BG-11 | 29.82 ± 0.74 a | 0.077 ± 0.001 a | 8.93 ± 0.14 b | 0.72 ± 0.02 a | |
| M-8/BG-11 | 19.13 ± 1.91 bc | 0.075 ± 0.003 a | 9.26 ± 0.38 b | 0.46 ± 0.05 bc | |
| Urea/BG-11 | 22.22± 1.07 b | 0.076 ± 0.004 a | 9.09 ± 0.50 b | 0.53 ± 0.03 b | |
| 3N-BBM+V/ BG-11 | 15.28 ± 2.97 c | 0.051 ± 0.005 b | 13.60 ± 1.21 a | 0.37 ± 0.07 c |
| Media Combinations | Carotenogenesis Strategy | Biomass Productivity (mg L−1 d−1) | Specific Growth Rate (u) (d−1) | Doubling Time (td) (d) | Biomass Yield (g L−1) |
|---|---|---|---|---|---|
| Medium A | Control | 47.78 ± 2.13 a | 0.092 ± 0.003 a | 7.52 ± 0.25 b | 1.29 ± 0.06 a |
| N-replete | 34.84 ± 2.76 b | 0.081 ± 0.003 b | 8.50 ± 0.34 b | 0.94 ± 0.07 b | |
| N-deficient | 31.97 ± 1.85 b | 0.079 ± 0.004 b | 8.80 ± 0.41 a | 0.86 ± 0.05 b | |
| Medium B | Control | 48.33 ± 1.48 a | 0.095 ± 0.002 a | 7.29 ± 0.16 b | 1.31 ± 0.04 a |
| N-replete | 46.48 ± 2.43 a | 0.094 ± 0.003 a | 7.39 ± 0.27 b | 1.26 ± 0.07 a | |
| N-deficient | 30.68 ± 2.63 b | 0.079 ± 0.004 b | 8.70 ± 0.47 a | 0.82 ± 0.07 b | |
| Medium C | Control | 31.97 ± 2.69 a | 0.083 ± 0.005 a | 8.35 ± 0.53 b | 0.86 ± 0.07 a |
| N-replete | 20.22 ± 1.76 b | 0.068 ± 0.004 b | 10.14 ± 0.55 a | 0.55 ± 0.05 b | |
| N-deficient | 15.59 ± 1.60 b | 0.061 ± 0.004 b | 11.49 ± 0.83 a | 0.42 ± 0.04 b | |
| Medium D | Control | 45.64 ± 1.74 a | 0.101 ± 0.004 a | 6.85 ± 0.29 b | 1.23 ± 0.05 a |
| N-replete | 36.56 ± 2.67 b | 0.093 ±0.004 ab | 7.42 ± 0.34 ab | 0.99 ± 0.07 b | |
| N-deficient | 32.31 ± 0.92 b | 0.089 ± 0.004 b | 7.76 ± 0.37 a | 0.87 ± 0.02 b |
| DNA Concentration (ng/µL) | Final Library DNA Concentration (ng/µL) | Average Library Size (bp) |
|---|---|---|
| 1.5 | 15.00 | 673 |
| Media | NO3 (g/L) | Urea (g/L) | PO4 (g/L) | N/P Ratio |
|---|---|---|---|---|
| BBM | 0.25 | - | 0.25 | 1.0 |
| BG-11 | 1.5 | - | 0.04 | 37.5 |
| M-8 | 3.0 | - | 1.0 | 3.0 |
| Urea | - | 0.55 | 0.12 | 4.58 |
| 3N-BBM+V | 0.75 | - | 0.15 | 5.05 |
| Concentration Combinations | NaNO3 (g/L) | K2HPO4 (g/L) | N/P Ratio |
|---|---|---|---|
| (A) N-replete + P-deficient | 1.5 | 0.04 | 37.5 |
| (B) N-deficient + P-replete | 0.38 | 0.16 | 2.38 |
| (C) N-deficient + P-deficient | 0.38 | 0.04 | 9.5 |
| (D) N-replete + P-replete | 1.5 | 0.16 | 9.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.E.A.; Vorisek, F.; Dowd, S.E.; Kesner, S.; Song, Y.; Qian, D.; Crocker, M. Formation of Lutein, β-Carotene and Astaxanthin in a Coelastrella sp. Isolate. Molecules 2022, 27, 6950. https://doi.org/10.3390/molecules27206950
Ali HEA, Vorisek F, Dowd SE, Kesner S, Song Y, Qian D, Crocker M. Formation of Lutein, β-Carotene and Astaxanthin in a Coelastrella sp. Isolate. Molecules. 2022; 27(20):6950. https://doi.org/10.3390/molecules27206950
Chicago/Turabian StyleAli, Hamdy Elsayed Ahmed, Fritz Vorisek, Scot E. Dowd, Stephanie Kesner, Yang Song, Dali Qian, and Mark Crocker. 2022. "Formation of Lutein, β-Carotene and Astaxanthin in a Coelastrella sp. Isolate" Molecules 27, no. 20: 6950. https://doi.org/10.3390/molecules27206950
APA StyleAli, H. E. A., Vorisek, F., Dowd, S. E., Kesner, S., Song, Y., Qian, D., & Crocker, M. (2022). Formation of Lutein, β-Carotene and Astaxanthin in a Coelastrella sp. Isolate. Molecules, 27(20), 6950. https://doi.org/10.3390/molecules27206950







