Lignin-Derivative Ionic Liquids as Corrosion Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Characterization
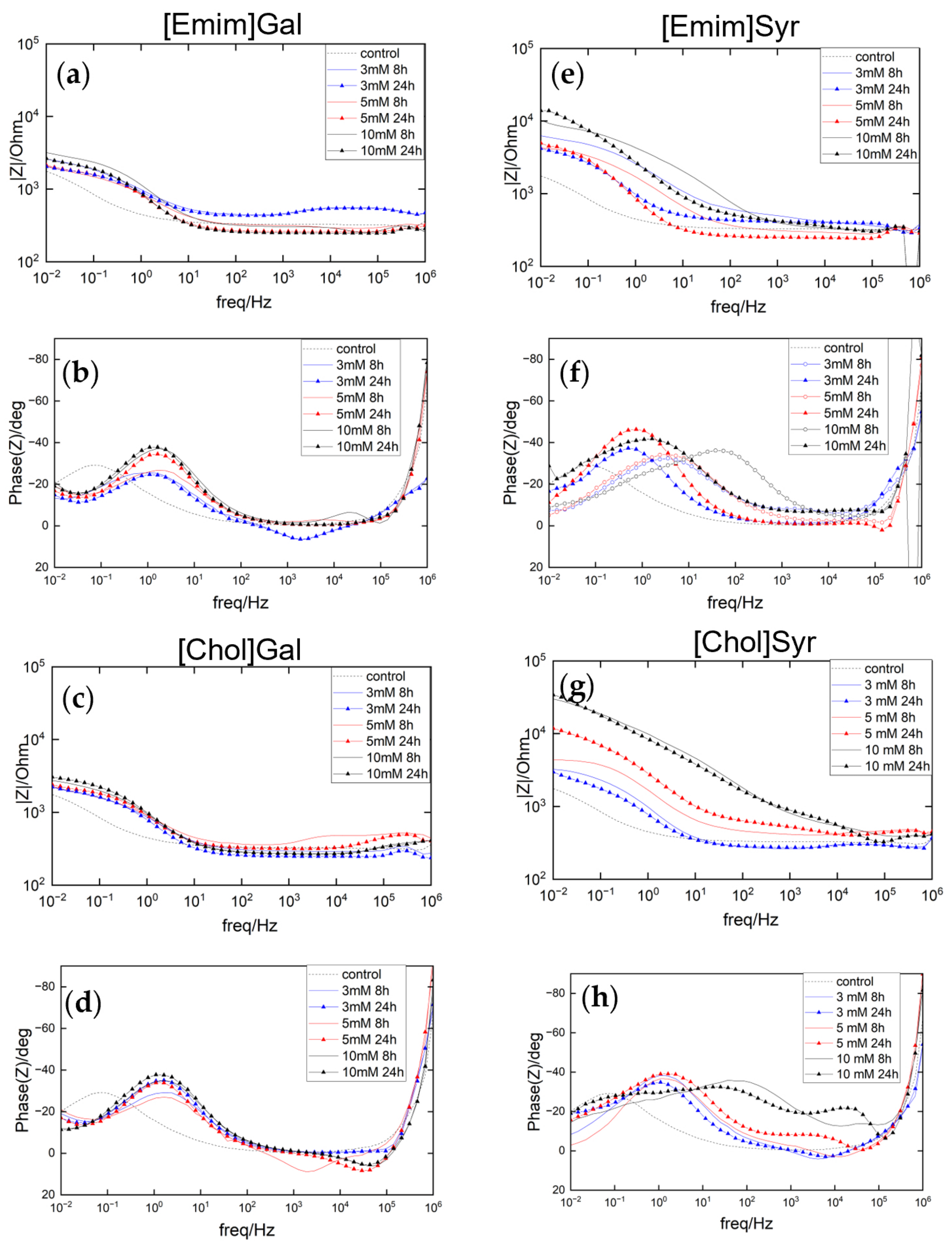
2.2. Electrochemical Characterization
2.2.1. Potentiostatic Electrochemical Impedance Spectroscopy (PEIS)
2.2.2. Cyclic Potentiodynamic Polarization (CPP)
2.3. Surface Characterization
3. Materials and Methods
3.1. Materials
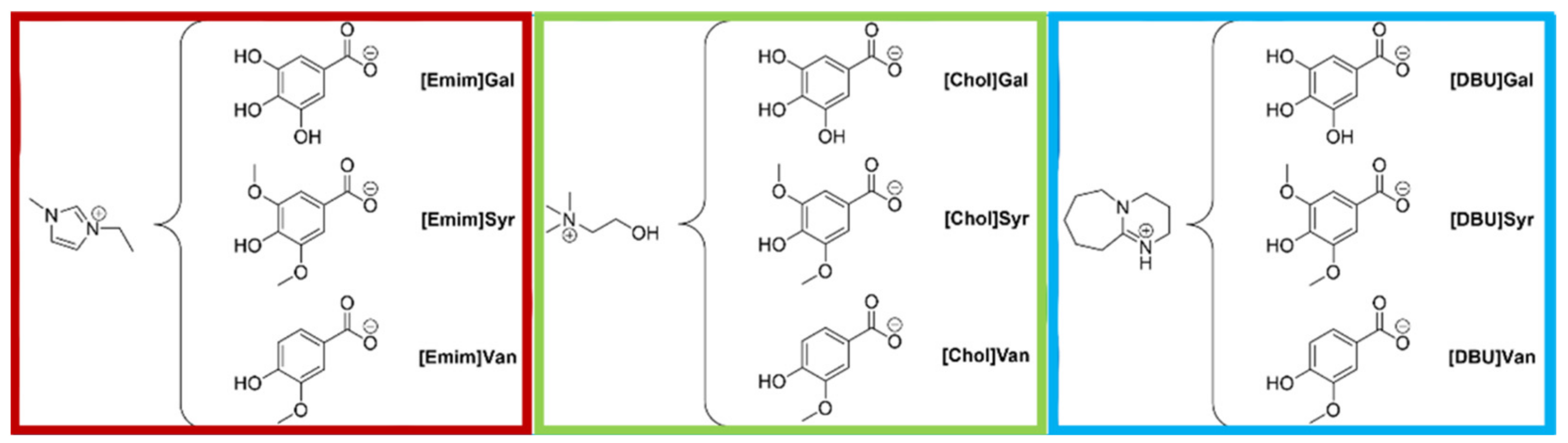
3.2. Synthetic Pathways
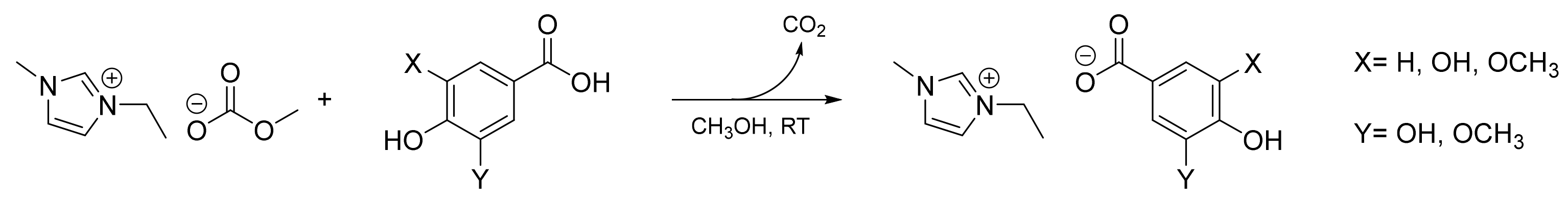
3.2.1. General Procedure for the Synthesis of Imidazolium Lignin-Based ILs
3-Ethyl-1-methyl-1H-imidazol-3 ium Gallate ([Emim]Gal)
3-Ethyl-1-methyl-1H-imidazol-3-ium Syringate ([Emim]Syr)
3-Ethyl-1-methyl-1H-imidazol-3-ium Vanillate ([Emim]Van)
3.2.2. General Procedure for the Synthesis of Cholinium Lignin-Based ILs
Trimethyl-β-hydroxyethyl-ammonium Syringate ([Chol]Syr)
Trimethyl-β-hydroxyethyl-ammonium Vanillate ([Chol]Van)
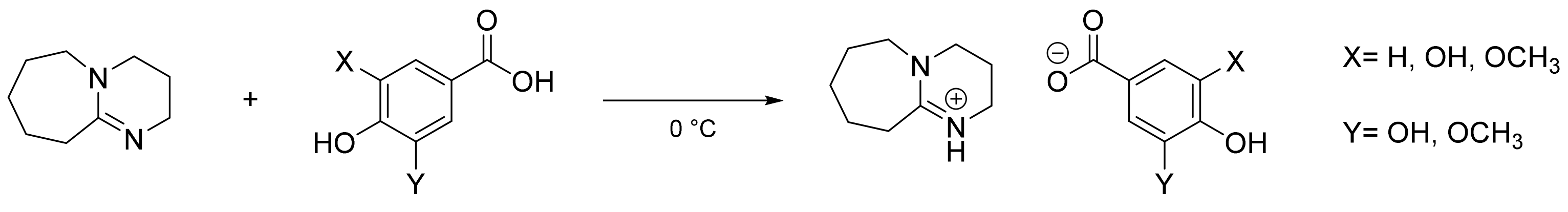
3.2.3. General Procedure for the Synthesis of Protic Lignin-Based ILs
1,5-Diazabicyclo [5.4.0]undec-7-eneium Gallate ([DBU]Gal)
1,5-Diazabicyclo [5.4.0]undec-7-eneium Syringate ([DBU]Syr)
1,5-Diazabicyclo [5.4.0]undec-7-eneium Vanillate ([DBU]Van)
3.3. Characterization Methods
3.3.1. Thermal Gravimetric Analysis (TGA)
3.3.2. Differential Scanning Calorimetry (DSC)
3.3.3. PEIS and CPP
3.3.4. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
3.3.5. Optical Microscope
3.3.6. Optical Profilometer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent developments in sustainable corrosion inhibitors: Design, performance and industrial scale applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Ma, A.W.; Ammar, S.; Kumar, S.S.A.; Ramesh, K.; Ramesh, S. A concise review on corrosion inhibitors: Types, mechanisms and electrochemical evaluation studies. J. Coat. Technol. Res. 2022, 19, 241–268. [Google Scholar] [CrossRef]
- Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from natural sources and biomass wastes. Molecules 2019, 24, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Heidarshenas, B.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K. Green Inhibitors for Steel Corrosion in Acidic Environment: State of Art. In Materials Today Sustainability; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 10. [Google Scholar] [CrossRef]
- Thomas, P.A.; Marvey, B.B. Room temperature ionic liquids as green solvent alternatives in the metathesis of oleochemical feedstocks. Molecules 2016, 21, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zullo, V.; Iuliano, A.; Guazzelli, L. Sugar-based ionic liquids: Multifaceted challenges and intriguing potential. Molecules 2021, 26, 2052. [Google Scholar] [CrossRef] [PubMed]
- Picchio, M.L.; Minudri, D.; Mantione, D.; Criado-Gonzalez, M.; Guzmán-González, G.; Schmarsow, R.; Müller, A.J.; Tomé, L.C.; Minari, R.J.; Mecerreyes, D. Natural Deep Eutectic Solvents Based on Choline Chloride and Phenolic Compounds as Efficient Bioadhesives and Corrosion Protectors. ACS Sustain. Chem. Eng. 2022, 10, 8135–8142. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Kumar, S.; Bahadur, I.; Verma, C.; Ebenso, E.E. Organic corrosion inhibitors for industrial cleaning of ferrous and non-ferrous metals in acidic solutions: A review. J. Mol. Liq. 2018, 256, 565–573. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Organic Corrosion Inhibitors. In Corrosion Inhibitors, Principles and Recent Applications; InTech: London, UK, 2018; p. 13. [Google Scholar] [CrossRef] [Green Version]
- Malinowski, S.; Wróbel, M.; Woszuk, A. Quantum chemical analysis of the corrosion inhibition potential by aliphatic amines. Materials 2021, 14, 6197. [Google Scholar] [CrossRef] [PubMed]
- TSintra, E.; Abranches, D.O.; Benfica, J.; Soares, B.P.; Ventura, S.P.M.; Coutinho, J.A.P. Cholinium-based ionic liquids as bioinspired hydrotropes to tackle solubility challenges in drug formulation. Eur. J. Pharm. Biopharm. 2021, 164, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Sales, I.; Abranches, D.; Sintra, T.E.; Mattedi, S.; Freire, M.G.; Coutinho, J.A.; Pinho, S.P. Selection of hydrotropes for enhancing the solubility of artemisinin in aqueous solutions. Fluid Phase Equilibria 2022, 562, 113556. [Google Scholar] [CrossRef]
- Kwolek, P.; Dychtoń, K.; Kościelniak, B.; Obłój, A.; Podborska, A.; Wojnicki, M. Gallic Acid as a Potential Green Corrosion Inhibitor for Aluminum in Acidic Solution. Metals 2022, 12, 250. [Google Scholar] [CrossRef]
- Obot, B.; Madhankumar, A. Enhanced corrosion inhibition effect of tannic acid in the presence of gallic acid at mild steel/HCl acid solution interface. J. Ind. Eng. Chem. 2015, 25, 105–111. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Tan, Y.J.; Bailey, S.; Kinsella, B. An investigation of the formation and destruction of corrosion inhibitor films using electrochemical impedance spectroscopy (EIS). Corros. Sci. 1996, 38, 1545–1561. [Google Scholar] [CrossRef]
- Fazary, E.; Taha, M.; Ju, Y.H. Iron complexation studies of gallic acid. J. Chem. Eng. Data 2009, 54, 35–42. [Google Scholar] [CrossRef]
- Mero, A.; Guglielmero, L.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L.; Koutsoumpos, S.; Tsonos, G.; Stavrakas, I.; Moutzouris, K.; Mezzetta, A. Influence of the cation partner on levulinate ionic liquids properties. J. Mol. Liq. 2022, 354, 118850. [Google Scholar] [CrossRef]
- Sintra, T.E.; Luís, A.; Rocha, S.N.; Ferreira, A.I.M.C.L.; Gonçalves, F.; Santos, L.M.N.B.F.; Neves, B.M.; Freire, M.G.; Ventura, S.P.M.; Coutinho, J.A.P. Enhancing the Antioxidant Characteristics of Phenolic Acids by Their Conversion into Cholinium Salts. ACS Sustain. Chem. Eng. 2015, 3, 2558–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2011.





| TGA | DSC | ||||
|---|---|---|---|---|---|
| ILs | Tstart (°C) | Tonset (°C) | Tpeak (°C) | Tm (°C) | Tg (°C) |
| [Emim]Gal | 201.0 | 189.8 253.5 | 202.4 280.33 | - | - |
| [Emim]Syr | 175.8 | 204.2 | 225.21 | 148.0 | 5.99 |
| [Emim]Van | 174.0 | 204.1 | 230.69 | −1.17 | |
| [Chol]Gal | 201.5 | 215.4 | 263.0 | 135.8 | 22.29 |
| [Chol]Syr | 178.2 | 186.5 | 206.4 | 157.4 | 18.63 |
| [Chol]Van | 204.7 | 212.14 | 223.4 | 181.6 | - |
| [DBU]Gal | 201.0 | 201.03 264.91 | 214.2 284.3 | 76.37 a | - |
| [DBU]Syr | 190.0 | 202.3 | 219.7 | - | - |
| [DBU]Van | 204.1 | 210.9 | 221.4 | - | - |
| Solution | MW | Concentration (mM) | Ecorr (mV) | icorr (µA/cm2) | IE (%) |
|---|---|---|---|---|---|
| control | 100 | −604 | 1.457 | - | |
| [Emim]Gal | 3 | −695 | 1.032 | 29 | |
| 280.28 | 5 | −689 | 0.923 | 37 | |
| 10 | −712 | 0.830 | 43 | ||
| [Emim]Syr | 3 | −471 | 1.170 | 20 | |
| 308.33 | 5 | −468 | 0.974 | 33 | |
| 10 | −522 | 0.312 | 79 | ||
| [Chol]Gal | 3 | −696 | 1.051 | 28 | |
| 273.29 | 5 | −684 | 0.733 | 49 | |
| 10 | −706 | 0.902 | 38 | ||
| [Chol]Syr | 3 | −486 | 1.151 | 21 | |
| 301.34 | 5 | −578 | 0.227 | 85 | |
| 10 | −491 | 0.066 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaci, S.; Minudri, D.; Guazzelli, L.; Mezzetta, A.; Mecerreyes, D.; Forsyth, M.; Somers, A. Lignin-Derivative Ionic Liquids as Corrosion Inhibitors. Molecules 2023, 28, 5568. https://doi.org/10.3390/molecules28145568
Monaci S, Minudri D, Guazzelli L, Mezzetta A, Mecerreyes D, Forsyth M, Somers A. Lignin-Derivative Ionic Liquids as Corrosion Inhibitors. Molecules. 2023; 28(14):5568. https://doi.org/10.3390/molecules28145568
Chicago/Turabian StyleMonaci, Sharon, Daniela Minudri, Lorenzo Guazzelli, Andrea Mezzetta, David Mecerreyes, Maria Forsyth, and Anthony Somers. 2023. "Lignin-Derivative Ionic Liquids as Corrosion Inhibitors" Molecules 28, no. 14: 5568. https://doi.org/10.3390/molecules28145568
APA StyleMonaci, S., Minudri, D., Guazzelli, L., Mezzetta, A., Mecerreyes, D., Forsyth, M., & Somers, A. (2023). Lignin-Derivative Ionic Liquids as Corrosion Inhibitors. Molecules, 28(14), 5568. https://doi.org/10.3390/molecules28145568










