Identification and Quantitation of the Bioactive Components in Wasted Aralia elata Leaves Extract with Endothelial Protective Activity
Abstract
:1. Introduction
2. Results and Discussion
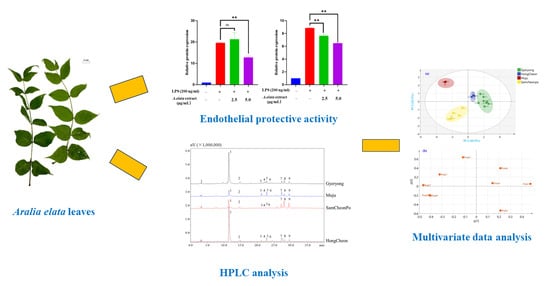
2.1. Cytotoxic Effects of LAE Extract in HUVECs
2.2. LAE Extract Attenuates Inflammatory Signaling Pathways in HUVECs
2.3. Chemical Profiling of Triterpenoid Saponins in LAE Extract
| No. | RT a (min) | Positive Mode (m/z) | Negative Mode (m/z) | MW b | Trivial Name | MS2, Ions m/z (Relative Abundance, %) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 32.13 | 1300.80 [M+ NH4]+ | 1327.75 [M + HCOOH − H]− | 1282.8 | Congmuyenoside III | 671.2 (25), 455.3 (75) | [39] |
| 2 | 36.57 | 1305.85 [M + Na]+ | 1327.90 [M + HCOOH − H]− | 1282.9 | Congmuyenoside G | 691.2 (30), 455.3 (70) | [39] |
| 3 | 39.42 | 981.60 [M + Na]+ | 1003.65 [M + HCOOH − H]− | 958.7 | unknown | 509.2 (15), 471.3 (85) | - |
| 4 | 40.28 | 981.70 [M + Na]+ | 1003.70 [M + HCOOH − H]− | 958.7 | Ecalbasaponin III | 673.4 (70), 493.3 (30) | [40] |
| 5 | 42.16 | 981.70 [M + Na]+ | 1003.70 [M + HCOOH − H]− | 958.7 | Quinoasaponin 2 | 819.5 (65), 455.3 (35) | [36] |
| 6 | 47.73 | 819.60 [M + NH4]+ | 841.70 [M + HCOOH − H]− | 796.6 | unknown | 641.4 (85), 439.4 (15) | - |
| 7 | 48.22 | 1108.75 [M + NH4]+ | 1089.23 [M − H]− | 1090.7 | Congmuyenoside IX | 1143.4 (80), 455.3 (20) | [41] |
| 8 | 50.21 | 1284.90 [M + NH4]+ | 1266.10 [M − H]− | 1266.9 | Congmuyenoside X | 1127.6 (85), 455.3 (15) | [41] |
| 9 | 51.09 | 1122.70 [M + NH4]+ | 1149.85 [M + HCOOH − H]− | 1104.9 | Aralia Saponin V | 981.5 (70), 455.4 (30) | [42] |
| 10 | 53.68 | 1122.70 [M + NH4]+ | 1103.90 [M + HCOOH − H]− | 1104.8 | Araloside G | 997.48 (60), 455.4 (40) | [43] |
| 11 | 54.08 | 965.60 [M + Na]+ | 987.55 [M + HCOOH − H]− | 942.6 | Silphioside | 803.5 (65), 439.4 (35) | [43] |
| 12 | 54.52 | 960.65 [M + NH4]+ | 987.55 [M + HCOOH − H]− | 942.6 | unknown | 641.4 (70, 439.4 (30) | - |
| 13 | 57.72 | 960.65 [M + NH4]+ | 987.55 [M + HCOOH − H]− | 942.6 | unknown | 657.4 (80), 455.4 (20) | - |
| 14 | 59.76 | 817.60 [M + NH4]+ | 793.75 [M − H]− | 794.8 | unknown | 643.4 (75), 455.4 (25) | - |
2.4. Changes in the Chemical Constituents in the Leaves of A. elata from Different Seasons and Cities
2.5. HCA Heatmap Analysis
2.6. Principal Component Analysis
2.7. Partial-Least-Squares Discriminate Analysis
3. Materials and Methods
3.1. Plant Materials
3.2. Chemical and Reagents
3.3. Preparation of Leaf Extracts
3.4. HPLC–DAD–ESI–MS/MS Analysis
3.5. Quality Control of LAE
3.6. Cell Culture
3.7. Cell Viability Assay
3.8. Western Blotting
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Levy, R.I.; Moskowitz, J. Cardiovascular research: Decades of progress, a decade of promise. Science 1982, 217, 121–129. [Google Scholar] [CrossRef]
- Zhou, Q.; Han, X.; Li, R.; Zhao, W.; Bai, B.; Yan, C.; Dong, X. Anti-atherosclerosis of oligomeric proanthocyanidins from Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function. Phytomedicine 2018, 51, 171–180. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegetarian dietary patterns and cardiovascular disease. Prog. Cardiovasc. Dis. 2018, 61, 54–61. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Wu, W.; Shi, C.; Hu, S.; Yin, T.; Ma, Q.; Han, T.; Zhang, Y.; Tian, F. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca2+–XO–ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic. Biol. Med. 2016, 95, 278–292. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K.; Leducq Transatlantic Network on Atherothrombosis. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaperonis, E.; Liapis, C.; Kakisis, J.; Dimitroulis, D.; Papavassiliou, V. Inflammation and atherosclerosis. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 386–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Sacanella, E.; Badia, E.; Antúnez, E.; Nicolás, J.M.; Fernández-Solá, J.; Rotilio, D.; De Gaetano, G.; Rubin, E.; Urbano-Márquez, A. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: A prospective randomized crossover trial: Effects of wine on inflammatory markers. Atherosclerosis 2004, 175, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Palmefors, H.; DuttaRoy, S.; Rundqvist, B.; Börjesson, M. The effect of physical activity or exercise on key biomarkers in atherosclerosis—A systematic review. Atherosclerosis 2014, 235, 150–161. [Google Scholar] [CrossRef]
- Hein, T.W.; Singh, U.; Vasquez-Vivar, J.; Devaraj, S.; Kuo, L.; Jialal, I. Human C-reactive protein induces endothelial dysfunction and uncoupling of eNOS in vivo. Atherosclerosis 2009, 206, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Teoh, H.; Quan, A.; Lovren, F.; Wang, G.; Tirgari, S.; Szmitko, P.E.; Szalai, A.J.; Ward, M.E.; Verma, S. Impaired endothelial function in C-reactive protein overexpressing mice. Atherosclerosis 2008, 201, 318–325. [Google Scholar] [CrossRef]
- Yosimichi, G.; Nakanishi, T.; Nishida, T.; Hattori, T.; Takano-Yamamoto, T.; Takigawa, M. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK). Eur. J. Biochem. 2001, 268, 6058–6065. [Google Scholar] [CrossRef] [PubMed]
- Ijomone, O.M.; Iroegbu, J.D.; Aschner, M.; Bornhorst, J. Impact of environmental toxicants on p38-and ERK-MAPK signaling pathways in the central nervous system. Neurotoxicology 2021, 86, 166–171. [Google Scholar] [CrossRef]
- Lim, H.-S.; Kim, Y.J.; Kim, B.-Y.; Jeong, S.-J. Bakuchiol suppresses inflammatory responses via the downregulation of the p38 MAPK/ERK signaling pathway. Int. J. Mol. Sci. 2019, 20, 3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudaliar, H.; Rayner, B.; Billah, M.; Kapoor, N.; Lay, W.; Dona, A.; Bhindi, R. Remote ischemic preconditioning attenuates EGR-1 expression following myocardial ischemia reperfusion injury through activation of the JAK-STAT pathway. Int. J. Cardiol. 2017, 228, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Bolli, R.; Dawn, B.; Xuan, Y.-T. Role of the JAK–STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc. Med. 2003, 13, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Zhou, G.; Zhang, X.; Zhang, W.; Cai, L.; Zhao, C. Protective effect of total aralosides of Aralia elata (Miq) Seem (TASAES) against diabetic cardiomyopathy in rats during the early stage, and possible mechanisms. Exp. Mol. Med. 2009, 41, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, X.; Xu, H.; Wen, F.; Zhang, X.; Sun, H.; Yao, F.; Sun, G.; Sun, X. Effect of the total saponins of Aralia elata (Miq) Seem on cardiac contractile function and intracellular calcium cycling regulation. J. Ethnopharmacol. 2014, 155, 240–247. [Google Scholar] [CrossRef]
- Hwang, K.-A.; Hwang, Y.-J.; Kim, G.R.; Choe, J.-S. Extracts from Aralia elata (Miq) Seem alleviate hepatosteatosis via improving hepatic insulin sensitivity. BMC Complement. Altern. Med. 2015, 15, 347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, H.; Xue, Y.; Zheng, Q. Cardioprotective and antioxidant activities of a polysaccharide from the root bark of Aralia elata (Miq.) Seem. Carbohydr. Polym. 2013, 93, 442–448. [Google Scholar] [CrossRef]
- Luo, Y.; Dong, X.; Yu, Y.; Sun, G.; Sun, X. Total aralosides of Aralia elata (Miq) Seem (TASAES) ameliorate nonalcoholic steatohepatitis by modulating IRE1α-mediated JNK and NF-κB pathways in ApoE–/–mice. J. Ethnopharmacol. 2015, 163, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-q.; Zhao, H.-t.; Zhang, X.-l.; Zhang, W.-t.; Liu, X.-c.; Gao, S.-h. Comparison of different extraction techniques and optimization of the microwave-assisted extraction of saponins from Aralia elata (Miq.) Seem fruits and rachises. Chem. Pap. 2020, 74, 3077–3087. [Google Scholar] [CrossRef]
- Luo, Y.; Lu, S.; Ai, Q.; Zhou, P.; Qin, M.; Sun, G.; Sun, X. SIRT1/AMPK and Akt/eNOS signaling pathways are involved in endothelial protection of total aralosides of Aralia elata (Miq) Seem against high-fat diet-induced atherosclerosis in ApoE−/− mice. Phytother. Res. 2019, 33, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xie, W.; Luo, Y.; Lu, S.; Dai, Z.; Wang, R.; Sun, G.; Sun, X. Protective effects of total saponins of Aralia elata (Miq.) on endothelial cell injury induced by TNF-α via modulation of the PI3K/Akt and NF-κB signalling pathways. Int. J. Mol. Sci. 2018, 20, 36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, W.; He, H.; Song, X.-y.; Yao, G.-d.; Song, S.-j. Triterpene saponins with neuroprotective effects from a wild vegetable Aralia elata. J. Funct. Foods 2018, 45, 313–320. [Google Scholar] [CrossRef]
- Wang, W.; Yao, G.-D.; Shang, X.-Y.; Gao, J.-C.; Zhang, Y.; Song, S.-J. Eclalbasaponin I from Aralia elata (Miq.) Seem. reduces oxidative stress-induced neural cell death by autophagy activation. Biomed. Pharmacother. 2018, 97, 152–161. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Meng, Y.; Sun, Y.; Wang, Q.; Yang, C.; Wang, Q.; Yang, B.; Kuang, H. Determination and pharmacokinetic study of two triterpenoid saponins in rat plasma after oral administration of the extract of Aralia elata leaves by UHPLC–ESI–MS/MS. J. Chromatogr. B 2015, 985, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tao, Y.; Jiang, Y. Apelin activates the expression of inflammatory cytokines in microglial BV2 cells via PI-3K/Akt and MEK/Erk pathways. Sci. China Life Sci. 2015, 58, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.-L.; Yu, X.-J.; Feng, Y.-Q.; Yang, Y.; Hu, H.-B.; Zhao, Y.-Y.; Zhang, J.-H.; Liu, K.-L.; Zhang, Y.; Fu, L.-Y. Luteolin Attenuates Hypertension via Inhibiting NF-κB-Mediated Inflammation and PI3K/Akt Signaling Pathway in the Hypothalamic Paraventricular Nucleus. Nutrients 2023, 15, 502. [Google Scholar] [CrossRef]
- An, W.; Yang, J.; Ao, Y. Metallothionein mediates cardioprotection of isoliquiritigenin against ischemia-reperfusion through JAK2/STAT3 activation. Acta Pharmacol. Sin. 2006, 27, 1431–1437. [Google Scholar] [CrossRef]
- Burysek, L.; Syrovets, T.; Simmet, T. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J. Biol. Chem. 2002, 277, 33509–33517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesaga, M.; Pyrzynska, K. Liquid chromatography/tandem mass spectrometry studies of the phenolic compounds in honey. J. Chromatogr. A 2009, 1216, 6620–6626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Y.; Li, L.; Zhao, L.; Hu, Y.; Hu, C.; Song, S. Studies on cytotoxic triterpene saponins from the leaves of Aralia elata. Food Chem. 2013, 138, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.X.; Wang, Z.B.; Wang, Q.H.; Yang, B.Y.; Xiao, H.B.; Okada, Y.; Okuyama, T. Triterpene glucosides from the leaves of Aralia elata and their cytotoxic activities. Chem. Biodivers. 2013, 10, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, X.; Niu, W.; Feng, Y.; Bian, J.; Xiao, H. Acute and sub-chronic toxicity study of the ethanol extract from leaves of Aralia elata in rats. J. Ethnopharmacol. 2015, 175, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Timalsina, D.; Devkota, H.P. Eclipta prostrata (L.) L.(Asteraceae): Ethnomedicinal uses, chemical constituents, and biological activities. Biomolecules 2021, 11, 1738. [Google Scholar] [CrossRef]
- Mroczek, A. Phytochemistry and bioactivity of triterpene saponins from Amaranthaceae family. Phytochem. Rev. 2015, 14, 577–605. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Zeng, Y.; Jin, S.; Liu, W.; Li, Z.; Qin, X.; Bai, Y. Traditional uses, phytochemistry, pharmacology, toxicity and quality control of medicinal genus Aralia: A review. J. Ethnopharmacol. 2022, 284, 114671. [Google Scholar]
- Han, F.; Liang, J.; Yang, B.-Y.; Kuang, H.-X.; Xia, Y.-G. Identification and comparison of triterpene saponins in Aralia elata leaves and buds by the energy-resolved MSAll technique on a liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2021, 203, 114176. [Google Scholar] [CrossRef]
- Qi, M.; Hua, X.; Peng, X.; Yan, X.; Lin, J. Comparison of chemical composition in the buds of Aralia elata from different geographical origins of China. R. Soc. Open Sci. 2018, 5, 180676. [Google Scholar] [CrossRef] [Green Version]
- Petrochenko, A.A.; Orlova, A.; Frolova, N.; Serebryakov, E.B.; Soboleva, A.; Flisyuk, E.V.; Frolov, A.; Shikov, A.N. Natural Deep Eutectic Solvents for the Extraction of Triterpene Saponins from Aralia elata var. mandshurica (Rupr. & Maxim.) J. Wen. Molecules 2023, 28, 3614. [Google Scholar]
- Guo, M.; Zhang, L.; Liu, Z. Analysis of saponins from leaves of Aralia elata by liquid chromatography and multi-stage tandem mass spectrometry. Anal. Sci. 2009, 25, 753–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, H.; Ri, H.C.; An, Z.; Wang, X.; Zhou, J.-N.; Zheng, D.; Wu, H.; Wang, P.; Yang, J. Deletion and tandem duplications of biosynthetic genes drive the diversity of triterpenoids in Aralia elata. Nat. Commun. 2022, 13, 2224. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vinh, L.B.; Cho, C.W.; Cho, K.W.; Kim, Y.H.; Kang, J.S. Discrimination and quality evaluation of fifteen components in Stauntonia hexaphylla leaves from different harvest time by HPLC–PDA–ESI–MS/MS and ELSD coupled with multivariate statistical analysis and anti-inflammatory activity evaluation. Appl. Biol. Chem. 2020, 63, 60. [Google Scholar] [CrossRef]
- Gao, D.; Cho, C.-W.; Kim, J.-H.; Bao, H.; Kim, H.-M.; Li, X.; Kang, J.-S. Phenolic Profile and Fingerprint Analysis of Akebia quinata Leaves Extract with Endothelial Protective Activity. Molecules 2022, 27, 4636. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G. Aralia elata var. mandshurica (Rupr. & Maxim.) J. Wen: An overview of pharmacological studies. Phytomedicine 2016, 23, 1409–1421. [Google Scholar]
- Solar, A.; Colarič, M.; Usenik, V.; Stampar, F. Seasonal variations of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.). Plant Sci. 2006, 170, 453–461. [Google Scholar] [CrossRef]
- Trigui, M.; Gasmi, L.; Zouari, I.; Tounsi, S. Seasonal variation in phenolic composition, antibacterial and antioxidant activities of Ulva rigida (Chlorophyta) and assessment of antiacetylcholinesterase potential. J. Appl. Phycol. 2013, 25, 319–328. [Google Scholar] [CrossRef]
- Souhila, T.; Fatma Zohra, B.; Tahar, H.S. Identification and quantification of phenolic compounds of Artemisia herba-alba at three harvest time by HPLC–ESI–Q-TOF–MS. Int. J. Food Prop. 2019, 22, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Formato, M.; Scharenberg, F.; Pacifico, S.; Zidorn, C. Seasonal variations in phenolic natural products in Fagus sylvatica (European beech) leaves. Phytochemistry 2022, 203, 113385. [Google Scholar] [CrossRef]
- Fang, R.; Redfern, S.P.; Kirkup, D.; Porter, E.A.; Kite, G.C.; Terry, L.A.; Berry, M.J.; Simmonds, M.S. Variation of theanine, phenolic, and methylxanthine compounds in 21 cultivars of Camellia sinensis harvested in different seasons. Food Chem. 2017, 220, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, T.; Xavier, V.; Falcão, M.; Mondin, C.; Dos Santos, M.; Cassel, E.; Astarita, L.; Santarém, E. Seasonal changes in phenolic compounds and in the biological activities of Baccharis dentata (Vell.) GM Barroso. Ind. Crops Prod. 2013, 51, 355–359. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and successive harvests interaction affects phenolic acids and aroma profile of genovese basil for pesto sauce production. Foods 2021, 10, 278. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, B.; Sun, J.; Fan, X.; Li, X.; Jiang, L.; Hao, G.; Pei, H.; Zhou, X. Heat shock treatment promoted callus formation on postharvest sweet potato by adjusting active oxygen and phenylpropanoid metabolism. Agriculture 2022, 12, 1351. [Google Scholar] [CrossRef]
- Lim, D.W.; Han, T.; Jung, J.; Song, Y.; Um, M.Y.; Yoon, M.; Kim, Y.T.; Cho, S.; Kim, I.H.; Han, D. Chlorogenic Acid from Hawthorn berry (Crataegus pinnatifida fruit) prevents stress hormone-induced depressive behavior, through monoamine oxidase b-reactive oxygen species signaling in hippocampal astrocytes of mice. Mol. Nutr. Food Res. 2018, 62, 1800029. [Google Scholar] [CrossRef]
- Shimomura, M.; Yoshida, H.; Fujiuchi, N.; Ariizumi, T.; Ezura, H.; Fukuda, N. Continuous blue lighting and elevated carbon dioxide concentration rapidly increase chlorogenic acid content in young lettuce plants. Sci. Hortic. 2020, 272, 109550. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Rasiukevičiūtė, N.; Viršilė, A.; Miliauskienė, J.; Laužikė, K.; Valiuškaitė, A.; Dėnė, L.; Chrapačienė, S. Phenolic compounds content evaluation of lettuce grown under short-term preharvest daytime or nighttime supplemental LEDs. Plants 2022, 11, 1123. [Google Scholar] [CrossRef]
- Wargent, J.; Nelson, B.; McGhie, T.; Barnes, P. Acclimation to UV-B radiation and visible light in L actuca sativa involves up-regulation of photosynthetic performance and orchestration of metabolome-wide responses. Plant Cell Environ. 2015, 38, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.J.; Baumann, T.W. Distribution and utilization of chlorogenic acid in Coffea seedlings. J. Exp. Bot. 1994, 45, 497–503. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Soliman, M.H.; Abdulmajeed, A.M.; Alhaithloul, H.; Alharbi, B.M.; El-Esawi, M.A.; Hasanuzzaman, M.; Elkelish, A. Saponin biopriming positively stimulates antioxidants defense, osmolytes metabolism and ionic status to confer salt stress tolerance in soybean. Acta Physiol. Plant. 2020, 42, 114. [Google Scholar] [CrossRef]
- Lazo-Vélez, M.A.; Guajardo-Flores, D.; Mata-Ramírez, D.; Gutiérrez-Uribe, J.A.; Serna-Saldivar, S.O. Characterization and quantitation of triterpenoid saponins in raw and sprouted Chenopodium berlandieri spp.(Huauzontle) grains subjected to germination with or without selenium stress conditions. J. Food Sci. 2016, 81, C19–C26. [Google Scholar] [CrossRef] [PubMed]
- Falcão, E.L.; Muniz, B.C.; Bastos Filho, C.J.A.; Kapoor, R.; da Silva, F.S.B. Soil microbial respiration and pH modulated by arbuscular mycorrhizal fungi influence the biosynthesis of health-promoting compounds in Anadenanthera colubrina (Vell.) Brenan. Rhizosphere 2023, 26, 100685. [Google Scholar] [CrossRef]
- Weng, Y.; Yu, L.; Cui, J.; Zhu, Y.-R.; Guo, C.; Wei, G.; Duan, J.-L.; Yin, Y.; Guan, Y.; Wang, Y.-H. Antihyperglycemic, hypolipidemic and antioxidant activities of total saponins extracted from Aralia taibaiensis in experimental type 2 diabetic rats. J. Ethnopharmacol. 2014, 152, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, X.; Liu, H.; Gong, D.; Li, X. Comparison of the nutritional and phytochemical composition and antioxidant activities of Aralia elata (Miq.) Seem fruits in Northeast China. Arab. J. Chem. 2021, 14, 103448. [Google Scholar] [CrossRef]
- Hou, L.; Li, S.; Tong, Z.; Yuan, X.; Xu, J.; Li, J. Geographical variations in fatty acid and steroid saponin biosynthesis in Dioscorea zingiberensis rhizomes. Ind. Crops Prod. 2021, 170, 113779. [Google Scholar] [CrossRef]
- La Pierre, K.J.; Blumenthal, D.M.; Brown, C.S.; Klein, J.A.; Smith, M.D. Drivers of variation in aboveground net primary productivity and plant community composition differ across a broad precipitation gradient. Ecosystems 2016, 19, 521–533. [Google Scholar] [CrossRef]
- Toledo, M.; Poorter, L.; Peña-Claros, M.; Alarcón, A.; Balcázar, J.; Leaño, C.; Licona, J.C.; Llanque, O.; Vroomans, V.; Zuidema, P. Climate is a stronger driver of tree and forest growth rates than soil and disturbance. J. Ecol. 2011, 99, 254–264. [Google Scholar] [CrossRef]
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and heat stress impacts on phenolic acids accumulation in durum wheat cultivars. Foods 2021, 10, 2142. [Google Scholar] [CrossRef] [PubMed]
- Puente-Garza, C.A.; Meza-Miranda, C.; Ochoa-Martínez, D.; García-Lara, S. Effect of in vitro drought stress on phenolic acids, flavonols, saponins, and antioxidant activity in Agave salmiana. Plant Physiol. Biochem. 2017, 115, 400–407. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, M.C.; Pinto, D.C.; Figueiredo, C.; Santos, C.; Silva, A.M. Phenolic and lipophilic metabolite adjustments in Olea europaea (olive) trees during drought stress and recovery. Phytochemistry 2021, 185, 112695. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N. Neuropharmacological effects of quercetin: A literature-based review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Virgiliou, C.; Kanelis, D.; Pina, A.; Gika, H.; Tananaki, C.; Zotou, A.; Theodoridis, G. A targeted approach for studying the effect of sugar bee feeding on the metabolic profile of Royal Jelly. J. Chromatogr. A 2020, 1616, 460783. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Mansour, R.S.; Saleh, S.S.; Kamel, G. Investigating the molecular structure of placenta and plasma in pre-eclampsia by infrared microspectroscopy. J. Pharm. Biomed. Anal. 2020, 184, 113186. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Chen, J.; Xie, J.; Shen, S.; Deng, Y.; Zhu, J.; Yuan, H.; Jiang, Y. Characterization of the key aroma compounds in black teas with different aroma types by using gas chromatography electronic nose, gas chromatography-ion mobility spectrometry, and odor activity value analysis. LWT 2022, 163, 113492. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, D.; Cho, C.-W.; Yang, Z.; Li, X.; Kang, J.-S. Identification and Quantitation of the Bioactive Components in Wasted Aralia elata Leaves Extract with Endothelial Protective Activity. Molecules 2023, 28, 5907. https://doi.org/10.3390/molecules28155907
Gao D, Cho C-W, Yang Z, Li X, Kang J-S. Identification and Quantitation of the Bioactive Components in Wasted Aralia elata Leaves Extract with Endothelial Protective Activity. Molecules. 2023; 28(15):5907. https://doi.org/10.3390/molecules28155907
Chicago/Turabian StyleGao, Dan, Chong-Woon Cho, Zemin Yang, Xiwen Li, and Jong-Seong Kang. 2023. "Identification and Quantitation of the Bioactive Components in Wasted Aralia elata Leaves Extract with Endothelial Protective Activity" Molecules 28, no. 15: 5907. https://doi.org/10.3390/molecules28155907
APA StyleGao, D., Cho, C.-W., Yang, Z., Li, X., & Kang, J.-S. (2023). Identification and Quantitation of the Bioactive Components in Wasted Aralia elata Leaves Extract with Endothelial Protective Activity. Molecules, 28(15), 5907. https://doi.org/10.3390/molecules28155907