Synthesis of Temperature Sensing Nitrogen-Doped Carbon Dots and Their Application in Fluorescent Ink
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of N-CDs
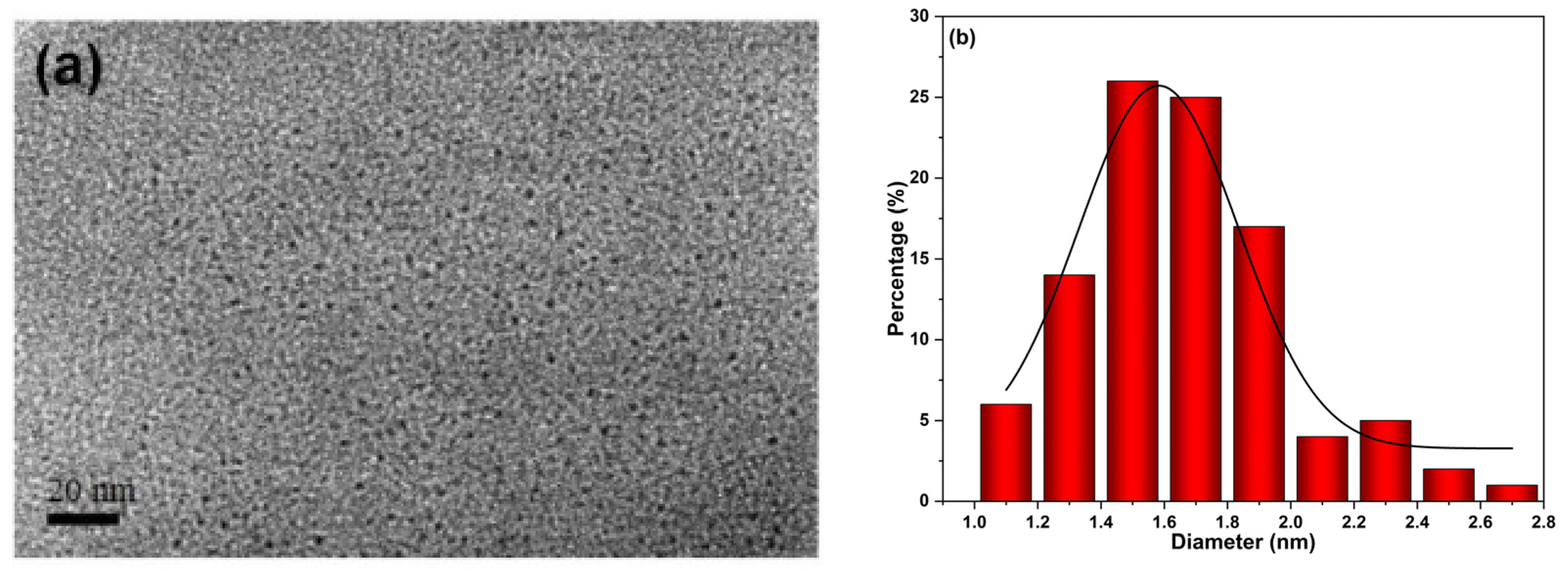
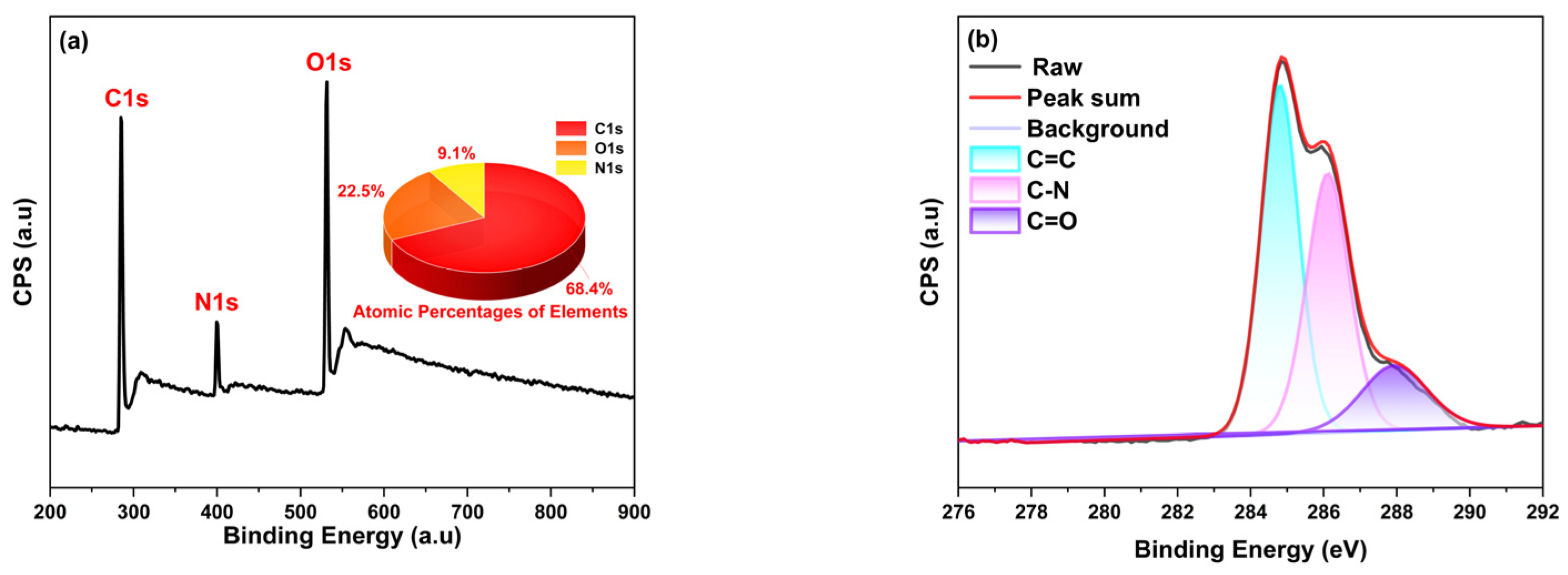
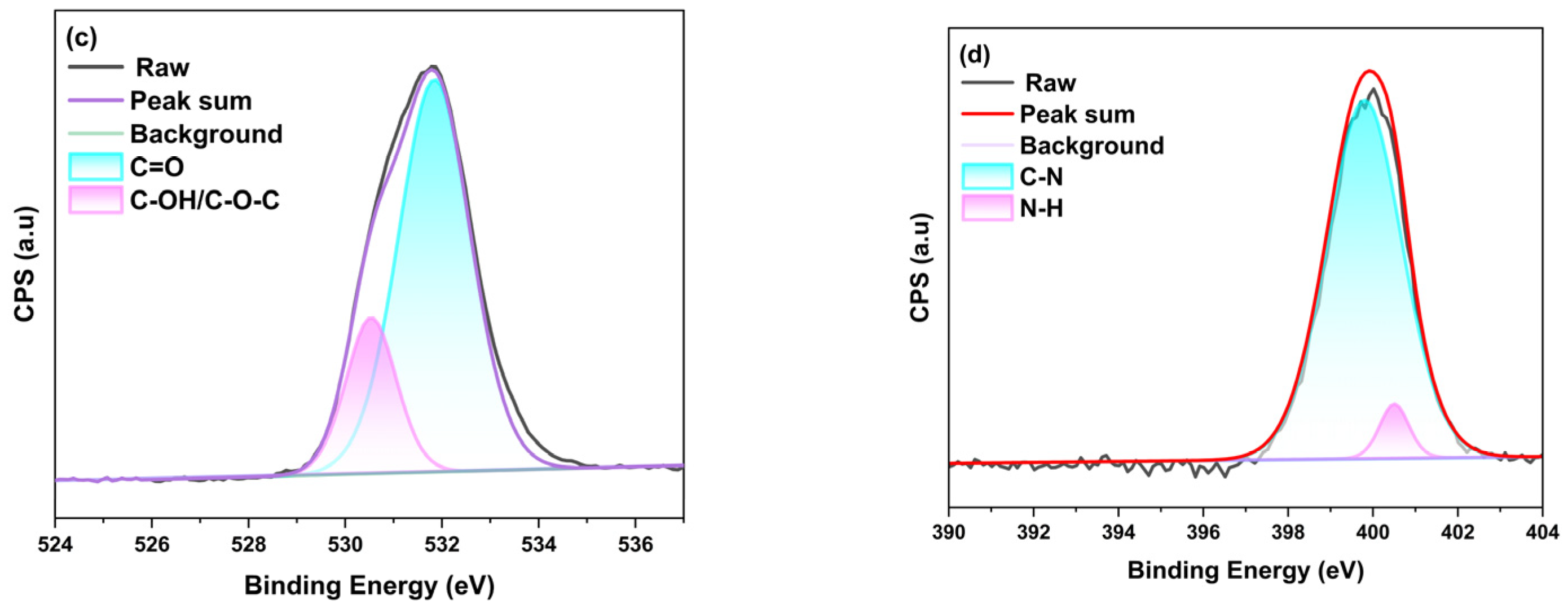
2.2. Characterization
2.3. Optical Properties
2.4. The Stability of the N-CDs
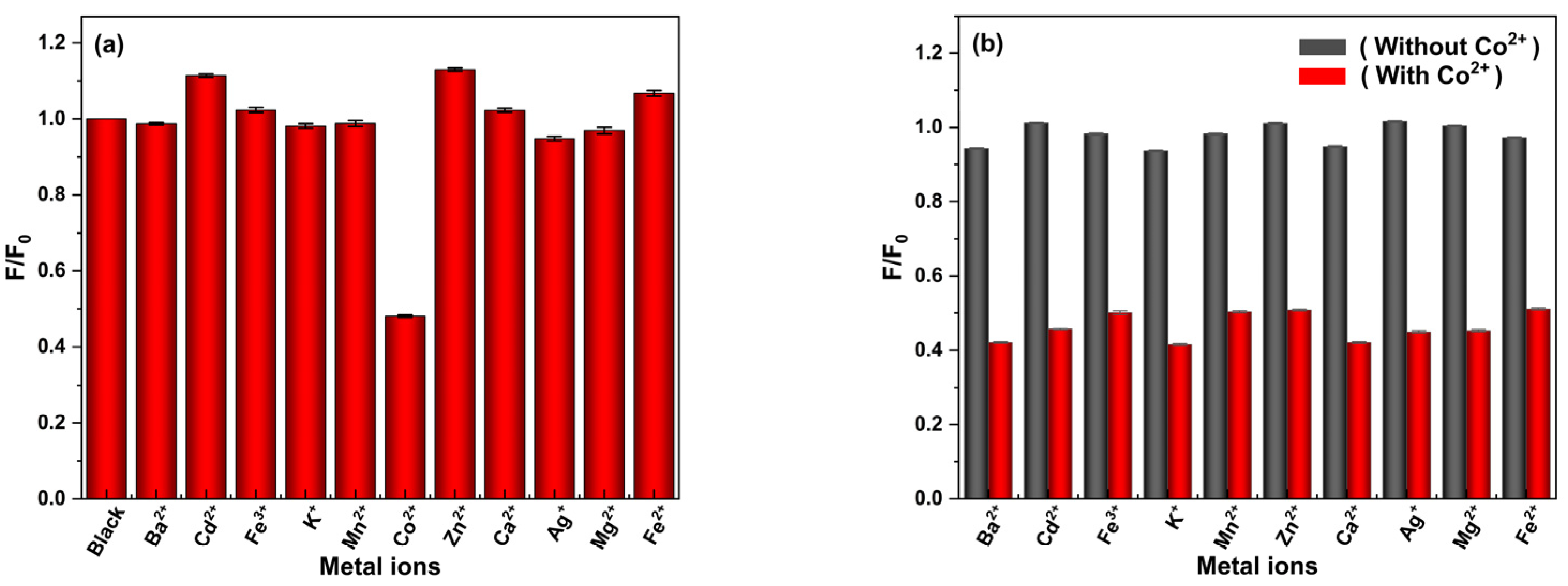
2.5. Detection of Co2+ and Method Selectivity
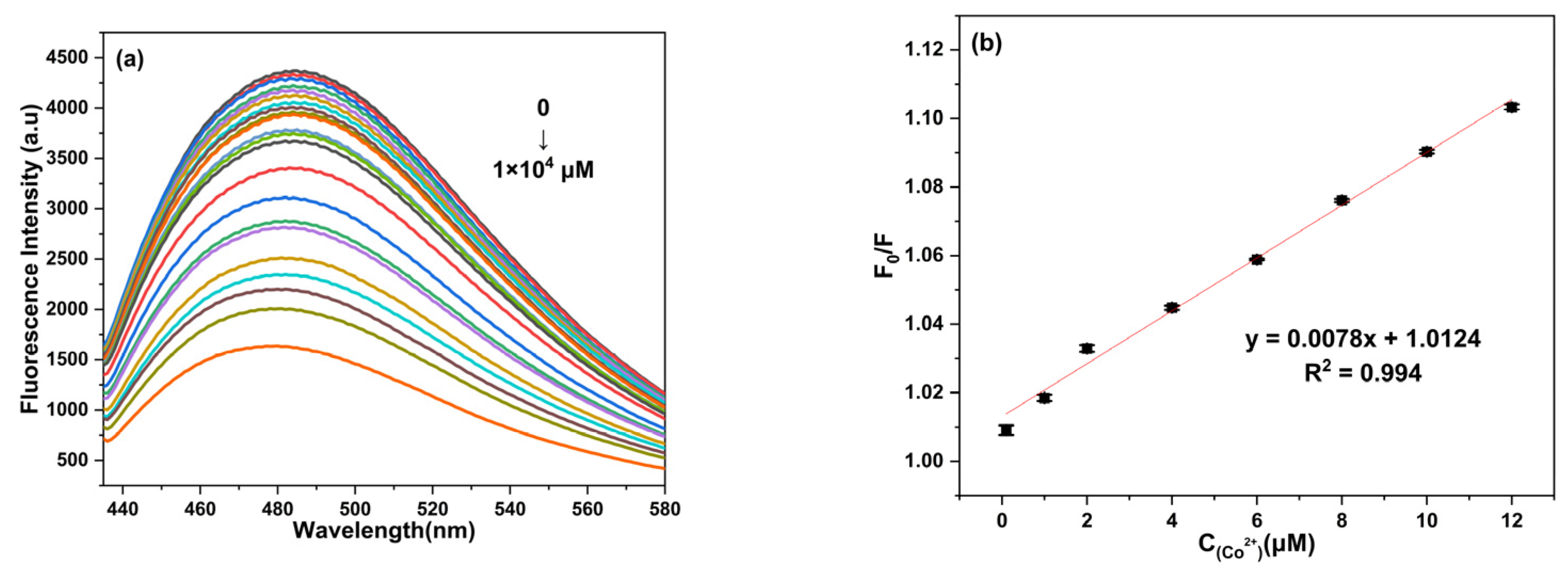
2.6. Detection Limit of Co2+
2.7. Possible Detection Mechanisms
2.8. Detection of Co2+ in Real Samples
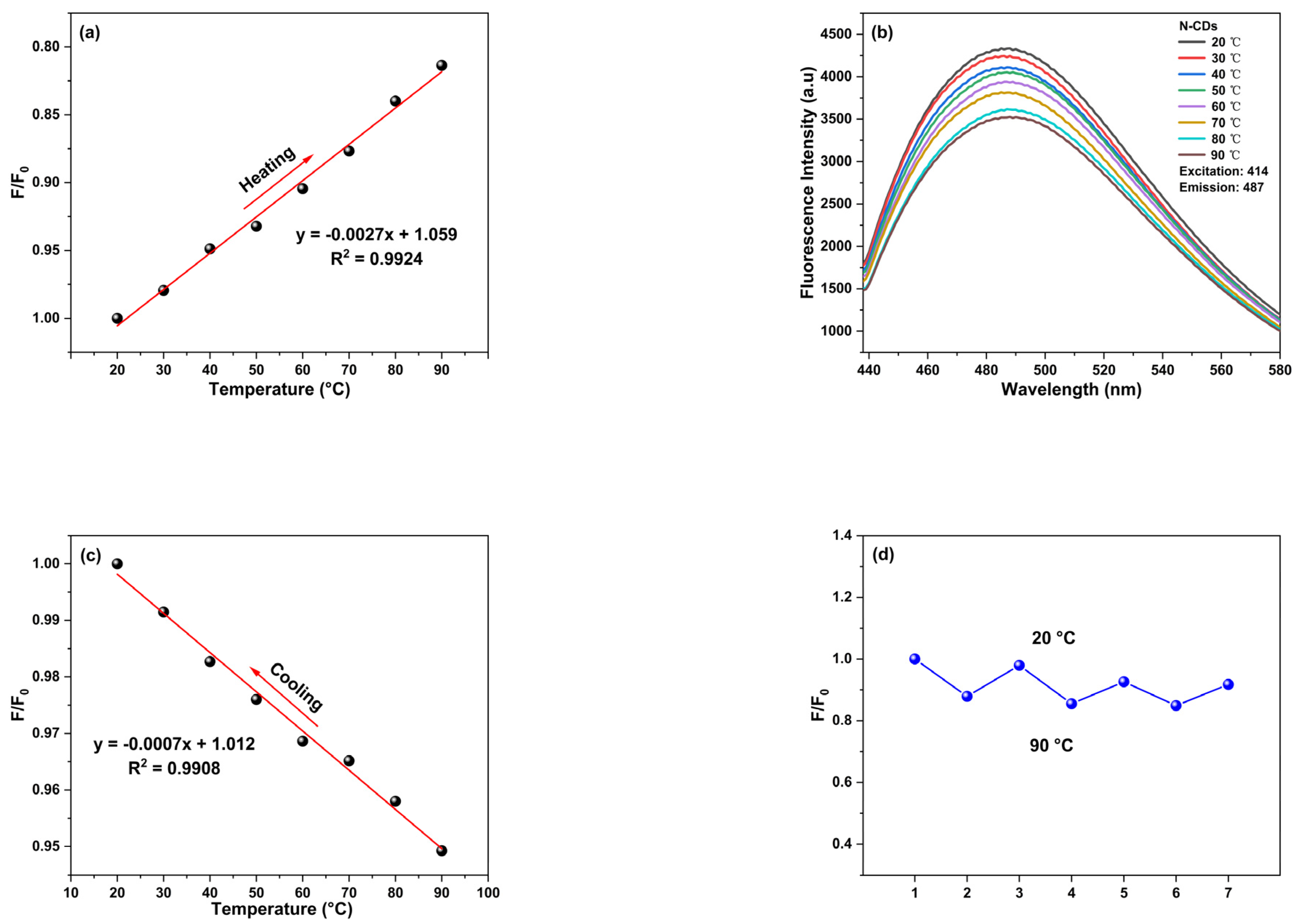
2.9. Research on Temperature Sensing Performance of N-CDs
2.10. Application of N-CDs in the Field of Fluorescent Ink
3. Experimental Instruments
3.1. Materials and Apparatus
3.2. Preparation of N-CDs
3.3. Detection of Co2+ and Interference Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lee, J.; Jang, J.; Yu, T. A facile aqueous-phase synthesis of Co-based nanostructures composed of cobalt hydroxide and cobalt oxide for enhanced photocatalytic activity of dye-sensitized H2 production. J. Alloys Compd. 2023, 942, 169078. [Google Scholar] [CrossRef]
- Li, S.; Zheng, X.; Jin, H.; Qian, L.; Wang, K.; Shen, Y.; Zhao, M.; Liu, R. Multivalent cobalt species supported on graphene aerogel for degradation of sulfamethoxazole via high-valent cobalt-oxo species. Chem. Eng. J. 2023, 463, 142367. [Google Scholar] [CrossRef]
- Shang, W.; Wang, H.; Yu, W.; He, Y.; Ma, Y.; Wu, Z.; Tan, P. Transforming the Electrochemical Behaviors of Cobalt Oxide from “Supercapacitator” to “Battery” by Atomic-Level Structure Engineering for Inspiring the Advance of Co-Based Batteries. Small 2023, 19, e2300647. [Google Scholar] [CrossRef]
- Zakrzewska, B.; Jabłońska, A.; Adamczyk, L.; Dembińska, B.; Kostuch, A.; Strawski, M.; Rutkowska, I.A.; Kulesza, P.J.; Marcinek, M.; Cox, J.A.; et al. Pyrolyzed cobalt hexacyanocobaltate dispersed on reduced-graphene-oxide as an electrocatalyst of the oxygen reduction reaction in an alkaline medium. J. Mater. Chem. A 2023, 11, 7286–7298. [Google Scholar] [CrossRef]
- Awual, M.R.; Ismael, M.; Yaita, T. Efficient detection and extraction of cobalt(II) from lithium ion batteries and wastewater by novel composite adsorbent. Sens. Actuators B 2014, 191, 9–18. [Google Scholar] [CrossRef]
- Liao, S.; Zhu, F.; Zhao, X.; Yang, H.; Chen, X. A reusable P, N-doped carbon quantum dot fluorescent sensor for cobalt ion. Sens. Actuators B 2018, 260, 156–164. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, J.; Liu, Y.; Wang, Y.; Xiao, Y.; Zhang, Y. One-pot synthesis of nitrogen-doped carbon dots for sensing of Co2+ and tetracycline antibiotics, biological imaging, and fluorescent inks. J. Nanoparticle Res. 2022, 24, 44. [Google Scholar] [CrossRef]
- Tsoutsi, D.; Guerrini, L.; Hermida-Ramon, J.M.; Giannini, V.; Liz-Marzán, L.M.; Wei, A.; Alvarez-Puebla, R.A. Simultaneous SERS detection of copper and cobalt at ultratrace levels. Nanoscale 2013, 5, 5841–5846. [Google Scholar] [CrossRef]
- Zamhari, M.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Simultaneous Electrochemical Detection of Co(II) and Cu(II) by 1-Diazo-2-Naphthol-4-Sulfonic Acid/MWCNTs Modified Electrode. Electroanalysis 2017, 29, 2348–2357. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.; Uttam, K.N. Synthesis of Sensitive and Robust Lignin Capped Silver Nanoparticles for the Determination of Cobalt(II), Chromium(III), and Manganese(II) Ions by Colorimetry and Manganese(II) Ions by Surface-Enhanced Raman Scattering (SERS) in Aqueous Media. Anal. Lett. 2021, 54, 2051–2069. [Google Scholar] [CrossRef]
- Kumar, A.; Asu, S.; Mukherjee, P.; Singh, P.; Kumari, A.; Sahu, S.K. Single-step synthesis of N-doped carbon dots and applied for dopamine sensing, in vitro multicolor cellular imaging as well as fluorescent ink. J. Photochem. Photobiol. A Chem. 2021, 406, 113019. [Google Scholar] [CrossRef]
- Ren, G.; Hou, X.; Kang, Y.; Zhang, R.; Zhang, M.; Liu, W.; Li, L.; Wei, S.; Wang, H.; Wang, B.; et al. Efficient preparation of nitrogen-doped fluorescent carbon dots for highly sensitive detection of metronidazole and live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118251. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, S.; Chen, B.; Wang, Y.; Shen, Z.; Qiu, M.; Pan, H.; Wang, W.; Wang, Y.; Li, X. Endoplasmic reticulum-targeted polymer dots encapsulated with ultrasonic synthesized near-infrared carbon nanodots and their application for in vivo monitoring of Cu2+. J. Colloid Interface Sci. 2022, 627, 705–715. [Google Scholar] [CrossRef]
- Yang, L.; Huang, H.; Wang, T.; Zhou, D.; Chen, Q.; Li, D.; Chen, S.; Lin, P. Endoplasmic reticulum-targetable selenium-doped carbon nanodots with redox-responsive fluorescence for in situ free-radical scavenging in cells and mice. Arab. J. Chem. 2023, 16, 105036. [Google Scholar] [CrossRef]
- Li, J.; Zuo, G.; Pan, X.; Wei, W.; Qi, X.; Su, T.; Dong, W. Nitrogen-doped carbon dots as a fluorescent probe for the highly sensitive detection of Ag+ and cell imaging. Luminescence 2018, 33, 243–248. [Google Scholar] [CrossRef]
- Hashemi, N.; Mousazadeh, M.H. Preparation of fluorescent nitrogen-doped carbon dots for highly selective on-off detection of Fe3+ ions in real samples. Opt. Mater. 2021, 121, 111515. [Google Scholar] [CrossRef]
- Sadhanala, H.K.; Aryal, S.; Sharma, K.; Orpaz, Z.; Michaeli, S.; Gedanken, A. Nitrogen-doped carbon dots as a highly selective and sensitive fluorescent probe for sensing Mg2+ ions in aqueous solution, and their application in the detection and imaging of intracellular Mg2+ ions. Sens. Actuators B 2022, 366, 131958. [Google Scholar] [CrossRef]
- Xu, H.; You, X.; Lu, Y.; Liang, P.; Luo, Z.; Wang, Y.; Zeng, S.; Zeng, H. Analysis of Mn2+ and Zn2+ Ions in Macroalgae with Heteroelement-Doped Carbon-Based Fluorescent Probe. Biosensors 2022, 12, 359. [Google Scholar] [CrossRef]
- Zi, L.; Huang, Y.; Yan, Z.; Liao, S. Thioglycolic acid-capped CuInS2/ZnS quantum dots as fluorescent probe for cobalt ion detection. J. Lumin. 2014, 148, 359–363. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, F.; Xu, J.; Zhang, Y.; Wu, Y.; Wang, L. Simple and sensitive detection method for Cobalt(II) in water using CePO4:Tb3+ nanocrystals as fluorescent probes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 151–155. [Google Scholar] [CrossRef]
- Bian, W.; Ma, J.; Liu, Q.; Wei, Y.; Li, Y.; Dong, C.; Shuang, S. A novel phosphorescence sensor for Co2+ ion based on Mn-doped ZnS quantum dots. Luminescence 2014, 29, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Feng, W.; Qi, X.; Li, J.; Chen, J.; Lu, L.; Deng, P.; Zeng, J.; Li, F. Differential knee skin temperature following total knee arthroplasty and its relationship with serum indices and outcome: A prospective study. J. Int. Med. Res. 2016, 44, 1023–1033. [Google Scholar] [CrossRef]
- Yang, J.; Yin, P.; Zhou, M.; Ou, C.Q.; Guo, Y.; Gasparrini, A.; Liu, Y.; Yue, Y.; Gu, S.; Sang, S.; et al. Cardiovascular mortality risk attributable to ambient temperature in China. Heart 2015, 101, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, Z.; Gu, Y.; Hu, Y.; Ji, Z.; Wang, S.; Lin, Z.; Li, X.; Xie, Z.; Pan, S. Glibenclamide Is Comparable to Target Temperature Management in Improving Survival and Neurological Outcome After Asphyxial Cardiac Arrest in Rats. J. Am. Hear. Assoc. 2016, 5, e003465. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Xin, S.X.; Chen, W. Temperature- and frequency-dependent dielectric properties of biological tissues within the temperature and frequency ranges typically used for magnetic resonance imaging-guided focused ultrasound surgery. Int. J. Hyperth 2014, 30, 56–65. [Google Scholar] [CrossRef]
- Pundi, A.; Chang, C.J. Recent Advances in Synthesis, Modification, Characterization, and Applications of Carbon Dots. Polymers 2022, 14, 2153. [Google Scholar] [CrossRef]
- Patir, K.; Barman, B.; Basumatary, S. One Pot Synthesis of Multicolor Emissive Nitrogen Doped Carbon Dots and its Application as Acetone and Picric Acid Sensor. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 1301–1310. [Google Scholar] [CrossRef]
- Li, L.; Shi, L.; Jia, J.; Chang, D.; Dong, C.; Shuang, S. Fe3+ detection, bioimaging, and patterning based on bright blue-fluorescent N-doped carbon dots. Analyst 2020, 145, 5450–5457. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Wu, Y.; Gu, J.; Ma, C.; Zhu, C.; Gao, H.; Zhang, Y.; Shang, Y.; Yang, Z.; et al. Highly Photoluminescent Carbon Dots with pH-Dependent Switchable Fluorescence and Sensitivity to Tetracycline. Nano 2021, 16, 2150036. [Google Scholar] [CrossRef]
- Liu, B.; Wei, S.; Liu, E.; Zhang, H.; Lu, P.; Wang, J.; Sun, G. Nitrogen-doped carbon dots as a fluorescent probe for folic acid detection and live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 268, 120661. [Google Scholar] [CrossRef]
- Amjadi, M.; Hallaj, T.; Mayan, M.A. Green synthesis of nitrogen-doped carbon dots from lentil and its application for colorimetric determination of thioridazine hydrochloride. RSC Adv. 2016, 6, 104467–104473. [Google Scholar] [CrossRef]
- Atchudan, R.; Gangadaran, P.; Perumal, S.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Rajendran, R.L.; Ahn, B.-C.; Lee, Y.R. Green Synthesis of Multicolor Emissive Nitrogen-Doped Carbon Dots for Bioimaging of Human Cancer Cells. J. Clust. Sci. 2023, 34, 1583–1594. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Yan, X.; Xiao, Y.; Zhang, Y. Simple synthesis of green luminescent N-doped carbon dots for malachite green determination. Anal. Methods 2022, 14, 2616–2622. [Google Scholar] [CrossRef]
- Issa, M.A.; Zentou, H.; Jabbar, Z.H.; Abidin, Z.Z.; Harun, H.; Halim, N.A.A.; Alkhabet, M.M.; Pudza, M.Y. Ecofriendly adsorption and sensitive detection of Hg (II) by biomass-derived nitrogen-doped carbon dots: Process modelling using central composite design. Environ. Sci. Pollut. Res. 2022, 29, 86859–86872. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, X.; Liu, Y.; Liang, L.; Peng, Y.; Wu, S.; Zhao, Y. N-Doped Carbon Dots as Fluorescent “Turn-Off” Nanosensors for Ascorbic Acid and Fe3+ Detection. ACS Appl. Nano Mater. 2022, 5, 7268–7277. [Google Scholar] [CrossRef]
- Xu, D.; Fu, N.; Xie, Y.; Wang, Y.; Xie, R.; Yang, H.; Sun, W.; Liu, X.; Han, A. Easy formation of nitrogen-doped carbon dots towards Hg2+ fluorescent measurement and multicolor intracellular imaging. Mater. Chem. Phys. 2021, 266, 124547. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.; Xiao, Y.; Liu, W. Hydrothermal synthesis of carbon dots codoped with nitrogen and phosphorus as a turn-on fluorescent probe for cadmium(II). Microchim. Acta 2019, 186, 147. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, J.; Liu, Y.; Wang, Y.; Zhang, Y. One-pot synthesis of nitrogen-doped carbon dots for highly sensitive determination of cobalt ions and biological imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 252, 119541. [Google Scholar] [CrossRef]
- Tammina, S.K.; Yang, D.; Koppala, S.; Cheng, C.; Yang, Y. Highly photoluminescent N, P doped carbon quantum dots as a fluorescent sensor for the detection of dopamine and temperature. J. Photochem. Photobiol. B Biol. 2019, 194, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Chen, C.; Wang, P.; Zhou, R.; Zhao, X.; Fan, H.; Huang, Z. Carbon dots derived from cellobiose for temperature and phosalone detection. Mater. Res. Bull. 2022, 151, 111790. [Google Scholar] [CrossRef]
- Guo, Z.; Luo, J.; Zhu, Z.; Sun, Z.; Zhang, X.; Wu, Z.-c.; Mo, F.; Guan, A. A facile synthesis of high-efficient N,S co-doped carbon dots for temperature sensing application. Dye. Pigment. 2020, 173, 107952. [Google Scholar] [CrossRef]
- Liu, S.; Liu, R.; Xing, X.; Yang, C.; Xu, Y.; Wu, D. Highly photoluminescent nitrogen-rich carbon dots from melamine and citric acid for the selective detection of iron(iii) ion. RSC Adv. 2016, 6, 31884–31888. [Google Scholar] [CrossRef]
- Jing, N.; Tian, M.; Wang, Y.; Zhang, Y. Nitrogen-doped carbon dots synthesized from acrylic acid and ethylenediamine for simple and selective determination of cobalt ions in aqueous media. J. Lumin. 2019, 206, 169–175. [Google Scholar] [CrossRef]
- Boonta, W.; Talodthaisong, C.; Sattayaporn, S.; Chaicham, C.; Chaicham, A.; Sahasithiwat, S.; Kangkaew, L.; Kulchat, S. The synthesis of nitrogen and sulfur co-doped graphene quantum dots for fluorescence detection of cobalt(ii) ions in water. Mater. Chem. Front. 2020, 4, 507–516. [Google Scholar] [CrossRef]
- Kong, D.; Yan, F.; Han, Z.; Xu, J.; Guo, X.; Chen, L. Cobalt(ii) ions detection using carbon dots as an sensitive and selective fluorescent probe. RSC Adv. 2016, 6, 67481–67487. [Google Scholar] [CrossRef]
- Du, F.; Cheng, Z.; Kremer, M.; Liu, Y.; Wang, X.; Shuang, S.; Dong, C. A label-free multifunctional nanosensor based on N-doped carbon nanodots for vitamin B12 and Co2+ detection, and bioimaging in living cells and zebrafish. J. Mater. Chem. B 2020, 8, 5089–5095. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Ga, L.; Wang, Y.; Ai, J. Synthesis of Temperature Sensing Nitrogen-Doped Carbon Dots and Their Application in Fluorescent Ink. Molecules 2023, 28, 6607. https://doi.org/10.3390/molecules28186607
Liu P, Ga L, Wang Y, Ai J. Synthesis of Temperature Sensing Nitrogen-Doped Carbon Dots and Their Application in Fluorescent Ink. Molecules. 2023; 28(18):6607. https://doi.org/10.3390/molecules28186607
Chicago/Turabian StyleLiu, Pingping, Lu Ga, Yong Wang, and Jun Ai. 2023. "Synthesis of Temperature Sensing Nitrogen-Doped Carbon Dots and Their Application in Fluorescent Ink" Molecules 28, no. 18: 6607. https://doi.org/10.3390/molecules28186607



