The Influence of Temperature on the Photoelectric Properties of GeSe Nanowires
Abstract
1. Introduction
2. Results and Discussion
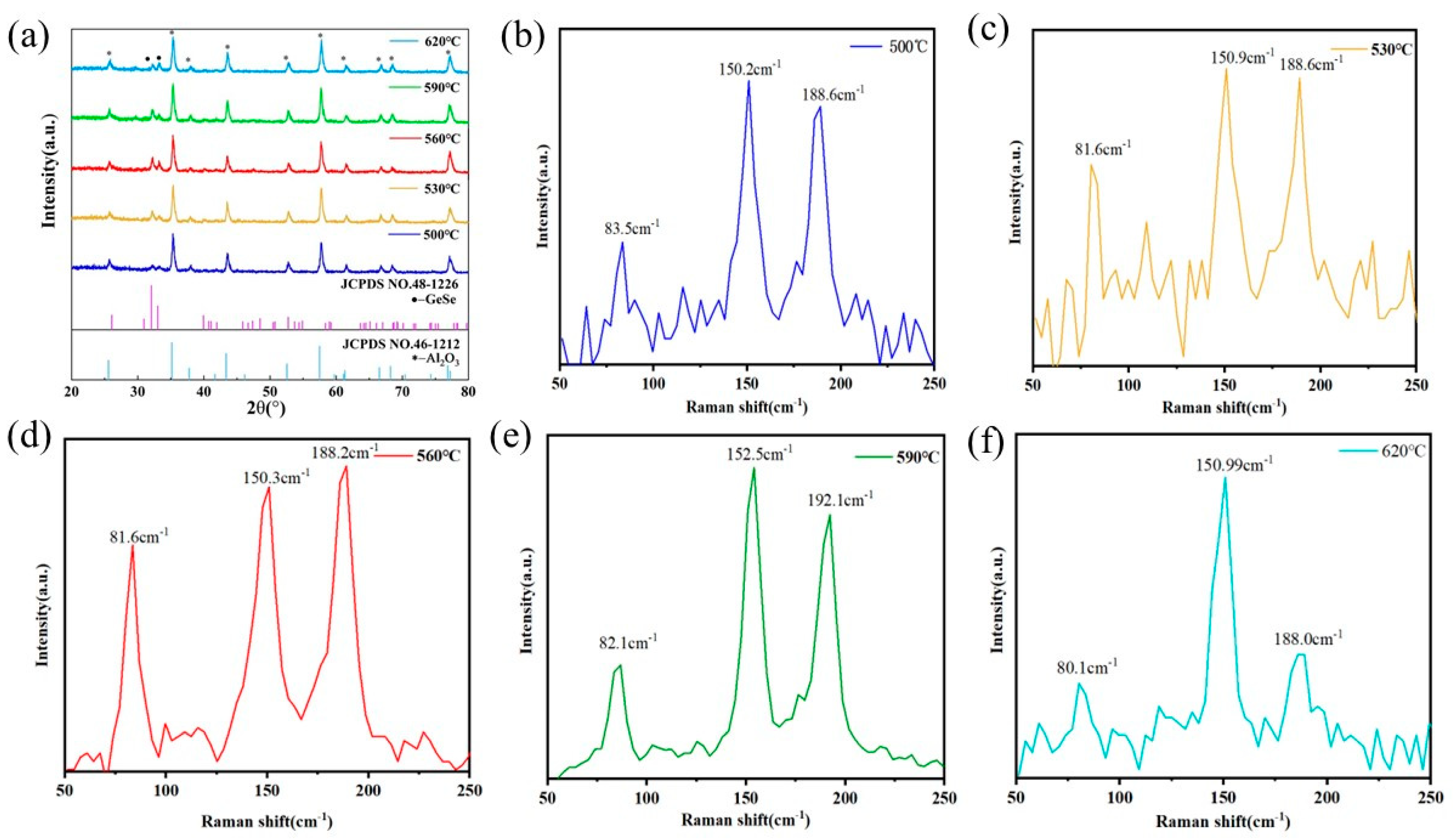
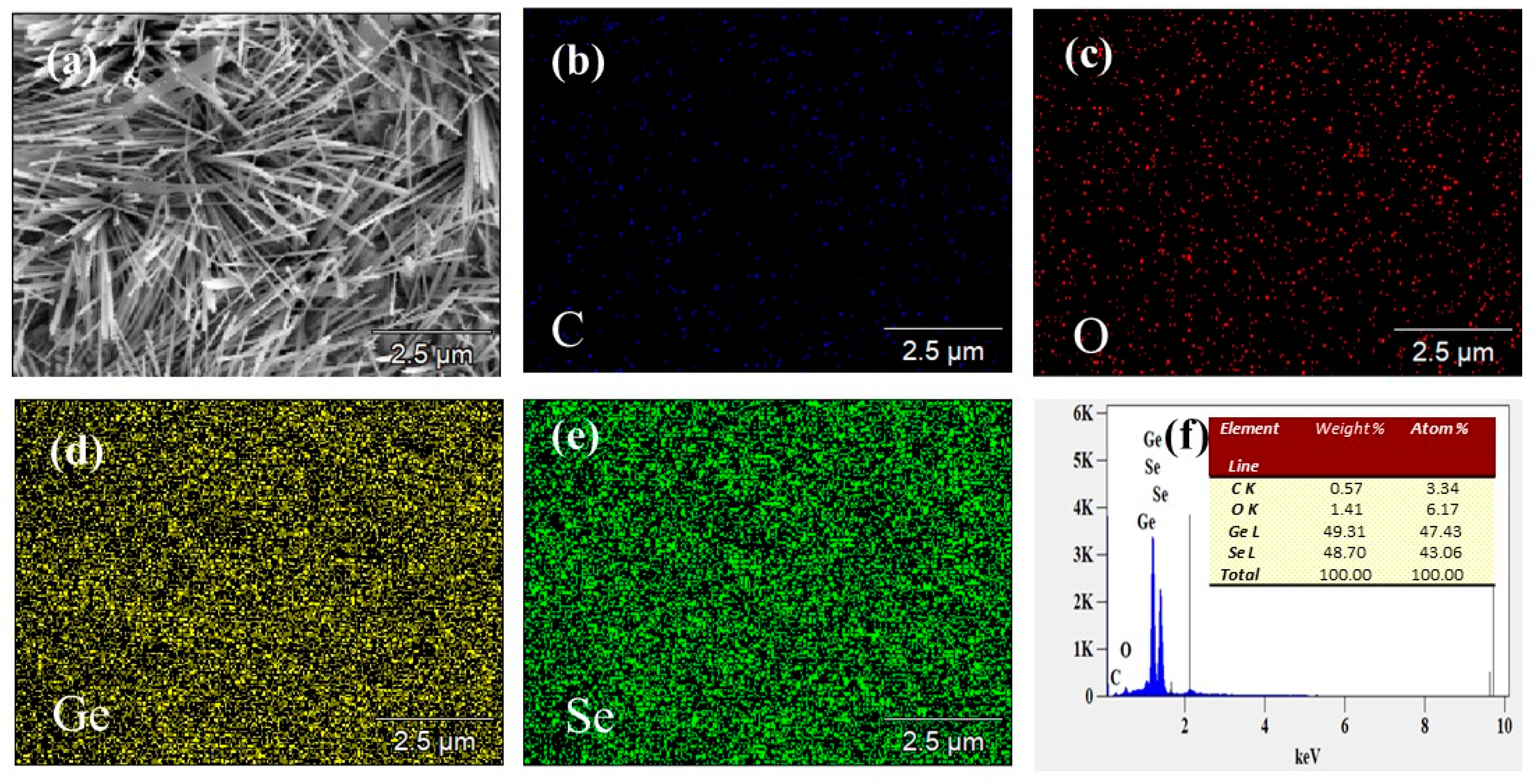
2.1. Structural Characterization
2.2. Computational Analysis
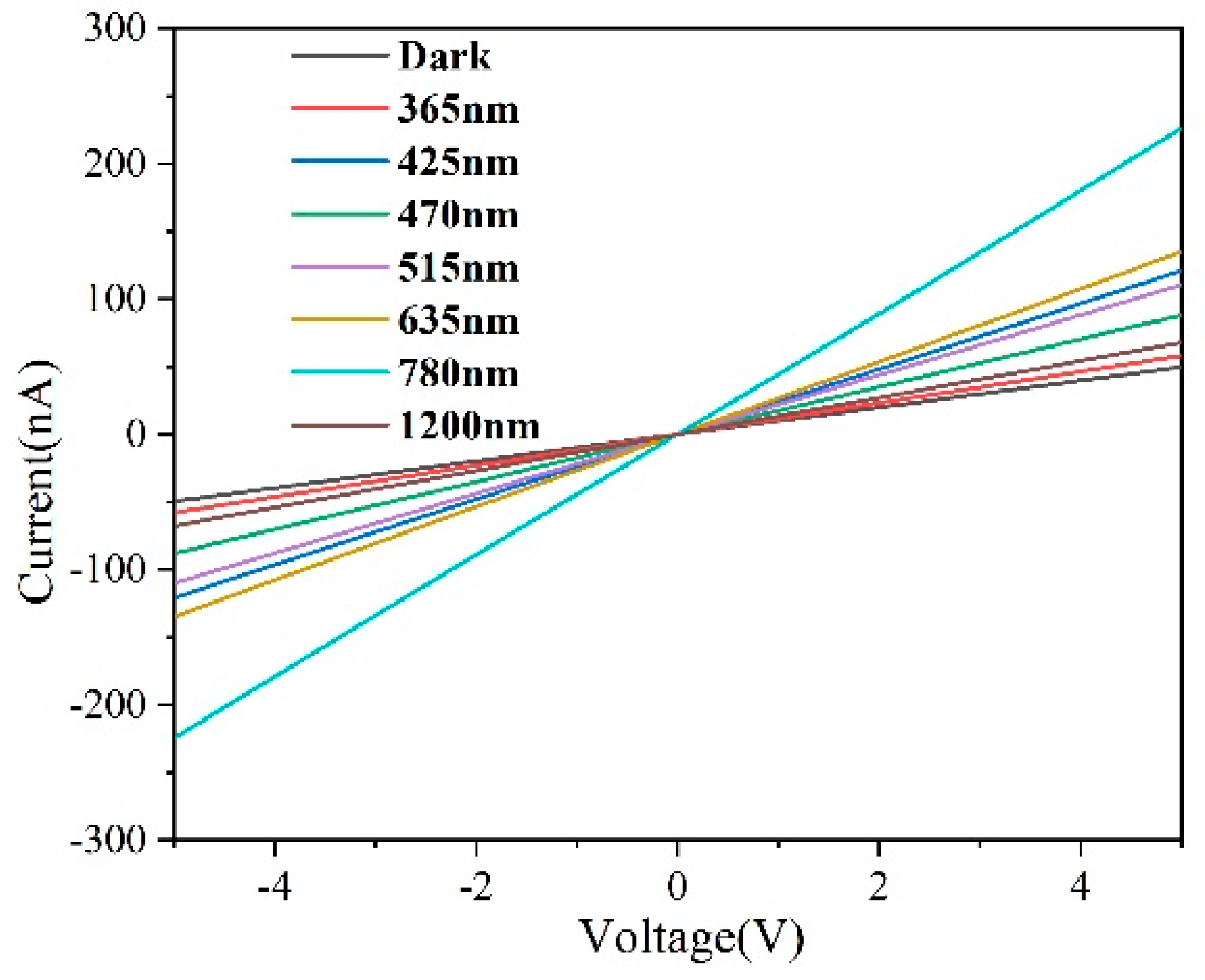
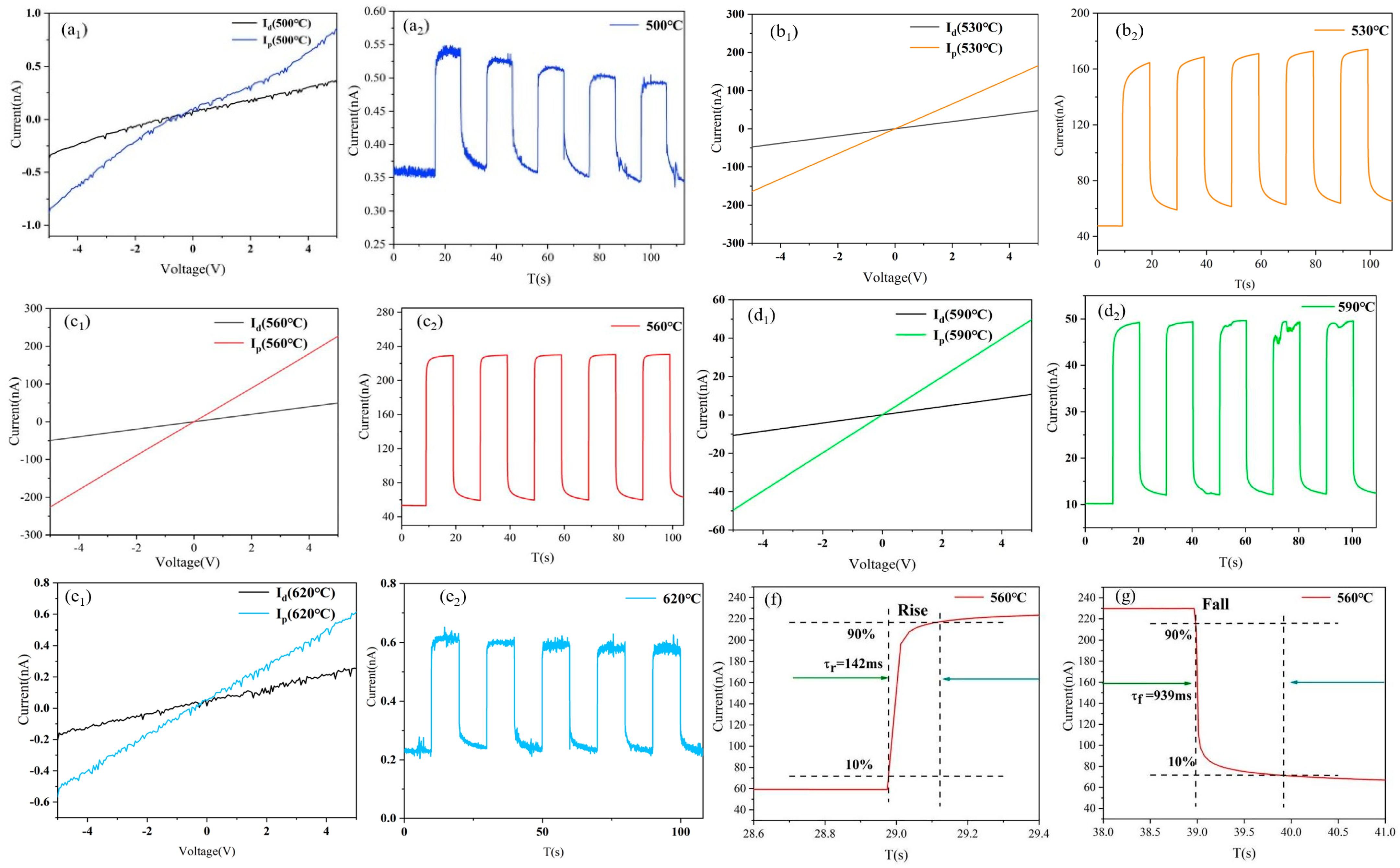
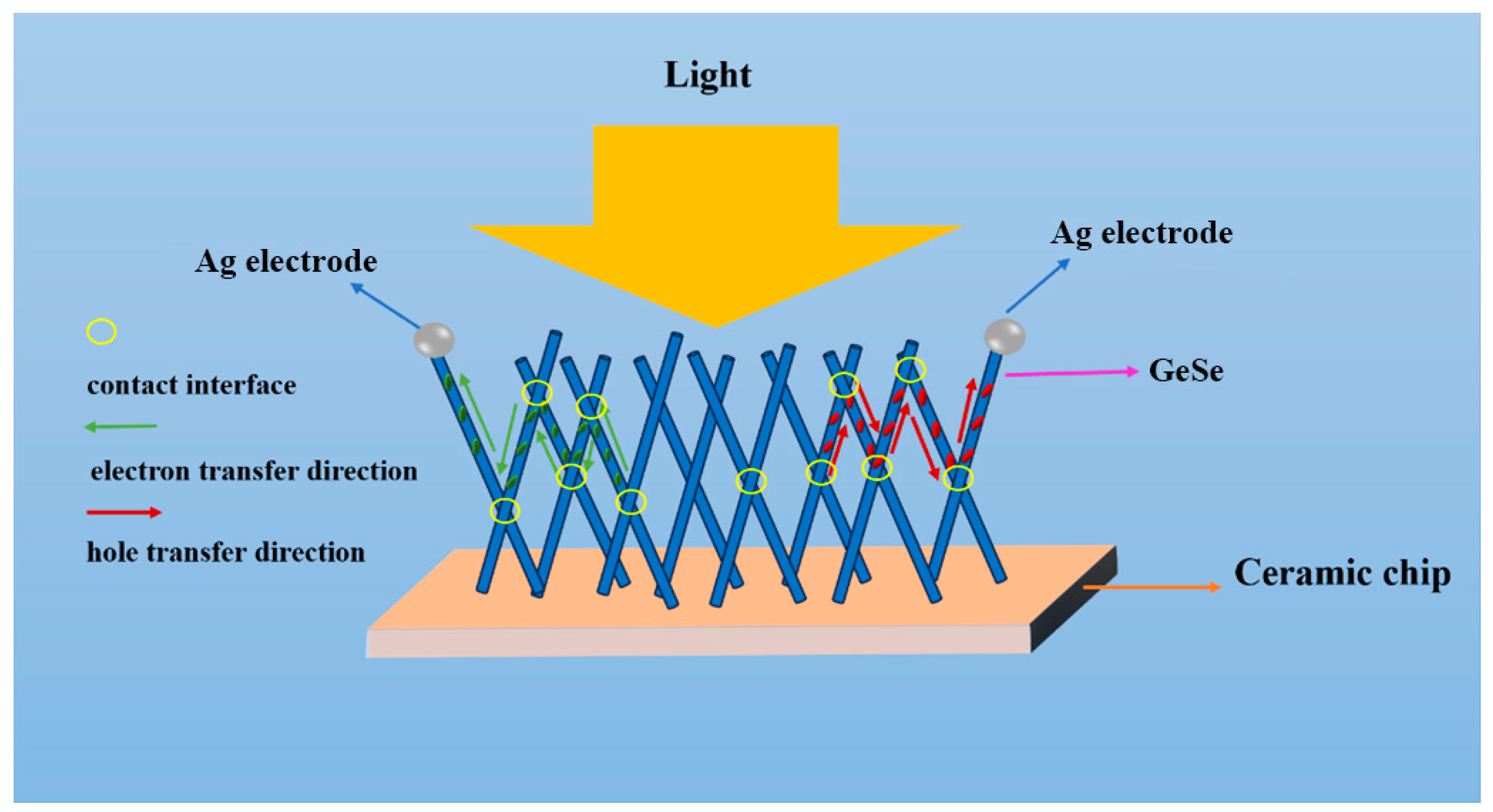
2.3. Photoelectric Performance Analysis
3. Experimental Section and Theoretical Calculations
3.1. Synthesis of Nanowires
3.2. Fabrication of Ag Electrodes
3.3. Characterization and Testing
3.4. Performance Testing of Photodetectors
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, F.; Zhang, X.; Tan, B.; Zhang, S.; Zhang, J.; Jia, D.; Zhou, Y.; Hu, P. Low Optical Writing Energy Multibit Optoelectronic Memory Based on SnS2/h-BN/Graphene Heterostructure. Small 2021, 17, 2104459. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Kaloni, T.P.; Lin, H.; Bedoya-Pinto, A.; Pandeya, A.K.; Kostanovskiy, I.; Zhao, K.; Zhong, Y.; Hu, X.; Xue, Q.K.; et al. 2D Ferroelectrics: Enhanced Spontaneous Polarization in Ultrathin SnTe Films with Layered Antipolar Structure. Adv. Mater. 2019, 31, 1970016. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Huang, L.; Jing, X.; Jiang, L.; Dong, H.; Wei, Z.; Li, J.; Hu, W. Short-Wave Near-Infrared Linear Dichroism of Two-Dimensional Germanium Selenide. J. Am. Chem. Soc. 2017, 139, 14976–14982. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hu, X.; Jin, B.; Yu, J.; Liu, K.; Li, H.; Zhai, T. Highly Anisotropic GeSe Nano sheets for Phototransistors with Ultrahigh Photoresponsivity. Adv. Sci. 2018, 5, 1800478. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, S.C.; Wang, Y.; Long, M.; Dai, C.; Chen, S.; Zhang, B.; Sun, Z.; Hu, C. In-Plane Optical Anisotropy of Low-Symmetry 2D GeSe. Adv. Opt. Mater. 2019, 7, 1801311.1–1801311.8. [Google Scholar] [CrossRef]
- Liu, S.C.; Yang, Y.; Li, Z.; Xue, D.; Hu, J. GeSe thin-film solar cells. Mater. Chem. Front. 2020, 4, 775–787. [Google Scholar] [CrossRef]
- Sarkar, D.; Ghosh, T.; Roychowdhury, S.; Arora, R.; Sajan, S.; Sheet, G.; Waghmare, U.; Biswas, K. Ferroelectric instability induced ultralow thermal conductivity and high thermoelectric performance in rhombohedral p-type GeSe crystal. J. Am. Chem. Soc. 2020, 142, 12237–12244. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, S.; Sun, S.; Xie, M.; Bo, C.; Zeng, H. GeSe monolayer semiconductor with tunable direct band gap and small carrier effective mass. Appl. Phys. Lett. 2015, 107, 122107. [Google Scholar] [CrossRef]
- Mao, Y.; Guo, Z.; Yuan, J.; Sun, T. 1D/2D van der Waals Heterojunctions Composed of Carbon Nanotubes and a GeSe Monolayer. Nanomaterials 2021, 11, 1565. [Google Scholar] [CrossRef]
- Tayari, V.; Senkovskiy, B.V.; Rybkovskiy, D.; Ehlen, N.; Fedorov, A.; Chen, C.; Avila, J.; Asensio, M.; Perucchi, A.; Pietro, P. Quasi-two-dimensional thermoelectricity in SnSe. Phys. Rev. B 2018, 97, 045424. [Google Scholar] [CrossRef]
- Zhao, H.; Mao, Y.; Mao, X.; Shi, X.; Xu, C.; Wang, C.; Zhang, S.; Zhou, D. Band Structure and Photoelectric Characterization of GeSe Monolayers. Adv. Funct. Mater. 2018, 28, 1704855. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, H.; Yang, Y.; Wang, C.; Zhou, D.; Shi, X.; Li, Y. Fast and broadband photoresponse of a few-layer GeSe field-effect transistor with direct band gaps. ACS Appl. Mater. Interfaces 2019, 11, 38031–38038. [Google Scholar] [CrossRef]
- Ramasamy, P.; Kwak, D.; Lim, D.; Ra, H.; Lee, J. Solution synthesis of GeS and GeSe nanosheets for high-sensitivity photodetectors. J. Mater. Chem. C Mater. Opt. Electron. Devices 2016, 4, 479–485. [Google Scholar] [CrossRef]
- He, X.; Ercius, P.; Varley, J.; Bailey, J.; Zapalac, G.; Nagle, T.; Poplavskyy, D.; Mackie, N.; Bayman, A.; Lordi, V. The role of oxygen doping on elemental intermixing at the PVD-CdS/Cu (InGa)Se2 heterojunction. Prog. Photovolt. Res. Appl. 2019, 27, 255–263. [Google Scholar] [CrossRef]
- Yoon, S.M.; Song, H.J.; Choi, H.C. p-Type Semiconducting GeSe Combs by a Vaporization–Condensation–Recrystallization (VCR) Process. Adv. Mater. 2010, 22, 2164–2167. [Google Scholar] [CrossRef]
- Sutter, P.; Komsa, H.P.; Kisslinger, K.; Sutter, E. Lateral Integration of SnS and GeSe van der Waals Semiconductors: Interface Formation, Electronic Structure, and Nanoscale Optoelectronics. ACS Nano 2023, 17, 9552–9564. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Huang, P.; Liu, K.; Jin, B.; Zhai, T. Salt-Assisted Growth of Ultrathin GeSe Rectangular Flakes for Phototransistors with Ultrahigh Responsivity. ACS Appl. Mater. Interfaces 2019, 11, 23353–23360. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, L.; Wu, H.; Shi, Y. Preparation of polycrystalline GeSe thin films and their applications in solar cells. Chin. J. Chem. 2021, 84, 814–819. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, X.; Chen, H.; Deng, J. Controllable preparation of ultrathin GeSe nanosheets for infrared photodetection. Infrared Phys. Technol. 2023, 132, 104736. [Google Scholar] [CrossRef]
- Zhao, F.; Luo, X.; Liu, L.; Lv, J.; Fan, X.; Qiao, X.; Xu, Y.; Kienle, L.; Zhang, X.; Qian, G. Visible-NIR Photodetectors Based on Low Dimensional GeSe Micro-Crystals: Designed Morphology and Improved Photoresponsivity. ChemPhysChem 2020, 21, 397–405. [Google Scholar] [CrossRef]
- Xue, D.J.; Liu, S.C.; Dai, C.M.; Chen, S.; He, C.; Zhao, L.; Hu, J.; Wan, L. GeSe thin-film solar cells fabricated by self-regulated rapid thermal sublimation. J. Am. Chem. Soc. 2017, 139, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, Q.H.; Wu, J.B.; Wei, S.; Tan, P. Review on the Raman spectroscopy of different types of layered materials. Nanoscale 2016, 8, 6435–6450. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Gao, X.P.A.; Zhang, Z. Broadband photodetection of GeSe films of vertically grown nanoflakes. ACS Appl. Electron. Mater. 2019, 1, 2236–2243. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, Q.; Liu, X.; Chang, L.; Qiu, J. Two-dimensional GeSe as an isostructural and isoelectronic analogue of phosphorene: Sonication-assisted synthesis, chemical stability, and optical properties. Chem. Mater. 2017, 29, 8361–8368. [Google Scholar] [CrossRef]
- Fukunaga, T.; Sugai, S.; Kinosada, T.; Murase, K. Observation of new Raman lines in GeSe and SnSe at low temperatures. Solid State Commun. 1981, 38, 1049–1052. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, S.; Huang, S.; Cai, B.; Xie, M.; Qu, L.; Zou, Y.; Hu, Z.; Yu, X.; Zeng, H. Structural and Electronic Properties of Atomically Thin Germanium Selenide Polymorphs. Sci. China Mater. 2015, 58, 929–935. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; He, W.; Peng, Y.; Xiang, G. Transition metal (TM = V, Cr, Mn, Fe, Co, Ni)-doped GeSe diluted magnetic semiconductor thin films with high-temperature ferromagnetism. Sci. China Mater 2024, 67, 279–288. [Google Scholar] [CrossRef]
- Uno, K.; Yamasaki, Y.; Tanaka, I. Growth mechanisms of zinc oxide and zinc sulfide films by mist chemical vapor deposition. Appl. Phys. Express 2017, 10, 015502.1–015502.4. [Google Scholar] [CrossRef]
- Myung, Y.; Im, H.; Kim, C.; Jung, C.; Cho, Y.; Jang, D.; Kim, H.; Back, S.; Park, J. Photo-induced cation exchange reaction of germanium chalcogenide nanocrystals synthesized using gas-phase laser photolysis reaction. Chem. Commun. 2013, 49, 187–189. [Google Scholar] [CrossRef]
- Wang, K.; Liu, M.; Huang, D.; Li, L.; Jiang, F. Rapid thermal deposited GeSe nanowires as a promising anode material for lithium-ion and sodium-ion batteries. J. Colloid Interface Sci. 2020, 571, 387–397. [Google Scholar] [CrossRef]
- Lam, S.M.; Sin, J.C.; Abdullah, A.Z.; Mohamed, A.R. Transition metal oxide loaded Zn O nanorods: Preparation, characterization and their UV-vis photocatalytic activities. Sep. Purif. Technol. 2014, 132, 378–387. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Lin, Y.; Li, M.; Cai, H.; Liang, Y.; Liu, M.; Lai, F.; Huang, F. Anisotropic Photoresponse of the Ultrathin GeSe Nanoplates Grown by Rapid Physical Vapor Deposition. ACS Appl. Mater. Interfaces 2019, 11, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, F.; Kang, J.; Liu, C.; Lei, M.; Pan, L.; Wang, H.; Wang, F.; Zhou, Z.; Cui, Y.; et al. Polarizer-free polarimetric image sensor through anisotropic two-dimensional GeSe. Sci. China Mater 2021, 64, 1230–1237. [Google Scholar] [CrossRef]
- Yin, Z.; Zeng, Y.; Yang, D.; Jiao, Y.; Song, J.; Hu, P.; Fan, H.; Teng, F. Multifunctional optoelectronic device based on CuO/ZnO heterojunction structure. J. Lumin. 2023, 257, 119762. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Tripathi, S. High-Performance SnS2 and CuO Nanocomposite-Based Broadband Photodetector. IEEE Trans. Electron Devices 2023, 70, 2378–2383. [Google Scholar] [CrossRef]
- Mukherjee, B.; Cai, Y.; Tan, H.R.; Feng, Y.; Tok, E.; Sow, C. NIR Schottky Photodetectors Based on Individual Single-Crystalline GeSe Nanosheet. ACS Appl. Mater. Interfaces 2013, 5, 9594–9604. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Wang, B.; Li, Y.; Wu, B.; Hao, C.; Zhang, Y.; Zeng, H. Piezo-phototronic effect regulated broadband photoresponse of a-Ga2O3/ZnO heterojunction. Nanoscale 2023, 15, 7068–7076. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhao, J.; Wang, R.; Xing, C.; Li, Z.; Huang, W.; Jiang, X.; Guo, Z.; Li, Y. Ultrathin GeSe Nanosheets: From Systematic Synthesis, to Studies of Carrier Dynamics and Applications for High Performance UV-Vis Photo-Detector. Acs Appl. Mater. Interfaces 2019, 11, 4278–4287. [Google Scholar] [CrossRef]
- Ma, X.; Liu, T.; Lu, B.; Zhang, Y. Synergistic effect of ZnMgAl-hydrotalcite nanomaterials electrocatalysts on high efficiency electrocatalytic reduction of CO2 to CO. J. Alloys Compd. 2024, 973, 172858. [Google Scholar] [CrossRef]
- Guo, S.; Yuan, L.; Liu, X.; Zhou, W.; Song, X.; Zhang, S. First-principles study of SO2 sensors based on phosphorene and its isoelectronic counterparts: GeS, GeSe, SnS, SnSe. Chem. Phys. Lett. 2017, 686, 83–87. [Google Scholar] [CrossRef]
- Guo, C.; Wang, T. Zigzag direction nanoarchitectonics of monolayer GeSe for SO2 gas sensors with high sensitivity and selectivity: A first-principles study. Appl. Phys. A 2022, 128, 975. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Q.; Wang, Z.; Ye, H.; Chen, X.; Fan, X.; Zhang, G. High Selective Gas Detection for small molecules based on Germanium selenide monolayer. Appl. Surf. Sci. 2018, 433, 575–581. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Zhang, Z.; Zhang, F.; Yang, Y. The Influence of Temperature on the Photoelectric Properties of GeSe Nanowires. Molecules 2024, 29, 2860. https://doi.org/10.3390/molecules29122860
Liu Q, Zhang Z, Zhang F, Yang Y. The Influence of Temperature on the Photoelectric Properties of GeSe Nanowires. Molecules. 2024; 29(12):2860. https://doi.org/10.3390/molecules29122860
Chicago/Turabian StyleLiu, Qiaoping, Zhiyong Zhang, Fuchun Zhang, and Yanning Yang. 2024. "The Influence of Temperature on the Photoelectric Properties of GeSe Nanowires" Molecules 29, no. 12: 2860. https://doi.org/10.3390/molecules29122860
APA StyleLiu, Q., Zhang, Z., Zhang, F., & Yang, Y. (2024). The Influence of Temperature on the Photoelectric Properties of GeSe Nanowires. Molecules, 29(12), 2860. https://doi.org/10.3390/molecules29122860





