Chitosan–Surfactant Composite Nanocoatings on Glass and Zinc Surfaces Prepared from Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
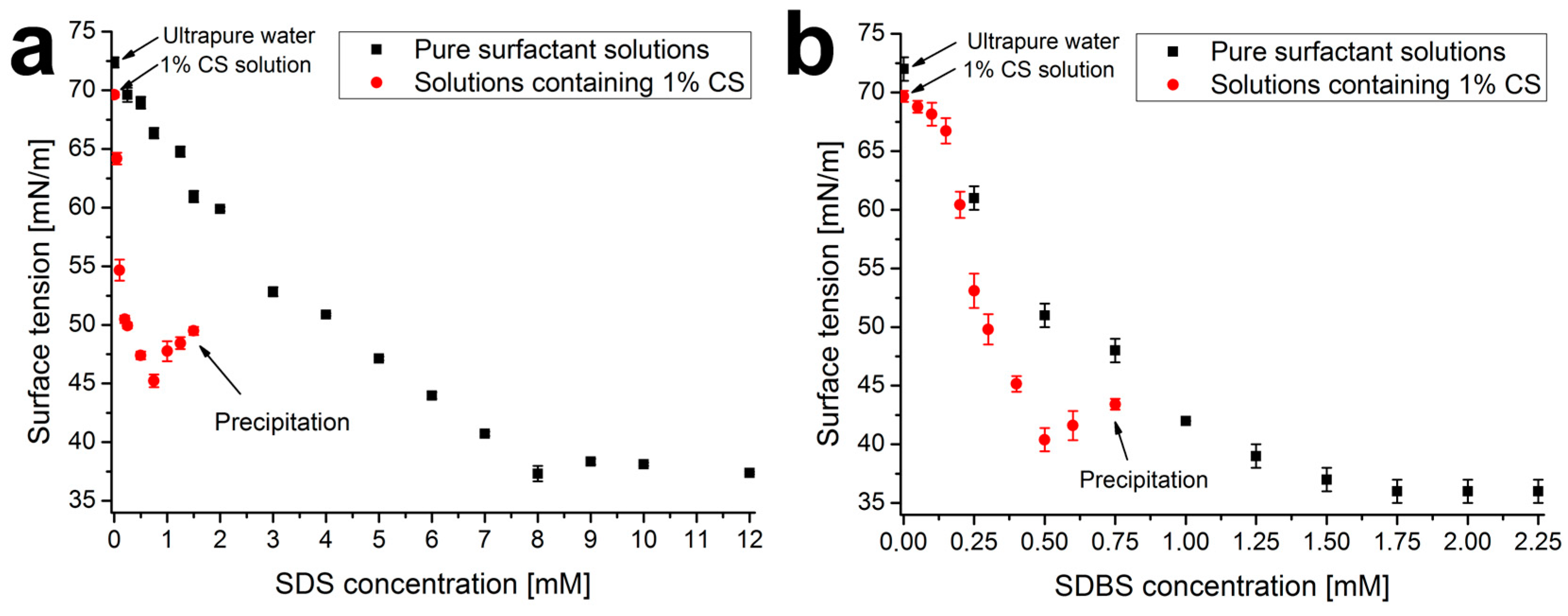
2.1. Surface Tension Measurements
2.2. UV-Vis Spectroscopy: Layer Thickness and Refractive Index
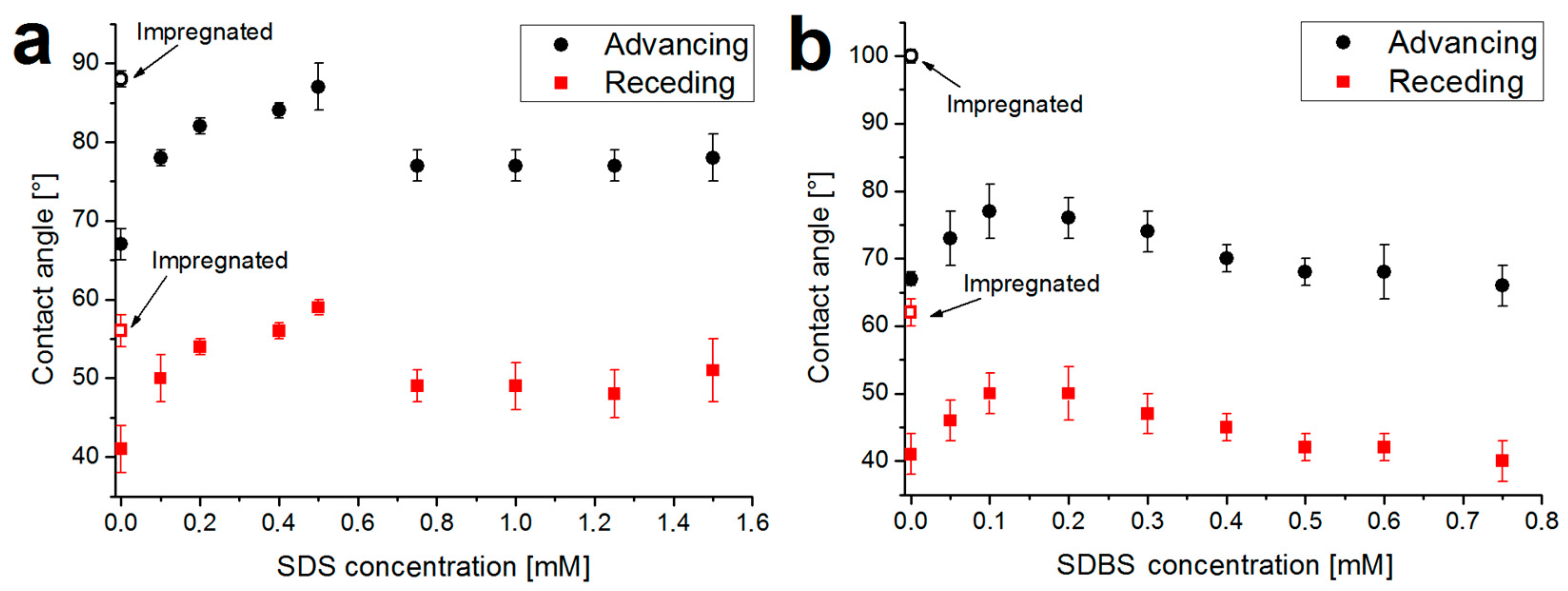
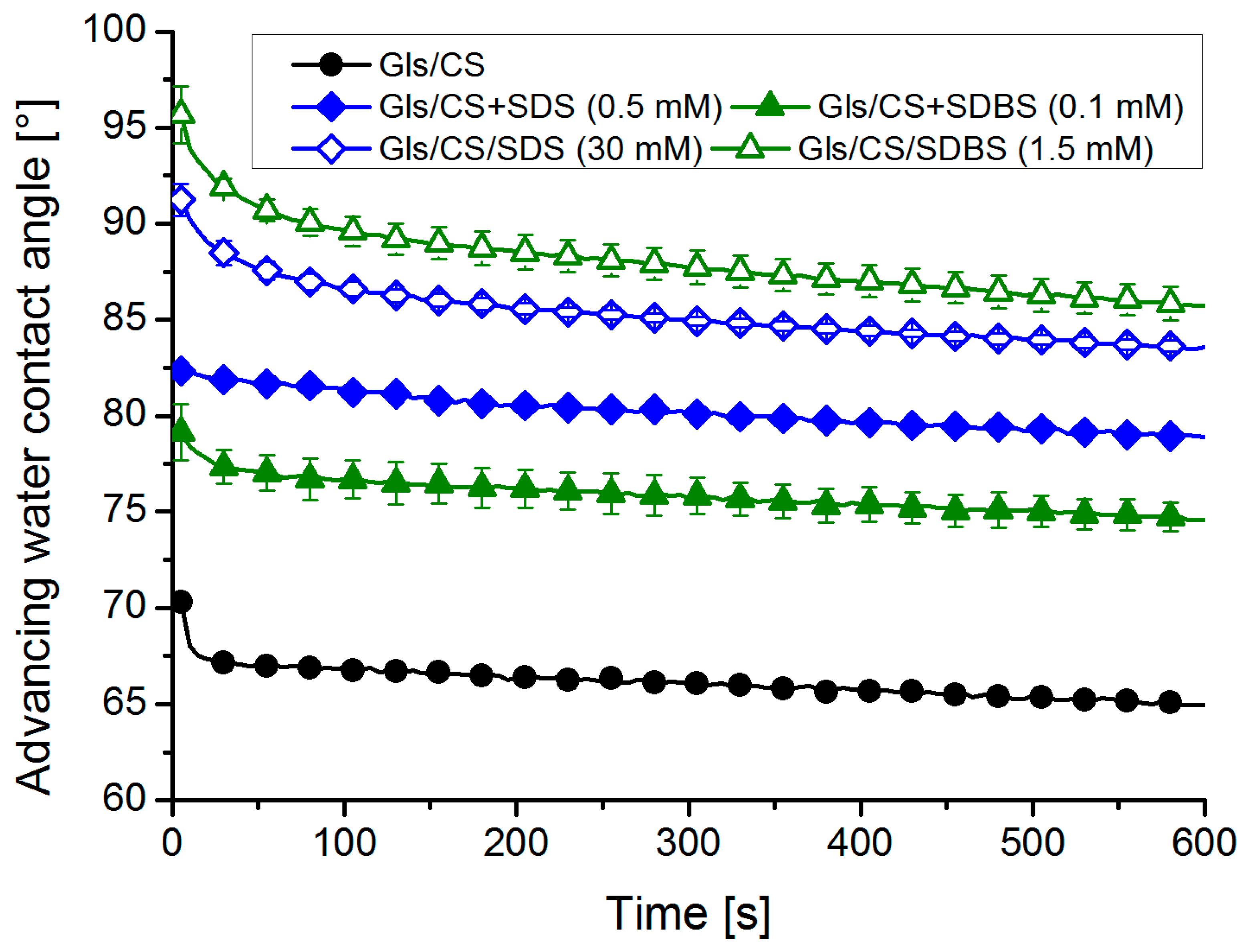
2.3. Wettability: Contact Angle Measurements
2.4. AFM Measurements: Surface Morphology
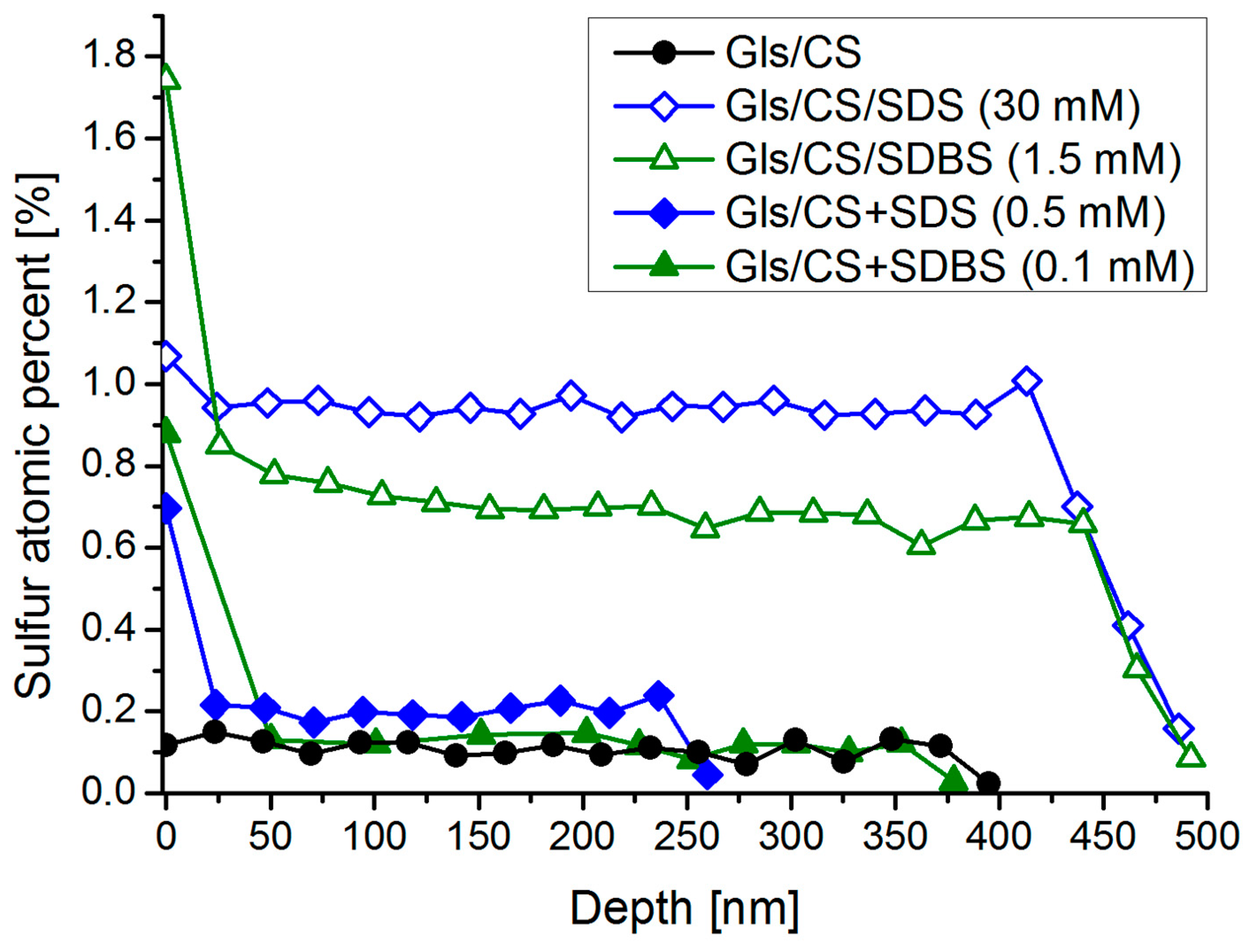
2.5. XPS Measurements: Distribution of Surfactants in the Layer
2.6. Electrochemical Tests: Barrier Properties and Corrosion Protection
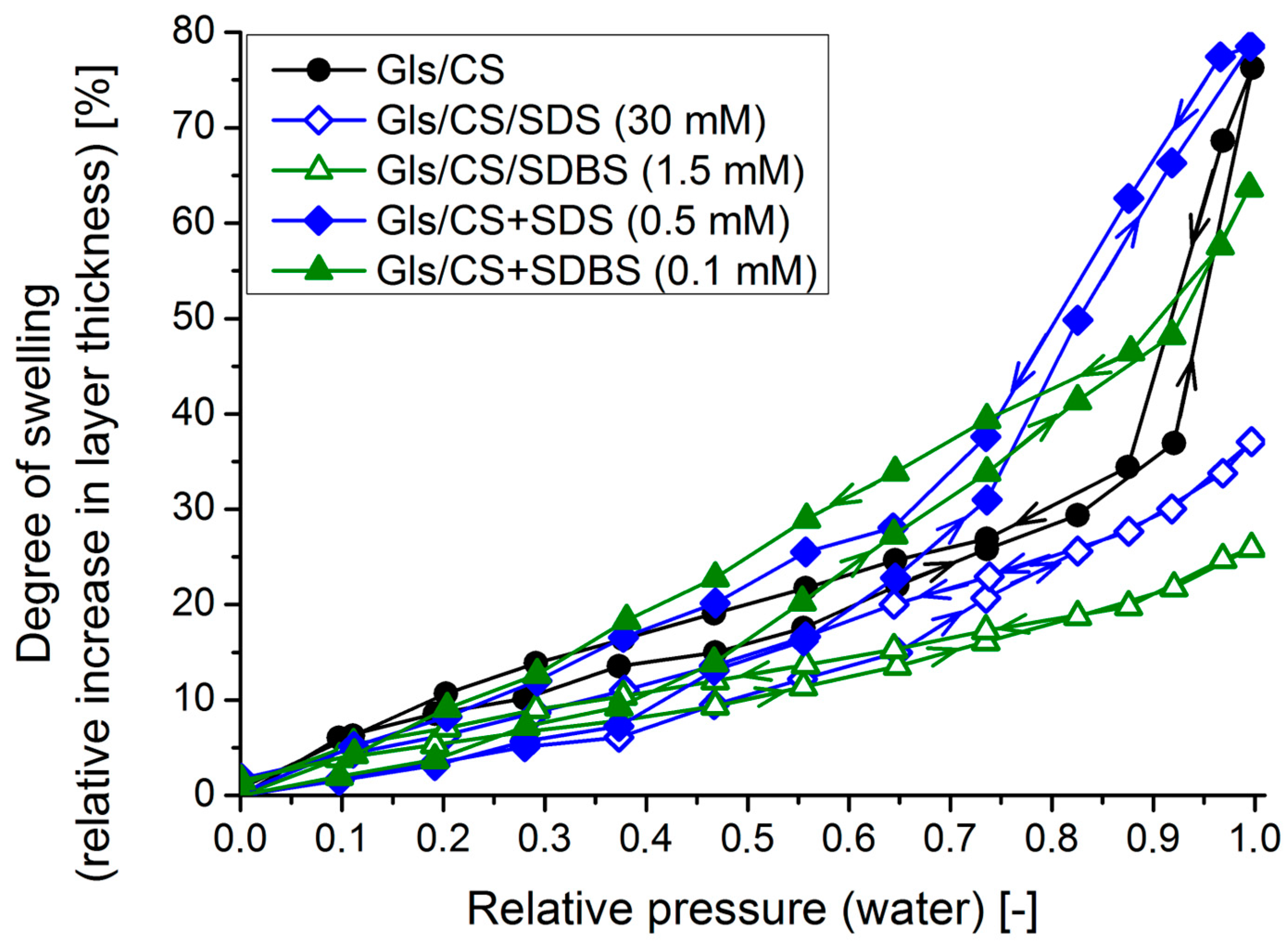
2.7. Spectroscopic Ellipsometry: Swelling in a Humid Environment
3. Materials and Methods
3.1. Materials
3.2. Coating Preparation
3.3. UV-Vis Spectroscopy
3.4. Surface Tension Measurements
3.5. Wettability Measurements
3.6. Rheological Measurements
3.7. AFM Measurements
3.8. Electrochemical Measurements
3.9. XPS Measurements
3.10. Spectroscopic Ellipsometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. UV-Visible Spectroscopy
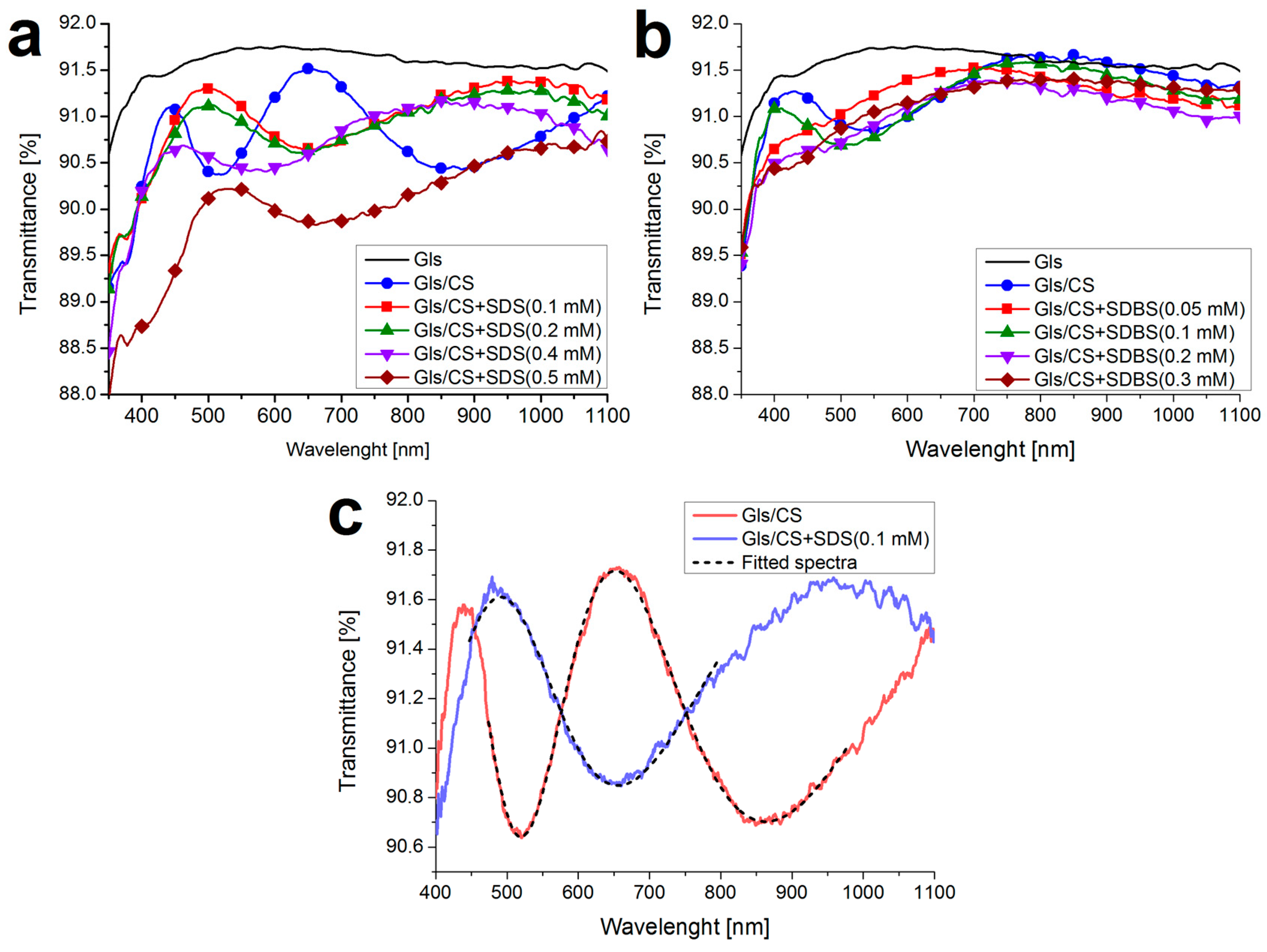
Appendix A.2. Rheological Measurements
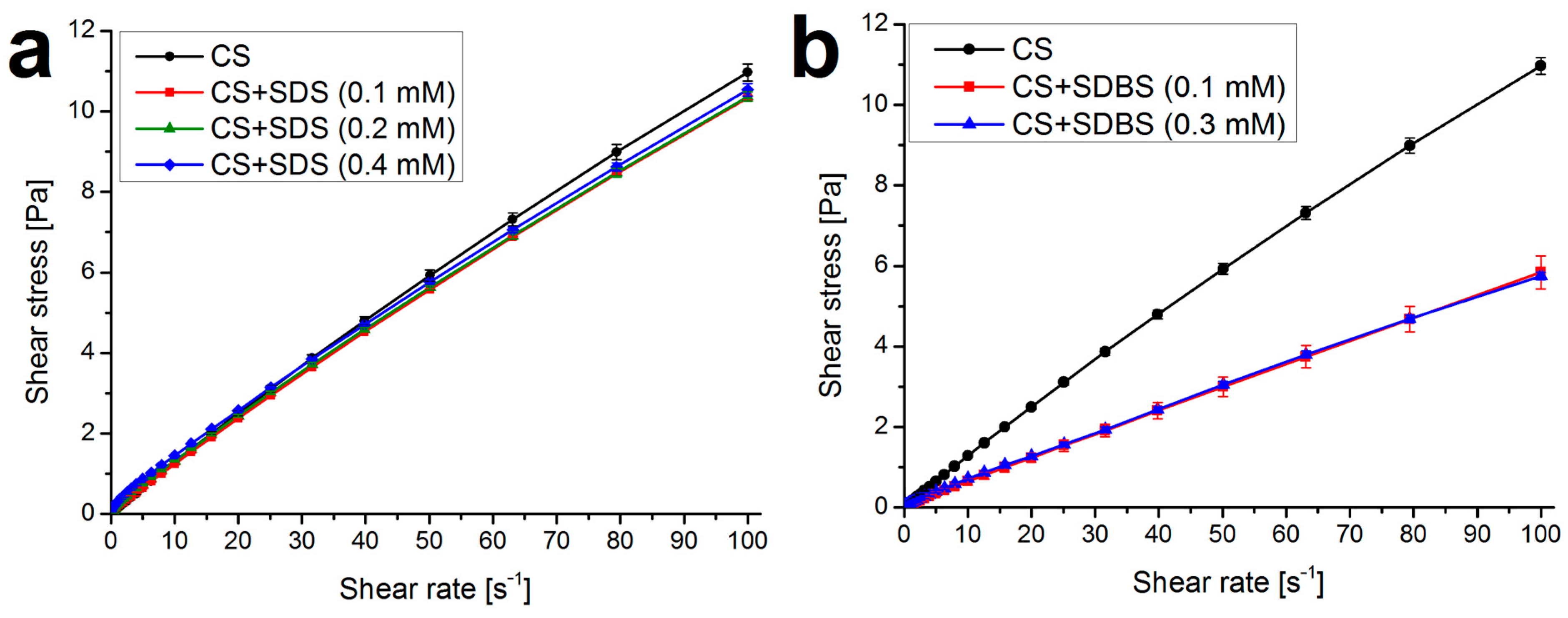
Appendix A.3. Atomic Force Microscopy: Validating Layer Thickness

Appendix A.4. X-ray Photoelectron Spectroscopy: Peak Decomposition

Appendix A.5. Electrochemical measurements
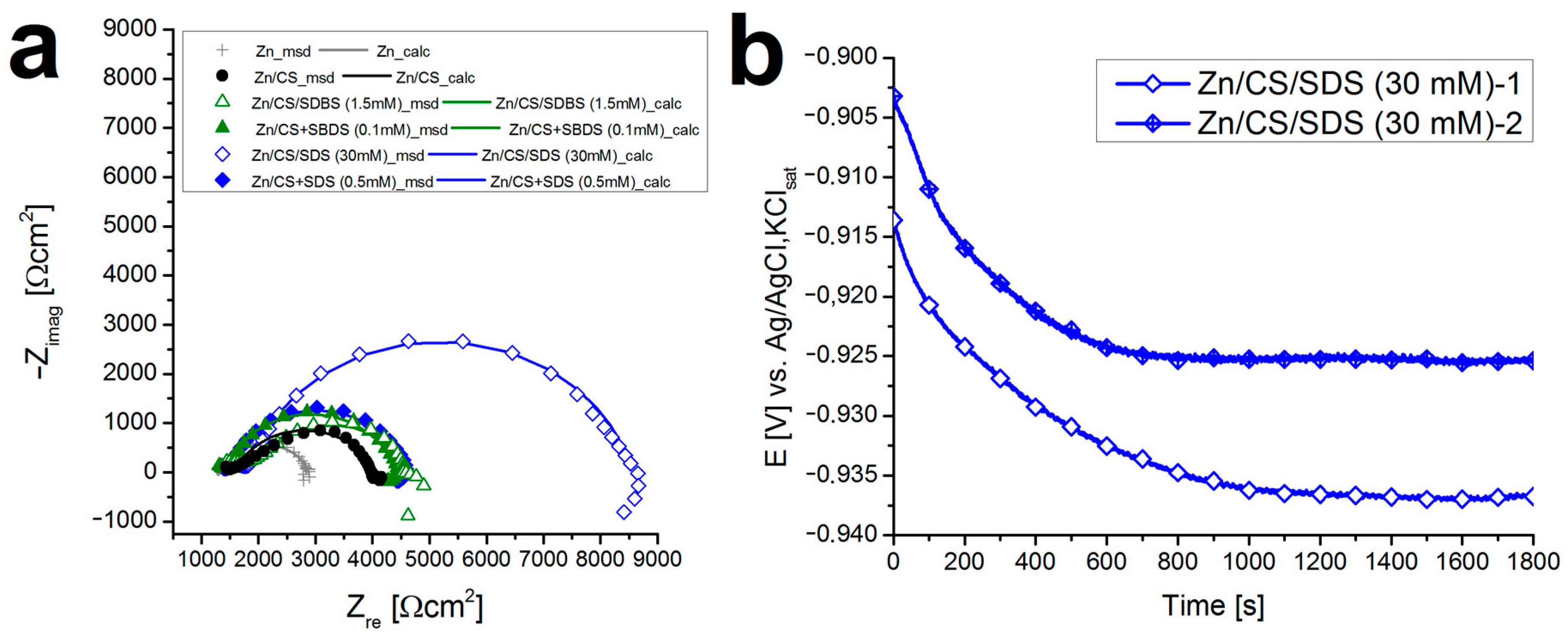
References
- Sebestyén, Z.; Jakab, E.; Domán, A.; Bokrossy, P.; Bertóti, I.; Madarász, J.; László, K. Thermal Degradation of Crab Shell Biomass, a Nitrogen-Containing Carbon Precursor. J. Therm. Anal. Calorim. 2020, 142, 301–308. [Google Scholar] [CrossRef]
- Przykaza, K.; Jurak, M.; Kalisz, G.; Mroczka, R.; Wiącek, A.E. Characteristics of Hybrid Bioglass-Chitosan Coatings on the Plasma Activated PEEK Polymer. Molecules 2023, 28, 1729. [Google Scholar] [CrossRef] [PubMed]
- Szafran, K.; Jurak, M.; Mroczka, R.; Wiącek, A.E. Preparation and Surface Characterization of Chitosan-Based Coatings for PET Materials. Molecules 2023, 28, 2375. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Mohamed, A.A. The Use of Chitosan-Based Composites for Environmental Remediation: A Review. Int. J. Biol. Macromol. 2023, 242, 124787. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Gänzle, M.G. Challenges and Opportunities Related to the Use of Chitosan as a Food Preservative. J. Appl. Microbiol. 2018, 126, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Kulka, K.; Sionkowska, A. Chitosan Based Materials in Cosmetic Applications: A Review. Molecules 2023, 28, 1817. [Google Scholar] [CrossRef] [PubMed]
- Ashassi-Sorkhabi, H.; Kazempour, A. Chitosan, Its Derivatives and Composites with Superior Potentials for the Corrosion Protection of Steel Alloys: A Comprehensive Review. Carbohydr. Polym. 2020, 237, 116110. [Google Scholar] [CrossRef] [PubMed]
- Bahari, H.S.; Ye, F.; Carrillo, E.A.T.; Leliopoulos, C.; Savaloni, H.; Dutta, J. Chitosan Nanocomposite Coatings with Enhanced Corrosion Inhibition Effects for Copper. Int. J. Biol. Macromol. 2020, 162, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Eddahhaoui, F.-Z.; Najem, A.; Elhawary, M.; Boudalia, M.; Campos, O.S.; Tabyaoui, M.; José Garcia, A.; Bellaouchou, A.; Amin, H.M.A. Experimental and Computational Aspects of Green Corrosion Inhibition for Low Carbon Steel in HCl Environment Using Extract of Chamaerops Humilis Fruit Waste. J. Alloys Compd. 2024, 977, 173307. [Google Scholar] [CrossRef]
- Galal, A.; Amin, H. Corrosion Protection of Metals and Alloys Using Graphene and Biopolymer Based Nanocomposites; Amin, H.M.A., Galal, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781315171364. [Google Scholar]
- Percot, A.; Viton, C.; Domard, A. Optimization of Chitin Extraction from Shrimp Shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef]
- Berezina, N. Production and Application of Chitin. Phys. Sci. Rev. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Murali, A.; Ganesan, M.; Satapathy, D.K.; Kumar, P.B.S. Penetrant-Induced Glass-like Transition in Thin Chitosan Films. J. Phys. Chem. B 2021, 125, 12617–12626. [Google Scholar] [CrossRef]
- Shamsheera, K.; Prasad, A.R.; PK, J.; Joseph, A. Development of Self-Assembled Monolayer of Stearic Acid Grafted Chitosan on Mild Steel and Inhibition of Corrosion in Hydrochloric Acid. Chem. Data Collect. 2020, 28, 100402. [Google Scholar] [CrossRef]
- Márton, P.; Szolnoki, B.; Nagy, N.; Deák, A.; Zámbó, D.; Szabó, G.S.; Hórvölgyi, Z. Wetting and Swelling Behaviour of N-Acetylated Thin Chitosan Coatings in Aqueous Media. Heliyon 2024, 10, e23201. [Google Scholar] [CrossRef] [PubMed]
- Goddard, E.D. Polymer/Surfactant Interaction: Interfacial Aspects. J. Colloid Interface Sci. 2002, 256, 228–235. [Google Scholar] [CrossRef]
- Claesson, P.M.; Dedinaite, A.; Mészáros, R.; Varga, I. Association between Polyelectrolytes and Oppositely Charged Surfactants in Bulk and at Solid/Liquid Interfaces; Wiley: Hoboken, NJ, USA, 2014; Volume 6, ISBN 9783527631193. [Google Scholar]
- Guzmán, E.; Llamas, S.; Maestro, A.; Fernández-Peña, L.; Akanno, A.; Miller, R.; Ortega, F.; Rubio, R.G. Polymer–Surfactant Systems in Bulk and at Fluid Interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. [Google Scholar] [CrossRef]
- Mezei, A.; Mészáros, R. Novel Method for the Estimation of the Binding Isotherms of Ionic Surfactants on Oppositely Charged Polyelectrolytes. Langmuir 2006, 22, 7148–7151. [Google Scholar] [CrossRef]
- Bekale, L.; Agudelo, D.; Tajmir-Riahi, H.A. Effect of Polymer Molecular Weight on Chitosan–Protein Interaction. Colloids Surf. B Biointerfaces 2015, 125, 309–317. [Google Scholar] [CrossRef]
- Darvas, M.; Gilányi, T.; Jedlovszky, P. Competitive Adsorption of Surfactants and Polymers at the Free Water Surface. A Computer Simulation Study of the Sodium Dodecyl Sulfate-Poly(Ethylene Oxide) System. J. Phys. Chem. B 2011, 115, 933–944. [Google Scholar] [CrossRef]
- Mezei, A.; Mészáros, R.; Varga, I.; Gilányi, T. Effect of Mixing on the Formation of Complexes of Hyperbranched Cationic Polyelectrolytes and Anionic Surfactants. Langmuir 2007, 23, 4237–4247. [Google Scholar] [CrossRef]
- Thongngam, M.; McClements, D.J. Characterization of Interactions between Chitosan and an Anionic Surfactant. J. Agric. Food Chem. 2004, 52, 987–991. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Lylyk, S.V.; Aksenenko, E.V.; Petkov, J.T.; Yorke, J.; Miller, R. Surface Tension Isotherms, Adsorption Dynamics and Dilational Visco-Elasticity of Sodium Dodecyl Sulphate Solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 8–15. [Google Scholar] [CrossRef]
- Hait, S.K.; Majhi, P.R.; Blume, A.; Moulik, S.P. A Critical Assessment of Micellization of Sodium Dodecyl Benzene Sulfonate (SDBS) and Its Interaction with Poly(Vinyl Pyrrolidone) and Hydrophobically Modified Polymers, JR 400 and LM 200. J. Phys. Chem. B 2003, 107, 3650–3658. [Google Scholar] [CrossRef]
- Chauhan, S.; Sharma, K. Effect of Temperature and Additives on the Critical Micelle Concentration and Thermodynamics of Micelle Formation of Sodium Dodecyl Benzene Sulfonate and Dodecyltrimethylammonium Bromide in Aqueous Solution: A Conductometric Study. J. Chem. Thermodyn. 2014, 71, 205–211. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Y. Switching Pickering Emulsion Stabilized by Chitosan-SDS Complexes through Ion Competition. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124316. [Google Scholar] [CrossRef]
- Bain, C.D.; Claesson, P.M.; Langevin, D.; Meszaros, R.; Nylander, T.; Stubenrauch, C.; Titmuss, S.; von Klitzing, R. Complexes of Surfactants with Oppositely Charged Polymers at Surfaces and in Bulk. Adv. Colloid Interface Sci. 2010, 155, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, R.; Thompson, L.; Bos, M.; Varga, I.; Gilányi, T. Interaction of Sodium Dodecyl Sulfate with Polyethyleneimine: Surfactant-Induced Polymer Solution Colloid Dispersion Transition. Langmuir 2003, 19, 609–615. [Google Scholar] [CrossRef]
- Jain, N.; Trabelsi, S.; Guillot, S.; McLoughlin, D.; Langevin, D.; Letellier, P.; Turmine, M. Critical Aggregation Concentration in Mixed Solutions of Anionic Polyelectrolytes and Cationic Surfactants. Langmuir 2004, 20, 8496–8503. [Google Scholar] [CrossRef] [PubMed]
- Hild, E.; Deák, A.; Naszályi, L.; Sepsi, Ö.; Ábrahám, N.; Hórvölgyi, Z. Use of the Optical Admittance Function and Its WKB Approximation to Simulate and Evaluate Transmittance Spectra of Graded-Index Colloidal Films. J. Opt. A Pure Appl. Opt. 2007, 9, 920–930. [Google Scholar] [CrossRef]
- Landau, L.; Levich, B. Dragging of a Liquid by a Moving Plate. Dyn. Curved Front. 1942, XVII, 141–153. [Google Scholar] [CrossRef]
- Barreiro-Iglesias, R.; Alvarez-Lorenzo, C.; Concheiro, A. Chitosan/Sodium Dodecylsulfate Interactions. J. Therm. Anal. Calorim. 2005, 82, 499–505. [Google Scholar] [CrossRef]
- Josyula, T.; Kumar Malla, L.; Thomas, T.M.; Kalichetty, S.S.; Sinha Mahapatra, P.; Pattamatta, A. Fundamentals and Applications of Surface Wetting. Langmuir 2024, 40, 8293–8326. [Google Scholar] [CrossRef] [PubMed]
- Millet, F.; Auvergne, R.; Caillol, S.; David, G.; Manseri, A.; Pébère, N. Improvement of Corrosion Protection of Steel by Incorporation of a New Phosphonated Fatty Acid in a Phosphorus-Containing Polymer Coating Obtained by UV Curing. Prog. Org. Coat. 2014, 77, 285–291. [Google Scholar] [CrossRef]
- Buier, R.; Szabó, G.S.; Katona, G.; Muntean, N.; Muresan, L.M. Influence of PH on the Inhibiting Characteristics of Cresol Red Incorporated in Chitosan Coatings on Zinc. Metals 2023, 13, 1958. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, C.; Wang, X.; Li, Z.; Yang, G. Structure and Properties of Chitosan/Sodium Dodecyl Sulfate Composite Films. RSC Adv. 2022, 12, 3969–3978. [Google Scholar] [CrossRef] [PubMed]
- Szőke, Á.F.; Szabó, G.S.; Hórvölgyi, Z.; Albert, E.; Végh, A.G.; Zimányi, L.; Muresan, L.M. Accumulation of 2-Acetylamino-5-Mercapto-1,3,4-Thiadiazole in Chitosan Coatings for Improved Anticorrosive Effect on Zinc. Int. J. Biol. Macromol. 2020, 142, 423–431. [Google Scholar] [CrossRef]
- Monte, M.L.; Moreno, M.L.; Senna, J.; Arrieche, L.S.; Pinto, L.A.A. Moisture Sorption Isotherms of Chitosan-Glycerol Films: Thermodynamic Properties and Microstructure. Food Biosci. 2018, 22, 170–177. [Google Scholar] [CrossRef]
- Tantala, J.; Rachtanapun, C.; Tongdeesoontorn, W.; Jantanasakulwong, K.; Rachtanapun, P. Moisture Sorption Isotherms and Prediction Models of Carboxymethyl Chitosan Films from Different Sources with Various Plasticizers. Adv. Mater. Sci. Eng. 2019, 2019, 4082439. [Google Scholar] [CrossRef]
- Available online: www.gwyddion.net (accessed on 26 June 2024).
- Feliu, S.; Bastidas, J.M.; Galvan, J.C.; Simancas, J.; Morcillo, M.; Almeida, E. Electrochemical Determination of Rusted Steel Surface Stability. J. Appl. Electrochem. 1993, 23, 157–161. [Google Scholar] [CrossRef]
- Boudalia, M.; Laourayed, M.; El Moudane, M.; Sekkat, Z.; Campos, O.S.; Bellaouchou, A.; Guenbour, A.; José Garcia, A.; Amin, H.M.A. Phosphate Glass Doped with Niobium and Bismuth Oxides as an Eco-Friendly Corrosion Protection Matrix of Iron Steel in HCl Medium: Experimental and Theoretical Insights. J. Alloys Compd. 2023, 938, 168570. [Google Scholar] [CrossRef]
- Gilbert, J.B.; Rubner, M.F.; Cohen, R.E. Depth-Profiling X-ray Photoelectron Spectroscopy (XPS) Analysis of Interlayer Diffusion in Polyelectrolyte Multilayers. Proc. Natl. Acad. Sci. USA 2013, 110, 6651–6656. [Google Scholar] [CrossRef] [PubMed]
- Taketa, T.B.; dos Santos, D.M.; Fiamingo, A.; Vaz, J.M.; Beppu, M.M.; Campana-Filho, S.P.; Cohen, R.E.; Rubner, M.F. Investigation of the Internal Chemical Composition of Chitosan-Based LbL Films by Depth-Profiling X-ray Photoelectron Spectroscopy (XPS) Analysis. Langmuir 2018, 34, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Maachou, H.; Genet, M.J.; Aliouche, D.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS Analysis of Chitosan-Hydroxyapatite Biomaterials: From Elements to Compounds. Surf. Interface Anal. 2013, 45, 1088–1097. [Google Scholar] [CrossRef]
- Rocha Neto, J.B.M.; Taketa, T.B.; Bataglioli, R.A.; Pimentel, S.B.; Santos, D.M.; Fiamingo, A.; Costa, C.A.R.; Campana-Filho, S.P.; Carvalho, H.F.; Beppu, M.M. Tailored Chitosan/Hyaluronan Coatings for Tumor Cell Adhesion: Effects of Topography, Charge Density and Surface Composition. Appl. Surf. Sci. 2019, 486, 508–518. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.; Ma, M.; Douthwaite, M.; He, C.; Miao, J.; Chen, J.; Li, C. SO2 Promoted in Situ Recovery of Thermally Deactivated Fe2(SO4)3/TiO2 NH3-SCR Catalysts: From Experimental Work to Theoretical Study. Chem. Eng. J. 2019, 361, 820–829. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Liu, Z. Significantly Enhanced Catalytic Performance of Fe2(SO4)3/CeO2 Catalyst for the Selective Catalytic Reduction of NOx by NH3. Ind. Eng. Chem. Res. 2021, 60, 15472–15478. [Google Scholar] [CrossRef]


| Sample | Refractive Index (–) | Layer Thickness (nm) | Relative Increase in Layer Thickness (Δdrel) (%) | |
|---|---|---|---|---|
| Native | Gls/CS | 1.5329 ± 0.0028 | 395 ± 7 | - |
| Mixed | Gls/CS+SDS (0.1 mM) | 1.5417 ± 0.0008 | 325 ± 11 | −18 ± 3 |
| Gls/CS+SDS (0.2 mM) | 1.5419 ± 0.0010 | 310 ± 4 | −22 ± 1 | |
| Gls/CS+SDS (0.4 mM) | 1.5478 ± 0.0010 | 293 ± 7 | −26 ± 2 | |
| Gls/CS+SDS (0.5 mM) | 1.5486 ± 0.0011 | 260 ± 1 | −34 ± 0 | |
| Gls/CS+SDBS (0.05 mM) | 1.5320 ± 0.0007 | 334 ± 11 | −15 ± 4 | |
| Gls/CS+SDBS (0.1 mM) | 1.5346 ± 0.0019 | 378 ± 10 | −4 ± 4 | |
| Gls/CS+SDBS (0.2 mM) | 1.5346 ± 0.0018 | 367 ± 11 | −7 ± 4 | |
| Gls/CS+SDBS (0.3 mM) | 1.5304 ± 0.0015 | 409 ± 11 | 4 ± 5 | |
| Impregnated | Gls/CS/SDS (30 mM) | 1.5238 ± 0.0027 | 486 ± 8 | 24 ± 2 |
| Gls/CS/SDBS (1.5 mM) | 1.5321 ± 0.0026 | 492 ± 10 | 25 ± 2 | |
| Sample | Rs (kΩcm2) | Qcoat (μSsn) | Rcoat (kΩcm2) | Qdl (μSsn) | Rct (kΩcm2) | Rp = Rcoat + Rct (kΩcm2) | χ² (–) | IE (%) |
|---|---|---|---|---|---|---|---|---|
| Zn | 1.29 | - | - | 24.79 | 1.58 | 1.58 | 6.51 × 10−4 | - |
| Zn/CS | 1.43 | 0.39 | 0.19 | 16.76 | 2.42 | 2.61 | 2.50 × 10−3 | 39.5 |
| Zn/CS+SDS (0.5 mM) | 1.22 | 0.08 | 0.37 | 14.33 | 3.11 | 3.35 | 6.35 × 10−4 | 52.8 |
| Zn/CS+SDBS (0.1 mM) | 1.21 | 0.10 | 0.23 | 16.54 | 2.97 | 3.20 | 7.77 × 10−4 | 52.4 |
| Zn/CS/SDS (30 mM) | 1.49 | 0.12 | 0.33 | 17.31 | 6.74 | 7.07 | 1.01 × 10−3 | 77.7 |
| Zn/CS/SDBS (1.5 mM) | 1.39 | 0.12 | 0.45 | 22.94 | 2.86 | 3.32 | 2.49 × 10−3 | 50.6 |
| Sample | Linear Polarization | Tafel Interpretation | |||||
|---|---|---|---|---|---|---|---|
| Ecorr (V) | Rp (Ω) | P (%) | ba (V/dec) | Ecorr (V) | icorr (mA/cm2) | IE (%) | |
| Zn | −0.985 | 3206 (Rps) | - | 0.167 | −0.880 | 13.93 | - |
| Zn/CS | −0.970 | 3240 | 94 | 0.258 | −0.959 | 8.34 | 40.2 |
| Zn/CS+SDS (0.5 mM) | −0.954 | 4225 | 68 | 0.230 | −0.942 | 9.20 | 33.9 |
| Zn/CS+SDBS (0.1 mM) | −0.955 | 3954 | 73 | 0.304 | −0.942 | 6.16 | 55.8 |
| Zn/CS/SDS (30 mM) | −0.925 | 6897 | 37 | 0.334 | −0.903 | 5.38 | 61.4 |
| Zn/CS/SDBS (1.5 mM) | −0.911 | 4690 | 52 | 0.214 | −0.885 | 6.31 | 54.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márton, P.; Áder, L.; Kemény, D.M.; Rácz, A.; Kovács, D.; Nagy, N.; Szabó, G.S.; Hórvölgyi, Z. Chitosan–Surfactant Composite Nanocoatings on Glass and Zinc Surfaces Prepared from Aqueous Solutions. Molecules 2024, 29, 3111. https://doi.org/10.3390/molecules29133111
Márton P, Áder L, Kemény DM, Rácz A, Kovács D, Nagy N, Szabó GS, Hórvölgyi Z. Chitosan–Surfactant Composite Nanocoatings on Glass and Zinc Surfaces Prepared from Aqueous Solutions. Molecules. 2024; 29(13):3111. https://doi.org/10.3390/molecules29133111
Chicago/Turabian StyleMárton, Péter, Liza Áder, Dávid Miklós Kemény, Adél Rácz, Dorina Kovács, Norbert Nagy, Gabriella Stefánia Szabó, and Zoltán Hórvölgyi. 2024. "Chitosan–Surfactant Composite Nanocoatings on Glass and Zinc Surfaces Prepared from Aqueous Solutions" Molecules 29, no. 13: 3111. https://doi.org/10.3390/molecules29133111







