Direct Regeneration of Degraded LiFePO4 Cathode via Reductive Solution Relithiation Regeneration Process
Abstract
1. Introduction
2. Results and Discussion
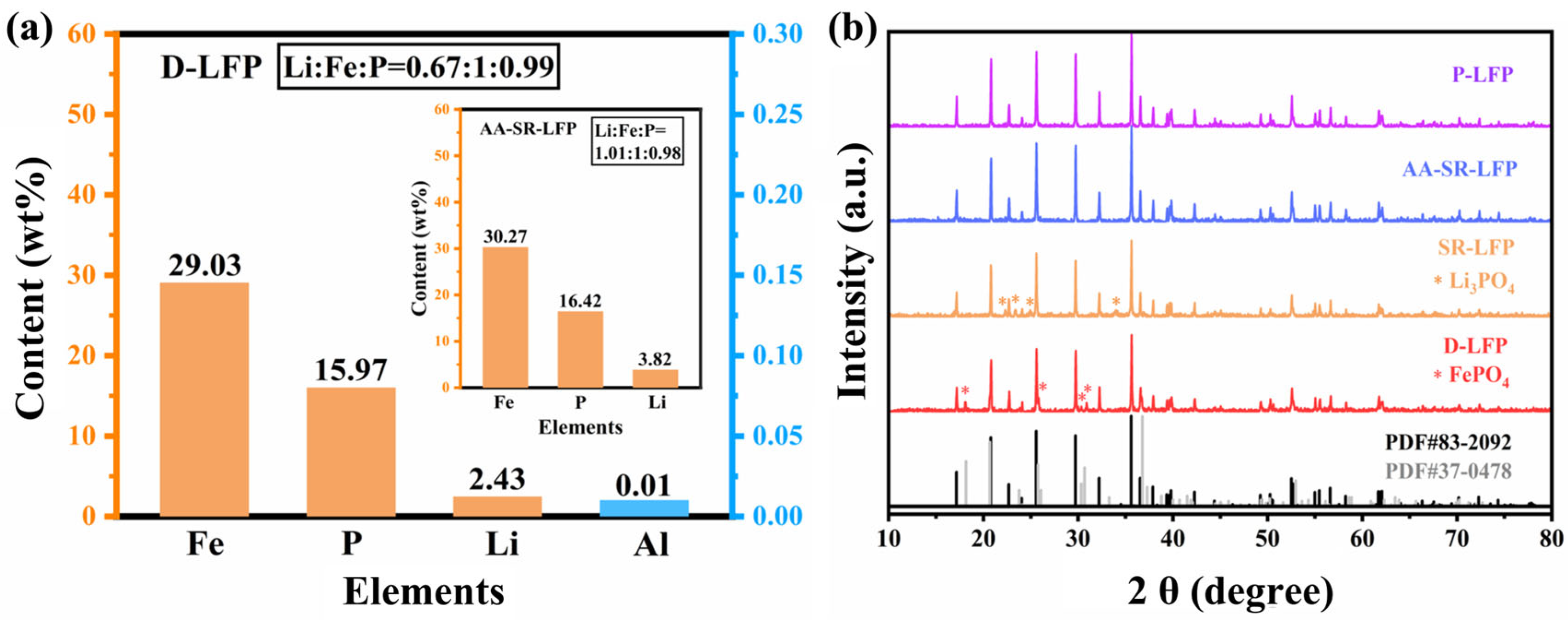
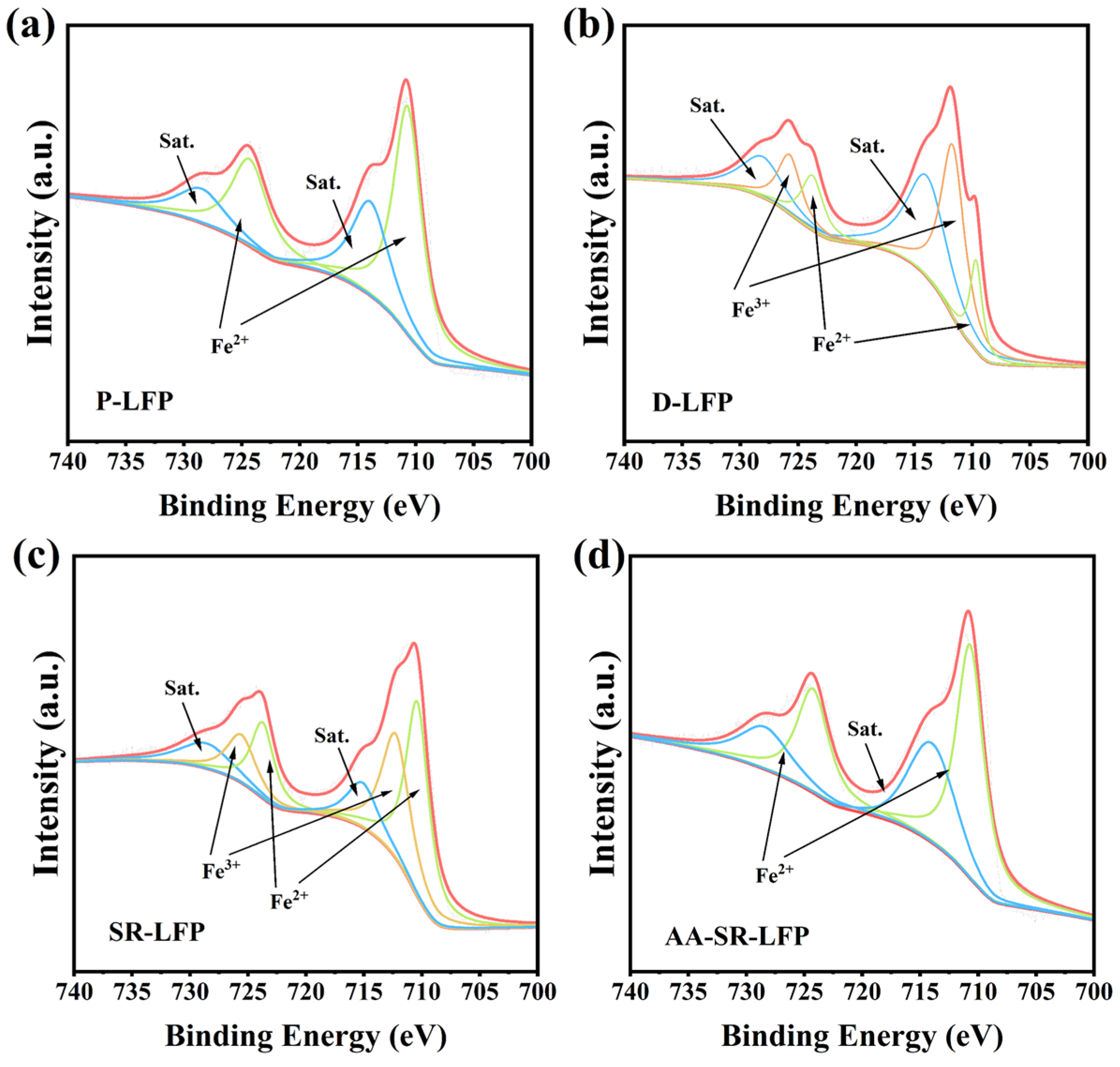
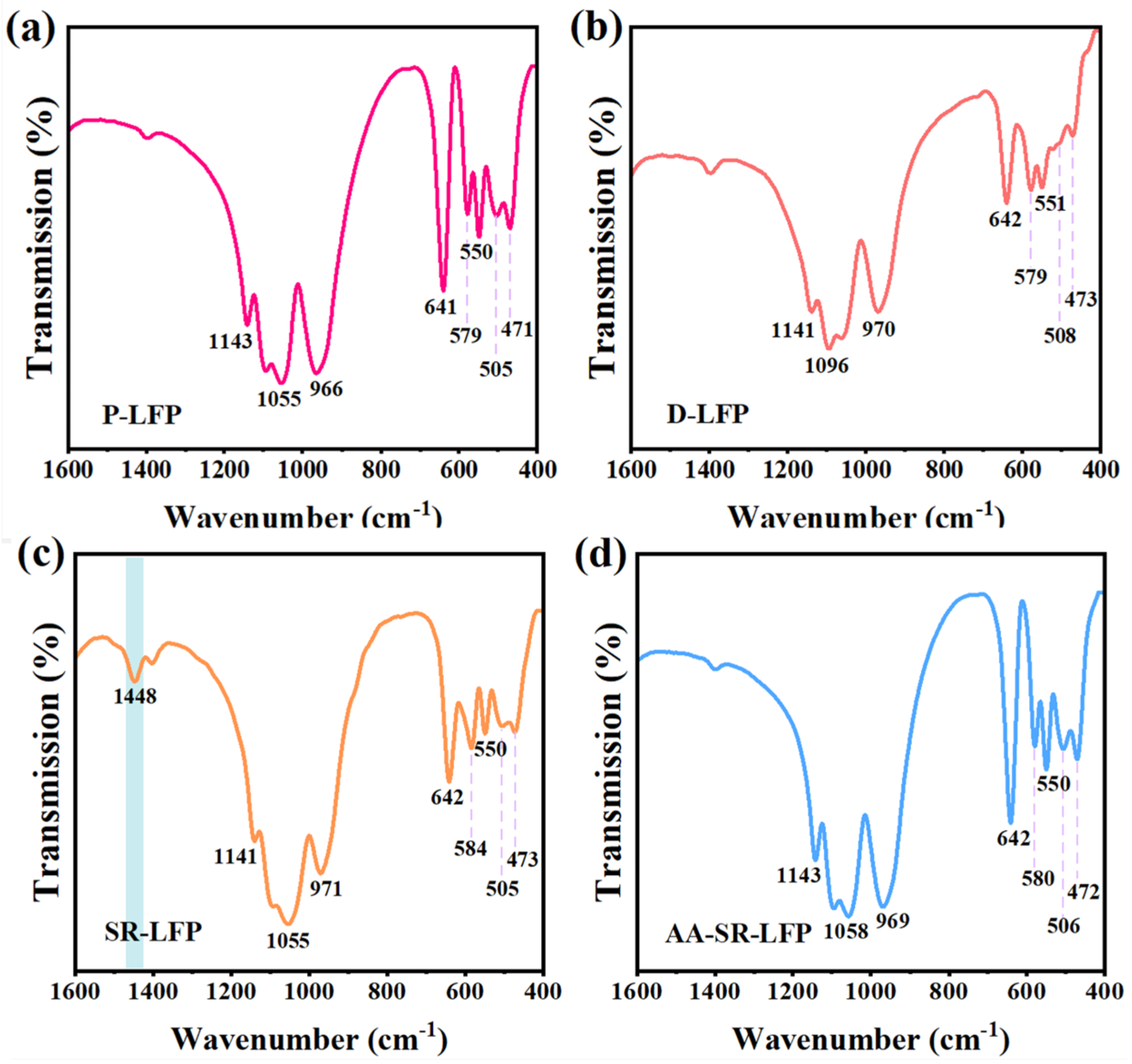
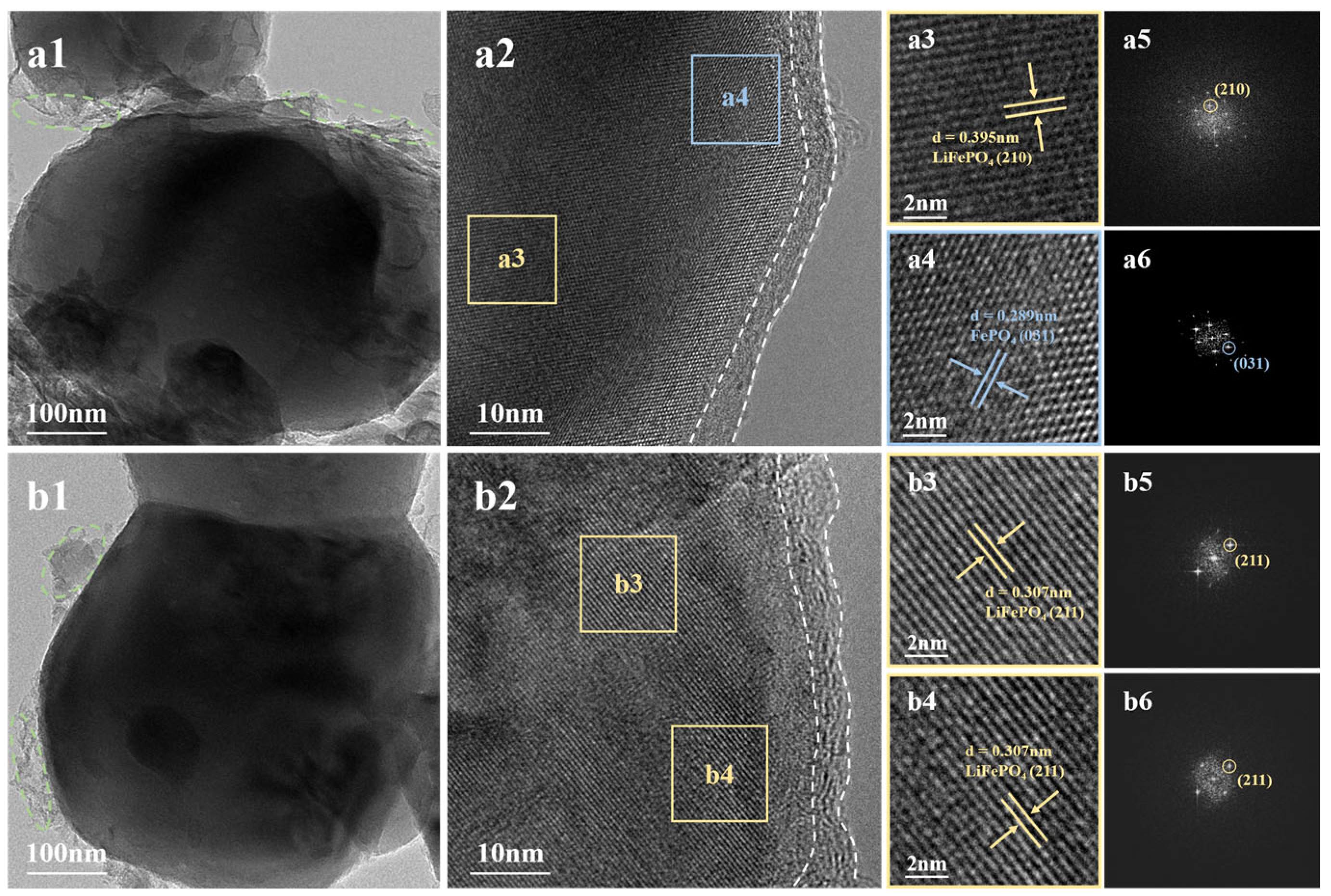
2.1. Structural and Morphology Characterization
2.2. Electrochemical Performance
3. Experimental Section
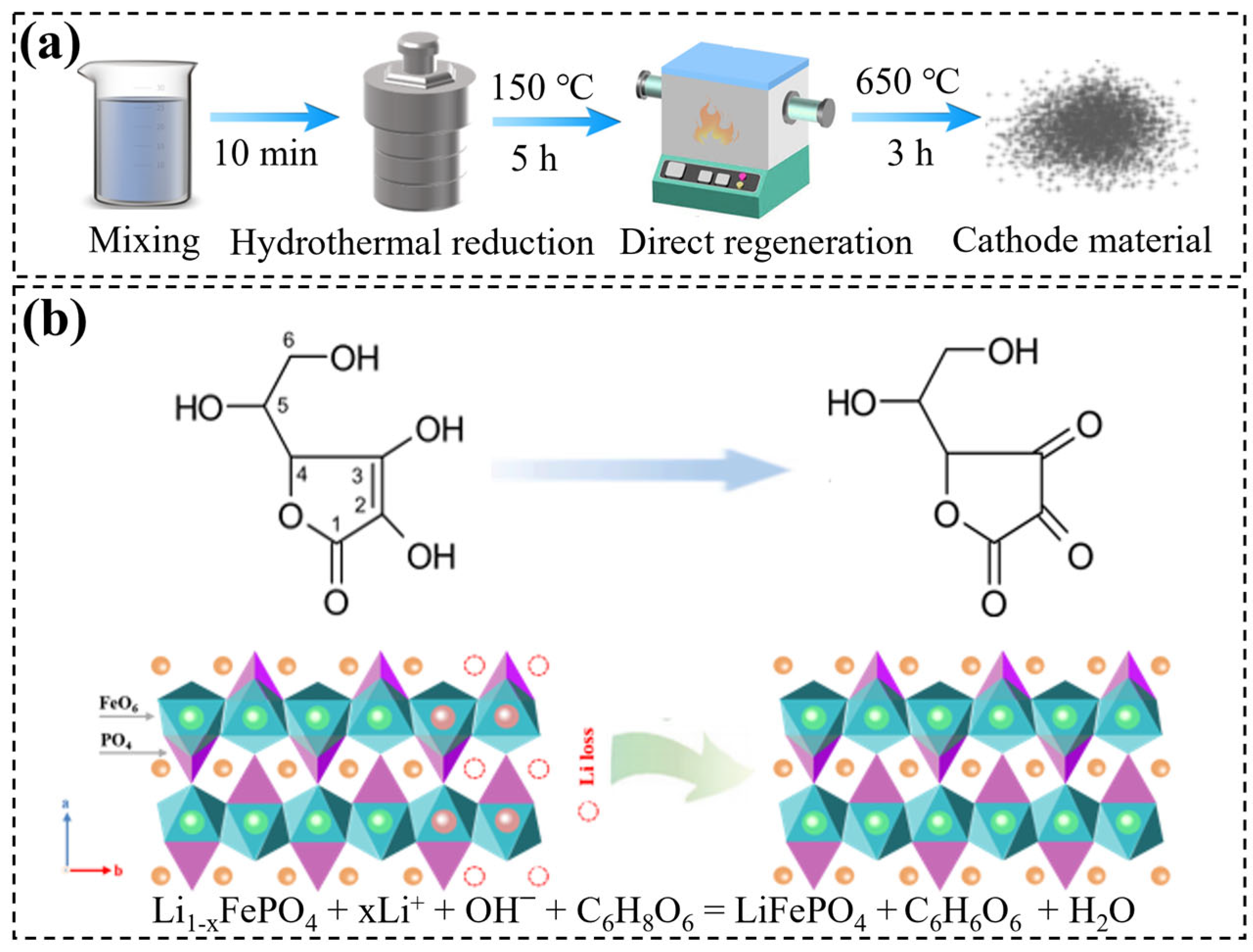
3.1. Pretreatment of Materials
3.2. Direct Regeneration of D-LFP
3.3. Material Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, X.; Azhari, L.; Wang, Y. Li-ion battery recycling challenges. Chem 2021, 7, 2843–2847. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Choux, M.; Pripp, S.W.; Kvalnes, F.; Hellström, M. To shred or to disassemble—A techno-economic assessment of automated disassembly vs. shredding in lithium-ion battery module recycling. Resour. Conserv. Recycl. 2024, 203, 107430. [Google Scholar] [CrossRef]
- Yao, X.; Li, D.; Guo, L.; Kallel, M.; Alahmari, S.D.; Ren, J.; Seok, I.; Roymahapatra, G.; Wang, C. Carbon-coated LiMn0.8Fe0.2PO4 cathodes for high-rate lithium-ion batteries. Adv. Compos. Hybrid Mater. 2024, 7, 63. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B.; Ge, Z.; Zhang, S.; Song, B.; Tian, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Advances and perspectives towards spent LiFePO4 battery recycling. J. Clean. Prod. 2024, 434, 140077. [Google Scholar] [CrossRef]
- Du, M.; Guo, J.-Z.; Zheng, S.-H.; Liu, Y.; Yang, J.-L.; Zhang, K.-Y.; Gu, Z.-Y.; Wang, X.-T.; Wu, X.-L. Direct reuse of LiFePO4 cathode materials from spent lithium-ion batteries: Extracting Li from brine. Chin. Chem. Lett. 2023, 34, 107706. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Zhang, H.; Wang, Y.; Chen, Y.; Wang, C. Simultaneous anodic de-lithiation/cathodic lithium-embedded regeneration method for recycling of spent LiFePO4 battery. Energy Storage Mater. 2024, 65, 103081. [Google Scholar] [CrossRef]
- Yu, F.; Xu, X.; Guo, Y. Recovery of metal ions in lithium iron phosphate powder and lithium nickel-cobalt-manganate powder by electrochemical oxidation. Sep. Purif. Technol. 2024, 344, 127134. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, X.; Zhang, B.; Di, A.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Start from the source: Direct treatment of a degraded LiFePO4 cathode for efficient recycling of spent lithium-ion batteries. Green Chem. 2022, 24, 7448–7457. [Google Scholar] [CrossRef]
- Fan, M.-C.; Wozny, J.; Gong, J.; Kang, Y.-Q.; Wang, X.-S.; Zhang, Z.-X.; Zhou, G.-M.; Zhao, Y.; Li, B.-H.; Kang, F.-Y. Lithium metal recycling from spent lithium-ion batteries by cathode overcharging process. Rare Met. 2022, 41, 1843–1850. [Google Scholar] [CrossRef]
- Biswal, B.K.; Zhang, B.; Thi Minh Tran, P.; Zhang, J.; Balasubramanian, R. Recycling of spent lithium-ion batteries for a sustainable future: Recent advancements. Chem. Soc. Rev. 2024, 53, 5552–5592. [Google Scholar] [CrossRef]
- Carreira, E.M. A look back at 2022 and forward to 2023. J. Am. Chem. Soc. 2023, 145, 3255–3256. [Google Scholar] [CrossRef]
- Yan, T.; Zhong, S.; Zhou, M.; Guo, X.; Hu, J.; Wang, F.; Zeng, F.; Zuo, S. High-efficiency method for recycling lithium from spent LiFePO4 cathode. Nanotechnol. Rev. 2020, 9, 1586–1593. [Google Scholar] [CrossRef]
- Fu, D.; Zhou, W.; Liu, J.; Zeng, S.; Wang, L.; Liu, W.; Yu, X.; Liu, X. A facile route for the efficient leaching, recovery, and regeneration of lithium and iron from waste lithium iron phosphate cathode materials. Sep. Purif. Technol. 2024, 342, 127069. [Google Scholar] [CrossRef]
- Fu, Y.; Schuster, J.; Petranikova, M.; Ebin, B.J.R. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction. Conserv. Recycl. 2021, 172, 105666. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Sharma, P.; Pandey, A.; Jang, M.; Jeon, B.H.; Varjani, S.; Kim, S.H. Recycling of cathode material from spent lithium-ion batteries: Challenges and future perspectives. J. Hazard. Mater. 2022, 429, 128312. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Neiber, R.R.; Park, J. Recent progress in sustainable recycling of LiFePO4-type lithium-ion batteries: Strategies for highly selective lithium recovery. Chem. Eng. J. 2022, 431, 133993. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, Z.; Hua, D.; Gu, H.; Wang, N. Theoretical-molar Fe3+ recovering lithium from spent LiFePO4 batteries: An acid-free, efficient, and selective process. J. Hazard. Mater. 2020, 396, 122707. [Google Scholar] [CrossRef]
- Rohr, S.; Wagner, S.; Baumann, M.; Muller, S.; Lienkamp, M. A techno-economic analysis of end of life value chains for lithium-ion batteries from electric vehicles. In Proceedings of the 2017 Twelfth International Conference on Ecological Vehicles and Renewable Energies (EVER), Monte-Carlo, Monaco, 11–13 April 2017; IEEE: Piscataway, NJ, USA, 2017. [Google Scholar]
- Roy, J.J.; Cao, B.; Madhavi, S.J.C. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Xiong, J.; He, L.; Zhao, Z.; Wang, D. Selective recovery of lithium and iron phosphate/carbon from spent lithium iron phosphate cathode material by anionic membrane slurry electrolysis. Waste Manag. 2020, 107, 1–8. [Google Scholar] [CrossRef]
- Chen, B.; Liu, M.; Cao, S.; Hu, H.; Chen, G.; Guo, X.; Wang, X. Direct regeneration and performance of spent LiFePO4 via a green efficient hydrothermal technique. J. Alloys Compd. 2022, 924, 166487. [Google Scholar] [CrossRef]
- Ouaneche, T.; Courty, M.; Stievano, L.; Monconduit, L.; Guéry, C.; Sougrati, M.T.; Recham, N. Room temperature efficient regeneration of spent LiFePO4 by direct chemical lithiation. J. Power Sources 2023, 579, 233248. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; He, Z. Electrochemical Relithiation for Direct Regeneration of LiCoO2 Materials from Spent Lithium-Ion Battery Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 11596–11605. [Google Scholar] [CrossRef]
- Qin, Z.; Li, X.; Shen, X.; Cheng, Y.; Wu, F.; Li, Y.; He, Z. Electrochemical selective lithium extraction and regeneration of spent lithium iron phosphate. Waste Manag. 2024, 174, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, J.; He, D.; Hu, W.; Peng, D.; Li, Y.; Zhao, Z.; Wang, S.; Li, P.; Su, S. Molten salt infiltration–oxidation synergistic controlled lithium extraction from spent lithium iron phosphate batteries: An efficient, acid free, and closed-loop strategy. Green Chem. 2023, 25, 6057–6066. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Silvester, D.S.; Banks, C.E.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Direct regeneration of cathode materials in spent lithium-ion batteries toward closed-loop recycling and sustainability. J. Power Sources 2024, 589, 233728. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, L.; Wang, W.; Liu, Y.; Ma, Q.; Mu, D.; Li, R.; Dai, C. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv. 2016, 49, 43613–43625. [Google Scholar] [CrossRef]
- Roldán-Ruiz, M.J.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Highly efficient p-toluenesulfonic acid-based deep-eutectic solvents for cathode recycling of Li-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 5437–5445. [Google Scholar] [CrossRef]
- Peeters, N.; Binnemans, K.; Riaño, S. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents. Green Chem. 2020, 22, 4210–4221. [Google Scholar] [CrossRef]
- Fei, Z.; Zhang, Y.; Meng, Q.; Dong, P.; Li, Y.; Fei, J.; Qi, H.; Yan, J. The auto-oxidative relithiation of spent cathode materials at low temperature environment for efficient and sustainable regeneration. J. Hazard. Mater. 2022, 432, 128664. [Google Scholar] [CrossRef]
- Tang, D.; Ji, G.; Wang, J.; Liang, Z.; Chen, W.; Ji, H.; Ma, J.; Liu, S.; Zhuang, Z.; Zhou, G. A Multifunctional Amino Acid Enables Direct Recycling of Spent LiFePO4 Cathode Material. Adv. Mater. 2024, 36, e2309722. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Fu, J.; Zhang, S.; He, X.; Zhao, B.; Ren, J.; Zhong, J.; Liu, Z. Advances in recycling LiFePO4 from spent lithium batteries: A critical review. Sep. Purif. Technol. 2024, 338, 126551. [Google Scholar] [CrossRef]
- Gou, Y.; Qi, C.; Li, R.; Liu, X.; Zhou, Z.; Zhang, M.; Sun, Q.; Song, L.; Jin, Y. Direct regeneration of high-value LiFePO4 cathode materials with nitrogen doped carbon coating. Electrochim. Acta 2024, 488, 144180. [Google Scholar] [CrossRef]
- Yin, C.; Pan, C.; Pan, Y.; Hu, J. Hierarchical spheroidal MOF-derived MnO@C as cathode components for high-performance aqueous zinc ion batteries. J. Colloid. Interface Sci. 2023, 642, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Tang, X.; Tang, M.; Wu, Q.; Li, J. Failure mechanism and voltage regulation strategy of low N/P ratio lithium iron phosphate battery. J. Energy Storage 2022, 51, 104588. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, K.; Gong, R.; Meng, Q.; Zhang, Y.; Dong, P. Direct regeneration of spent LiFePO4 materials via a green and economical one-step hydrothermal process. J. Environ. Manag. 2023, 348, 119384. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Pan, C.; Pan, Y.; Hu, J.; Fang, G. Proton Self-Doped Polyaniline with High Electrochemical Activity for Aqueous Zinc-Ion Batteries. Small Methods 2023, 7, 2300574. [Google Scholar] [CrossRef]
- Liu, K.; Wang, J.; Wang, M.; Zhang, Q.; Cao, Y.; Huang, L.; Valix, M.; Tsang, D.C.W. Low-carbon recycling of spent lithium iron phosphate batteries via a hydro-oxygen repair route. Green. Chem. 2023, 25, 6642–6651. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Yang, L.; Du, X.; Yang, Y. Capacity fade characteristics of lithium iron phosphate cell during dynamic cycle. Energy 2020, 206, 118155. [Google Scholar] [CrossRef]
- Lei, S.; Sun, W.; Yang, Y. Comprehensive Technology for Recycling and Regenerating Materials from Spent Lithium Iron Phosphate Battery. Environ. Sci. Technol. 2024, 58, 3609–3628. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Gong, R.; Zhang, Y.; Meng, Q.; Dong, P. Direct Regeneration of Degraded LiFePO4 Cathode via Reductive Solution Relithiation Regeneration Process. Molecules 2024, 29, 3340. https://doi.org/10.3390/molecules29143340
Li C, Gong R, Zhang Y, Meng Q, Dong P. Direct Regeneration of Degraded LiFePO4 Cathode via Reductive Solution Relithiation Regeneration Process. Molecules. 2024; 29(14):3340. https://doi.org/10.3390/molecules29143340
Chicago/Turabian StyleLi, Chenchen, Rui Gong, Yingjie Zhang, Qi Meng, and Peng Dong. 2024. "Direct Regeneration of Degraded LiFePO4 Cathode via Reductive Solution Relithiation Regeneration Process" Molecules 29, no. 14: 3340. https://doi.org/10.3390/molecules29143340
APA StyleLi, C., Gong, R., Zhang, Y., Meng, Q., & Dong, P. (2024). Direct Regeneration of Degraded LiFePO4 Cathode via Reductive Solution Relithiation Regeneration Process. Molecules, 29(14), 3340. https://doi.org/10.3390/molecules29143340




