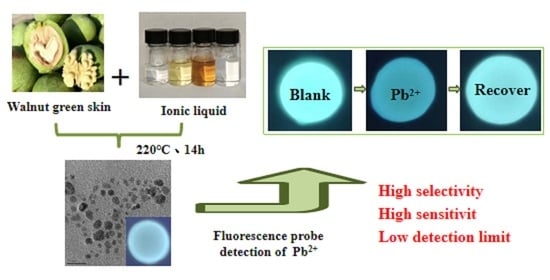
Fluorescence Detection of Pb2+ in Environmental Water Using Biomass Carbon Quantum Dots Modified with Acetamide-Glycolic Acid Deep Eutectic Solvent
Abstract
:1. Introduction
2. Result
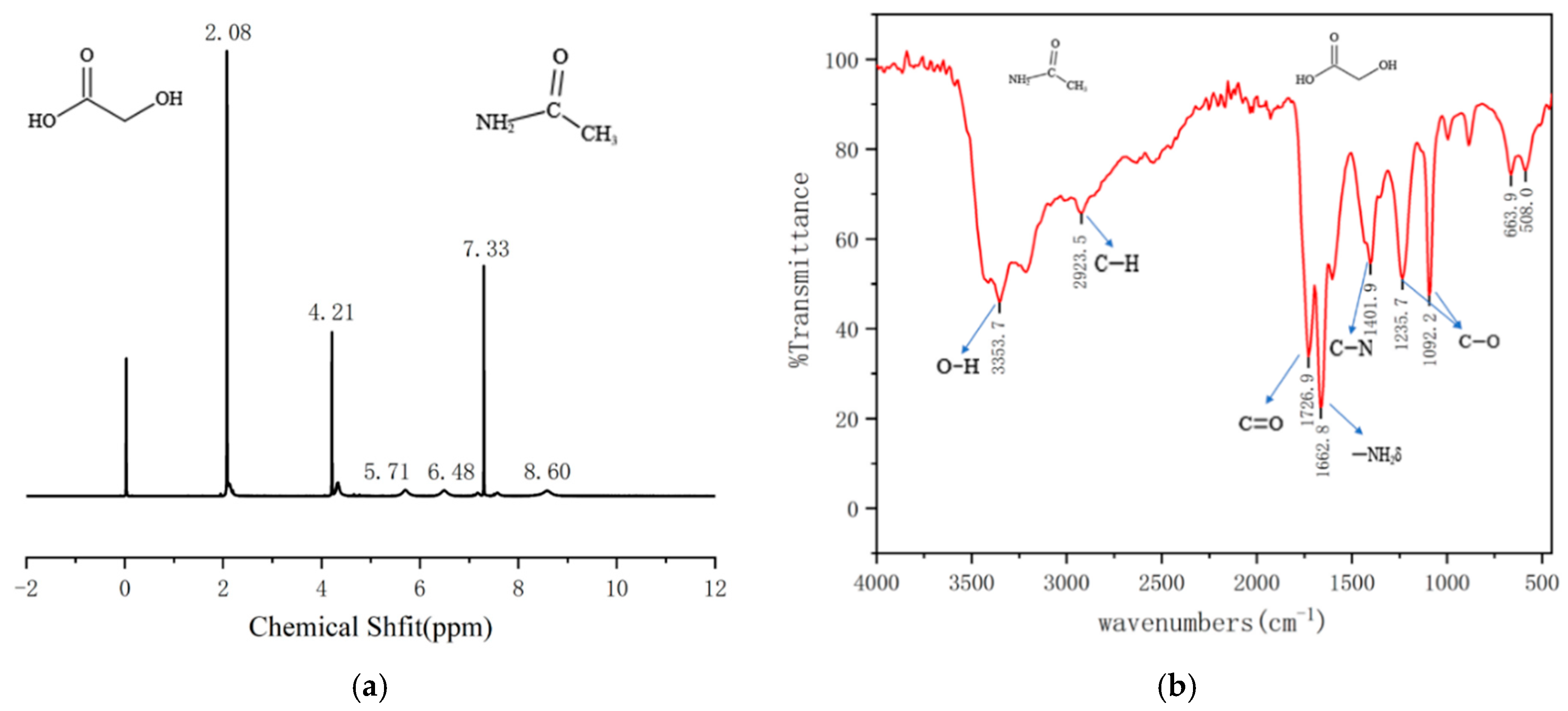
2.1. Structural Characterization of AGADES
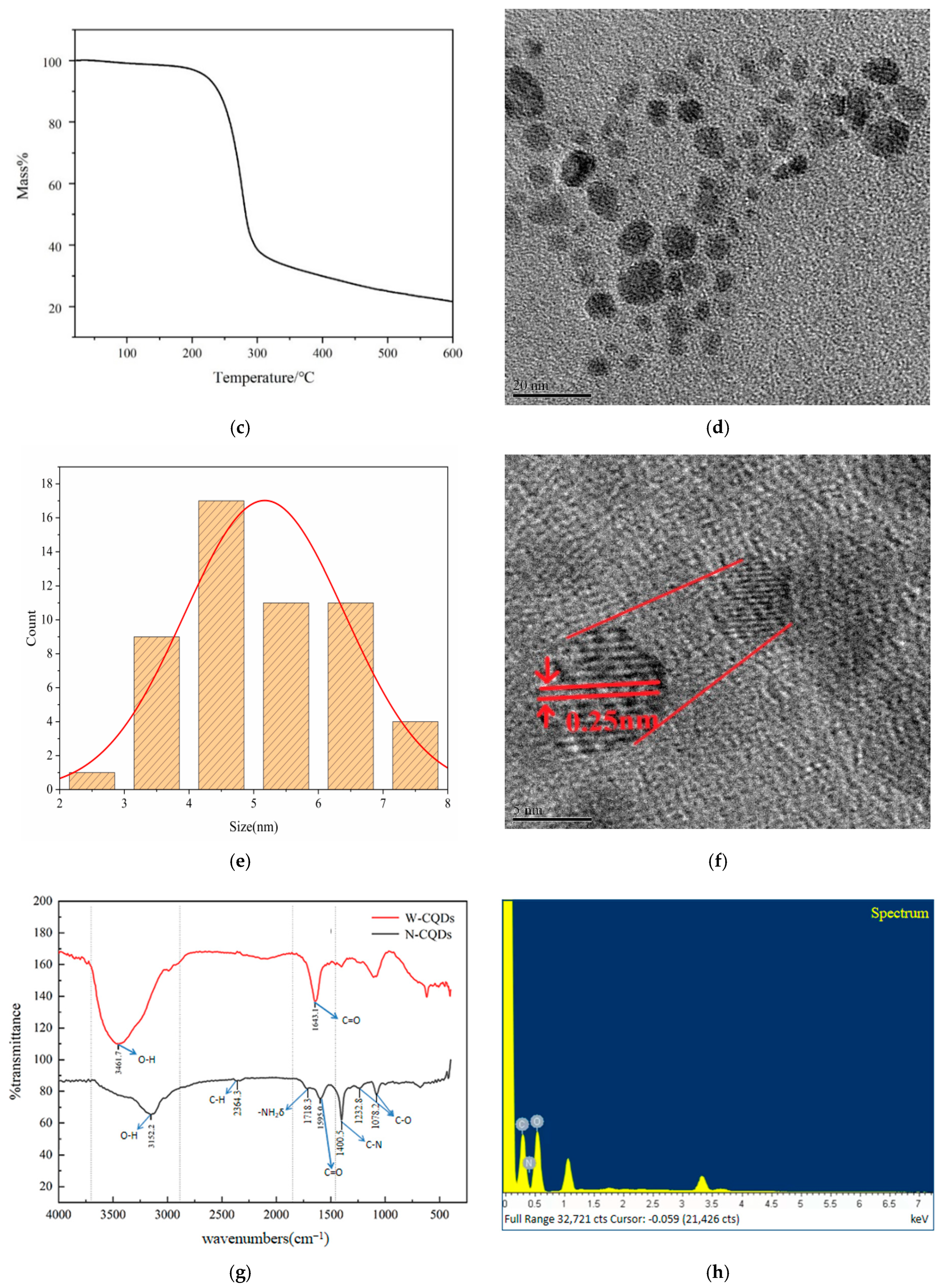
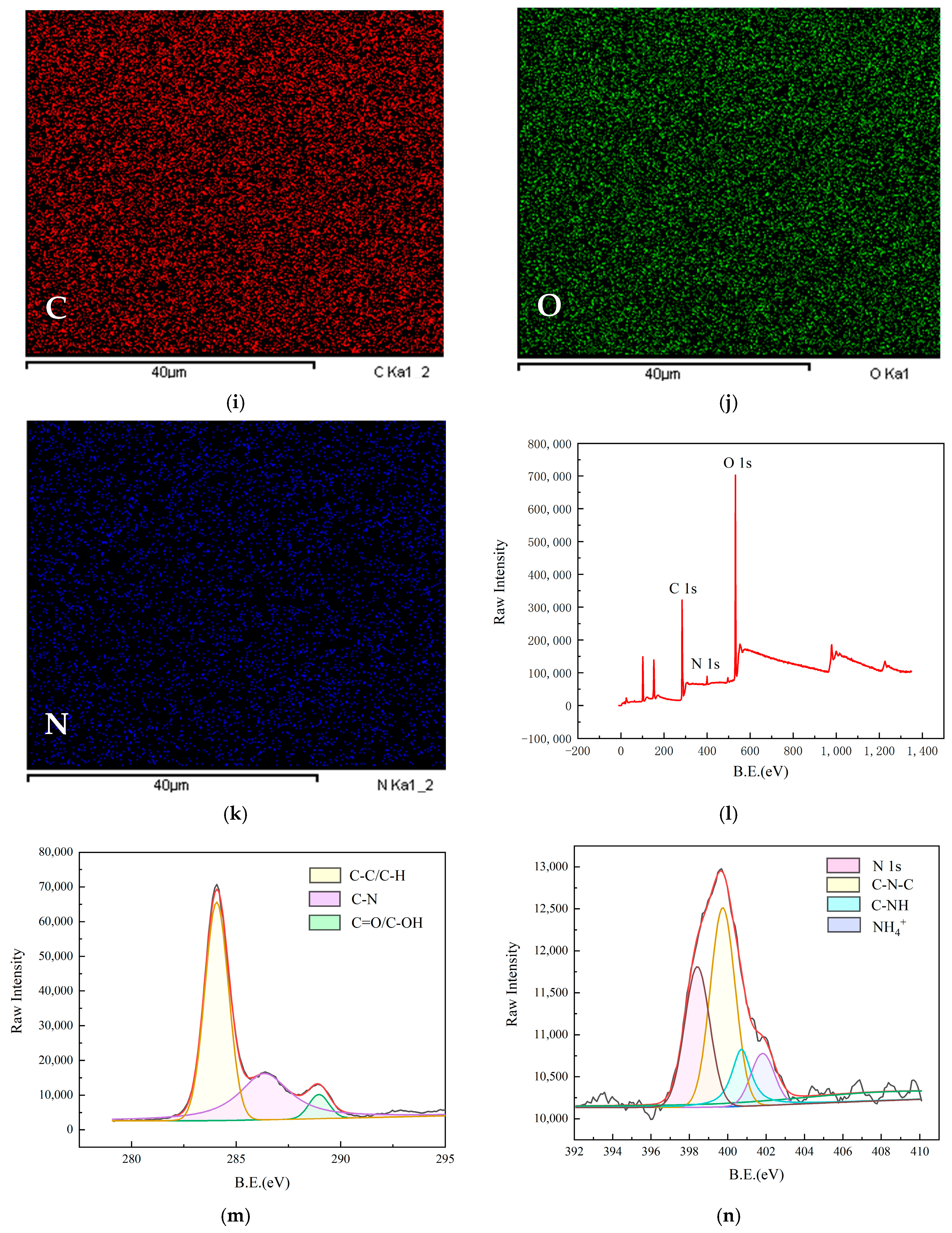
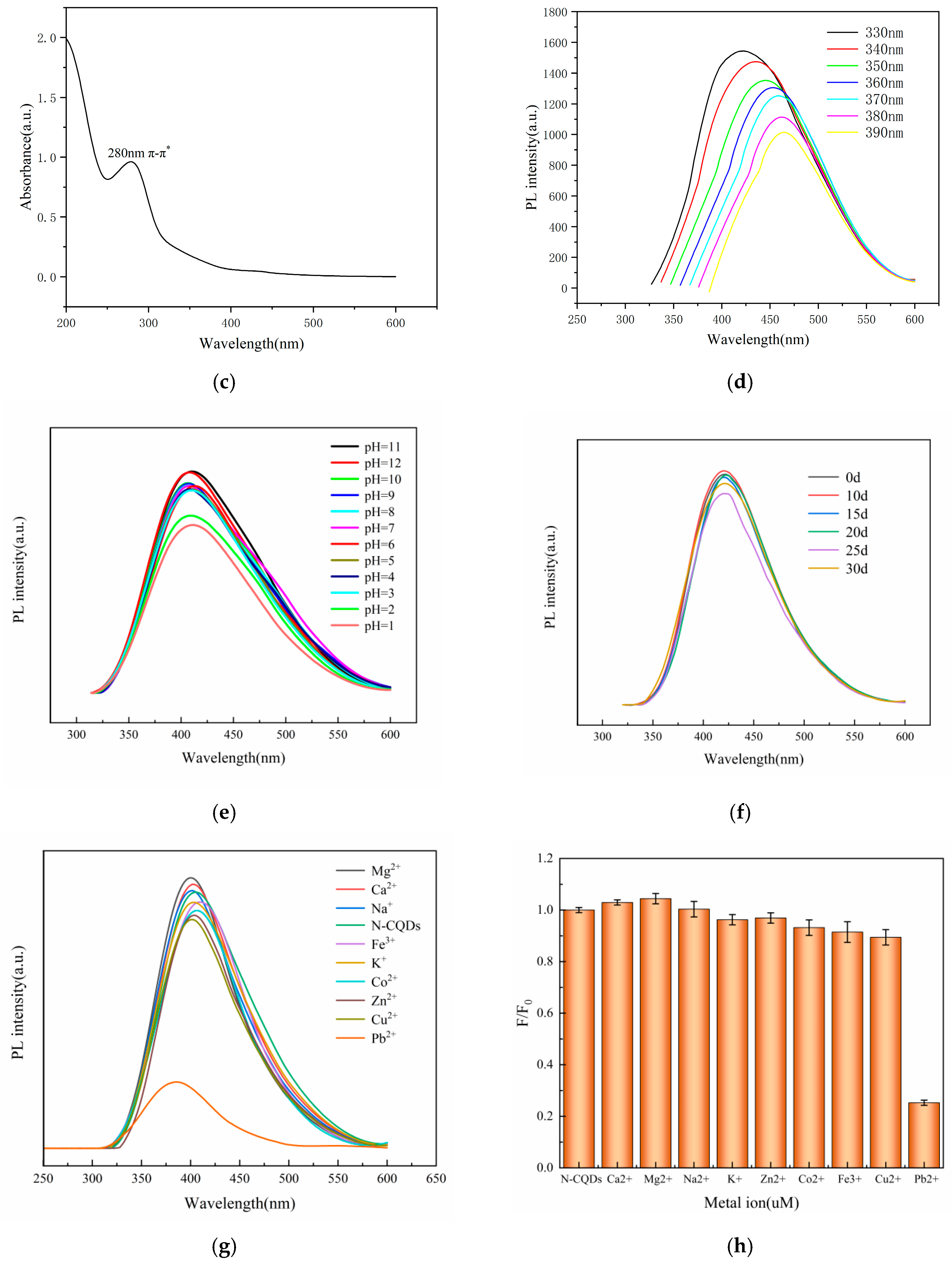
2.2. Properties of CQDs Prepared on the Basis of AGADES
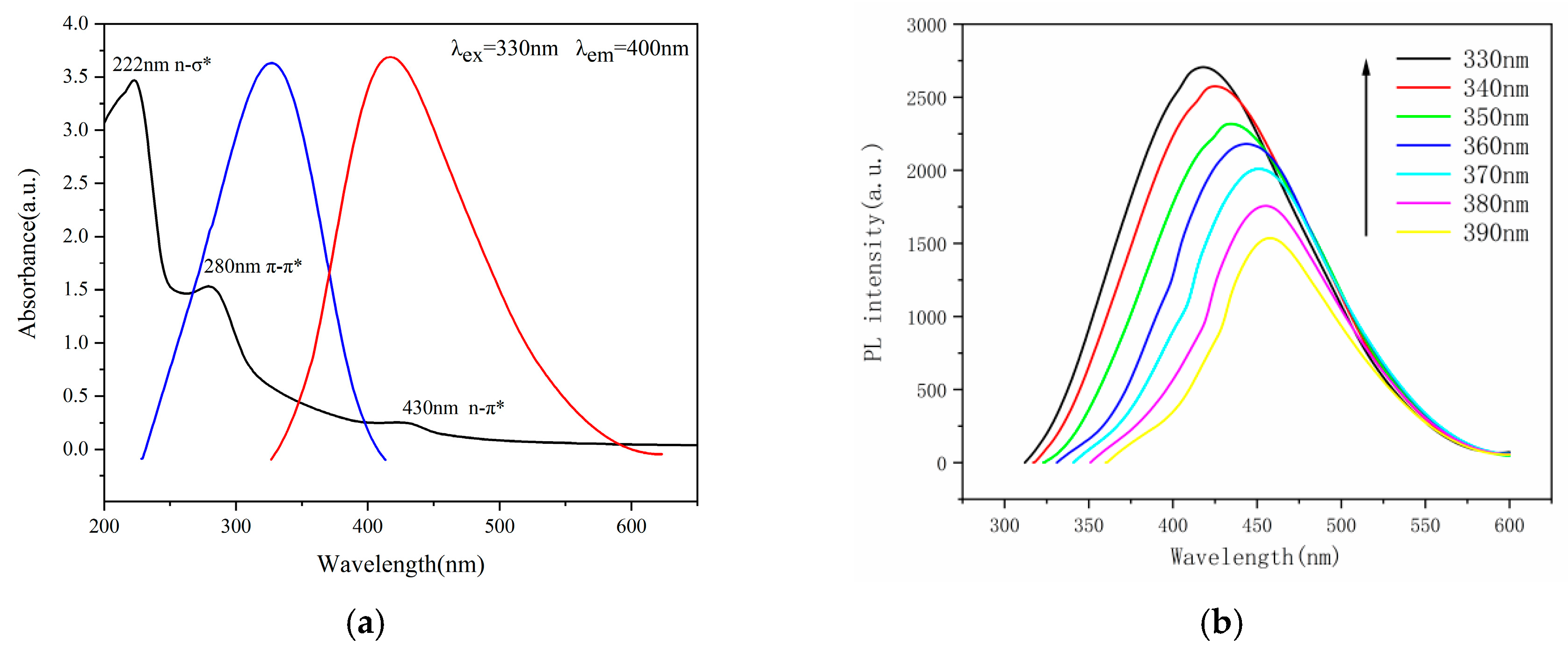
2.3. Optical Properties of N-CQDs
2.4. Effect of Reaction pH on N-CQDs
2.5. Temporal Stability of N-CQDs
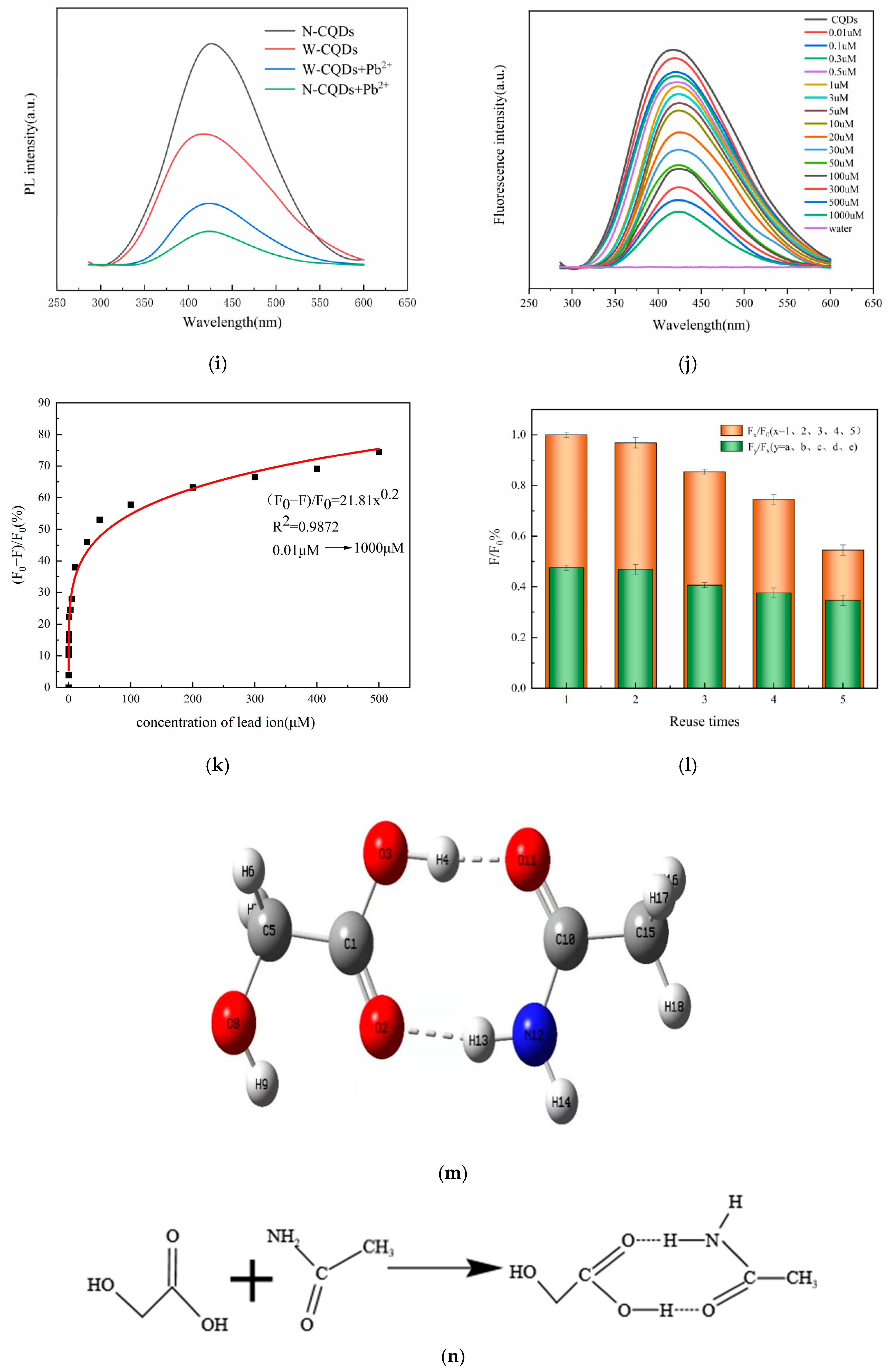
2.6. Specific selectivity of N-CQDs for Pb2+
2.7. Detection of Pb2+ in Actual Water Samples
2.8. Recycling Experiments with N-CQDs
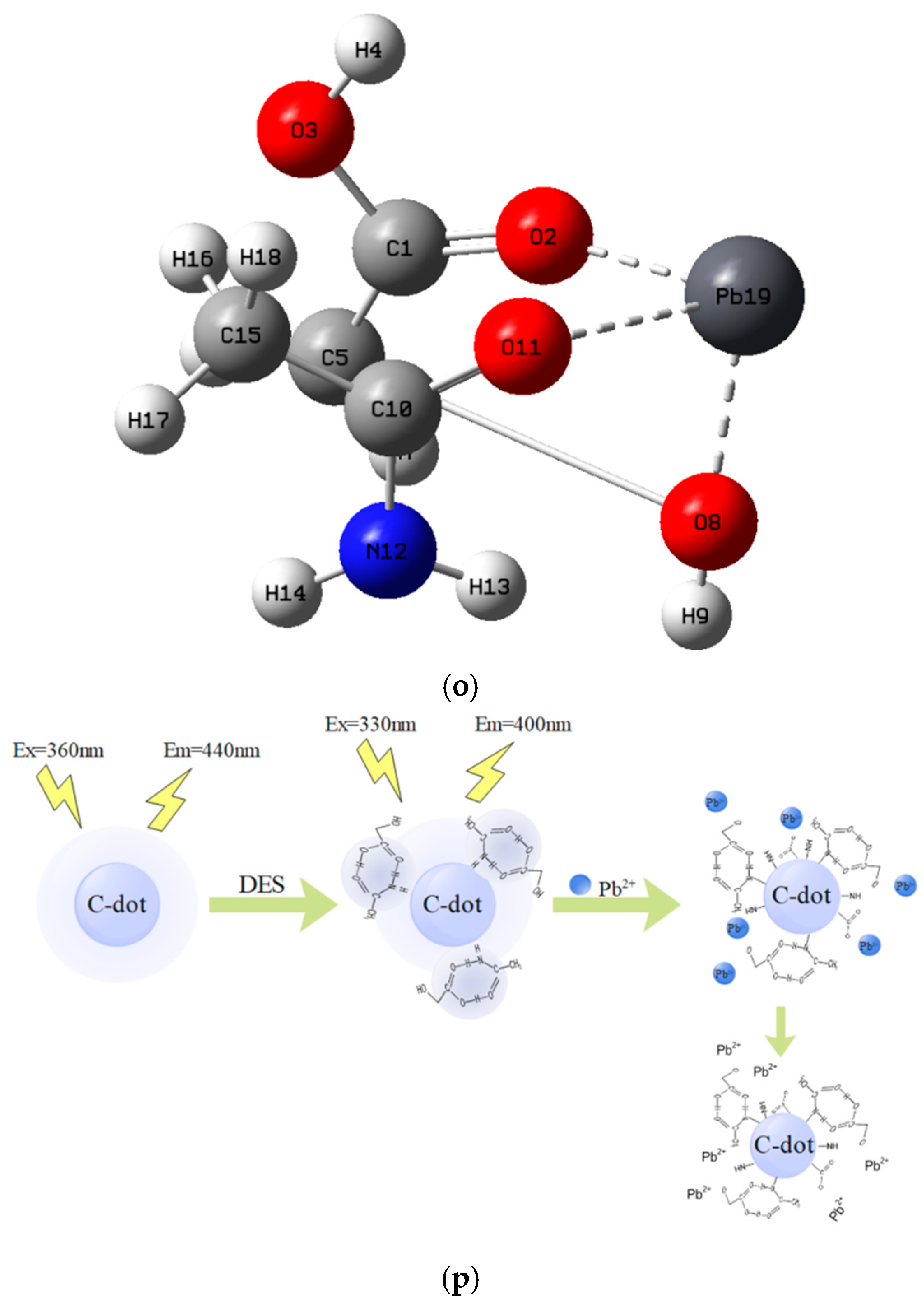
2.9. Principle of Pb2+ Detection by N-CQDs
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Preparation of AGADES
3.3. Preparation of N-CQDs and W-CQDs
3.3.1. Preparation of W-CQDs
3.3.2. Preparation of N-CQDs
3.4. Determination of the Fluorescence Stability Properties of N-CQDs
3.5. Selective Determination of N-CQDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bagal-Kestwal, D.; Karve, M.S.; Kakade, B.; Pillai, V.K. Invertase inhibition based electrochemical sensor for the detection of heavy metal ions in aqueous system: Application of ultra-microelectrode to enhance sucrose biosensor’s sensitivity. Biosens. Bioelectron. 2008, 24, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Boruah, A.; Saikia, M.; Das, T.; Goswamee, R.L.; Saikia, B.K. Blue-emitting fluorescent carbon quantum dots from waste biomass sources and their application in fluoride ion detection in water. J. Photochem. Photobiol. B Biol. 2020, 209, 111940. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-H.; Zou, Y.-N.; Cao, X.-C.; Lin, Z.-H. Color-tunable fluorescent nitrogen-doped graphene quantum dots derived from pineapple leaf fiber biomass to detect Hg2+. Chin. J. Anal. Chem. 2022, 50, 69–76. [Google Scholar] [CrossRef]
- Gong, Y.; Dong, Z. Transfer, transportation, and accumulation of cerium-doped carbon quantum dots: Promoting growth and development in wheat. Ecotoxicol. Environ. Saf. 2021, 226, 112852. [Google Scholar] [CrossRef] [PubMed]
- Sistani, S.; Shekarchizadeh, H. Fabrication of fluorescence sensor based on molecularly imprinted polymer on amine-modified carbon quantum dots for fast and highly sensitive and selective detection of tannic acid in food samples. Anal. Chim. Acta 2021, 1186, 339122. [Google Scholar] [CrossRef] [PubMed]
- Preethi, M.; Murugan, R.; Viswanathan, C.; Ponpandian, N. Potato starch derived N-doped carbon quantum dots as a fluorescent sensing tool for ascorbic acid. J. Photochem. Photobiol. A Chem. 2022, 431, 114009. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, Y.; Wang, X.; Xu, Q.; Li, Z.; Sheng, L.; Yan, L. Fluorescence probe based on boron-doped carbon quantum dots for high selectivity “on-off-on” mercury ion sensing and cell imaging. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129150. [Google Scholar] [CrossRef]
- Ahlawat, A.; Rana, P.S.; Solanki, P.R. Studies of photocatalytic and optoelectronic properties of microwave synthesized and polyethyleneimine stabilized carbon quantum dots. Mater. Lett. 2021, 305, 130830. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, G.; Gao, Y.; Chen, Q.; Bi, J. Up-conversion fluorescent carbon quantum dots decorated covalent triazine frameworks as efficient metal-free photocatalyst for hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 8739–8748. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Ding, J.; Wu, T.; Cai, S.; Zhang, W.; Cai, R.; Chen, C.; Yang, R. Synthesis of carbon quantum dots for application of alleviating amyloid-β mediated neurotoxicity. Colloids Surf. B Biointerfaces 2022, 212, 112373. [Google Scholar] [CrossRef]
- Masha, S.; Oluwafemi, O.S. Synthesis of blue and green emitting carbon-based quantum dots (CBQDs) and their cell viability against colon and bladder cancer cell lines. Mater. Lett. 2021, 283, 128790. [Google Scholar] [CrossRef]
- Mohammadi, R.; Naderi-Manesh, H.; Farzin, L.; Vaezi, Z.; Ayarri, N.; Samandari, L.; Shamsipur, M. Fluorescence sensing and imaging with carbon-based quantum dots for early diagnosis of cancer: A review. J. Pharm. Biomed. Anal. 2022, 212, 114628. [Google Scholar] [CrossRef]
- Praseetha, P.K.; Chandran, P.; Devi, V.A.; Alexander, P.; Vijayakumar, S. Biocompatible and nuclear penetrating carbon quantum dots for photoresistive bioimaging applications in animal cell lines. Gene Rep. 2022, 26, 101519. [Google Scholar] [CrossRef]
- Nagaraj, M.; Ramalingam, S.; Murugan, C.; Aldawood, S.; Jin, J.-O.; Choi, I.; Kim, M. Detection of Fe3+ ions in aqueous environment using fluorescent carbon quantum dots synthesized from endosperm of Borassus flabellifer. Environ. Res. 2022, 212, 113273. [Google Scholar] [CrossRef]
- Chung Hui, K.; Lun Ang, W.; Soraya Sambudi, N. Nitrogen and bismuth-doped rice husk-derived carbon quantum dots for dye degradation and heavy metal removal. J. Photochem. Photobiol. A Chem. 2021, 418, 113411. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Zhang, M.; Wang, M.; Zhao, Y. The optical fiber sensing platform for ferric ions detection: A practical application for carbon quantum dots. Sens. Actuators B Chem. 2022, 364, 131857. [Google Scholar] [CrossRef]
- Jo, M.-H.; Kim, K.-H.; Ahn, H.-J. P-doped carbon quantum dot graft-functionalized amorphous WO3 for stable and flexible electrochromic energy-storage devices. Chem. Eng. J. 2022, 445, 136826. [Google Scholar] [CrossRef]
- Raikwar, V.R. Synthesis and study of carbon quantum dots (CQDs) for enhancement of luminescence intensity of CQD@LaPO4:Eu3+ nanocomposite. Mater. Chem. Phys. 2022, 275, 125277. [Google Scholar] [CrossRef]
- Agrawal, N.; Bhagel, D.; Mishra, P.; Prasad, D.; Kohli, E. Post-synthetic modification of graphene quantum dots bestows enhanced biosensing and antibiofilm ability: Efficiency facet. RSC Adv. 2022, 12, 12310–12320. [Google Scholar] [CrossRef]
- John, V.L.; Joy, F.; Kollannoor, A.J.; Joseph, K.; Nair, Y.; Vinod, T.P. Amine functionalized carbon quantum dots from paper precursors for selective binding and fluorescent labelling applications. J. Colloid Interface Sci. 2022, 617, 730–744. [Google Scholar] [CrossRef]
- Kang, K.; Du, X.; Shi, L.; Peng, Z.; Zhang, X.; Liu, B.; Yue, G.; Wang, L.; Wang, Z.; Chen, S. Selective detection of ionic liquid fluorescence probes for visual colorimetry of different metal ions. Environ. Res. 2024, 242, 117791. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.Z. Preparation and structure tuning of graphene quantum dots for optical applications in chemosensing, biosensing, and bioimaging. In Sensing and Biosensing with Optically Active Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 41–77. [Google Scholar]
- Shishov, A.; Shakirova, F.; Markova, U.; Tolstoy, P.; Bulatov, A. A new hydrophobic deep eutectic solvent based on thymol and 4-(dimethylamino)benzaldehyde: Derivatization and microextraction of urea. J. Mol. Liq. 2022, 353, 118820. [Google Scholar] [CrossRef]
- de Andrade, D.C.; Monteiro, S.A.; Merib, J. A review on recent applications of deep eutectic solvents in microextraction techniques for the analysis of biological matrices. Adv. Sample Prep. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Alonso, D.A.; Burlingham, S.-J.; Chinchilla, R.; Guillena, G.; Ramón, D.J.; Tiecco, M. Asymmetric Organocatalysis in Deep Eutectic Solvents. Eur. J. Org. Chem. 2021, 2021, 4065–4071. [Google Scholar] [CrossRef]
- Sun, H.; Xin, R.; Qu, D.; Yao, F. Mechanism of deep eutectic solvents enhancing catalytic function of cytochrome P450 enzymes in biosynthesis and organic synthesis. J. Biotechnol. 2020, 323, 264–273. [Google Scholar] [CrossRef]
- Adi Kurnia, K.; Zunita, M.; Coutinho, J.A.P.; Gede Wenten, I.; Santoso, D. Development of quantitative structure-property relationship to predict the viscosity of deep eutectic solvent for CO2 capture using molecular descriptor. J. Mol. Liq. 2022, 347, 118239. [Google Scholar] [CrossRef]
- Phosiri, P.; Santaladchaiyakit, Y.; Burakham, R. Natural deep eutectic solvent-decorated magnetic layered double hydroxide as a sorbent for the enrichment of organochlorine pesticides in environmental samples. J. Chromatogr. A 2022, 1673, 463111. [Google Scholar] [CrossRef]
- Xue, T.; Shi, Y.; Guo, J.; Guo, M.; Yan, Y. Preparation of AgInS2 quantum dots and their application for Pb2+ detection based on fluorescence quenching effect. Vacuum 2021, 193, 110514. [Google Scholar] [CrossRef]
- Niu, X.; Zhong, Y.; Chen, R.; Wang, F.; Liu, Y.; Luo, D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens. Actuators B Chem. 2018, 255, 1577–1581. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Tan, Z.; Yin, X.; Wang, L. Electrochemiluminescence of CdTe quantum dots capped with glutathione and thioglycolic acid and its sensing of Pb2+. Electrochim. Acta 2012, 72, 28–31. [Google Scholar] [CrossRef]
- Wang, C.; Pei, L.; Chen, R.; Zhu, Y.; Su, J. A portable screen-printing electrode modified by COFDATA-TP with abundant carboxyl and secondary amine groups for simultaneous detection of Hg2+, Cu2+, Pb2+, and Cd2+. Ionics 2022, 28, 4025–4033. [Google Scholar] [CrossRef]
- Martín-Esteban, A.; Garcinuño, R.M.; Angelino, S.; Fernández, P.; Cámara, C. Determination of trace metals in waters and compost by on-line precipitation coupled to flame atomic absorption spectrophotometry or ion chromatography. Talanta 1999, 48, 959–966. [Google Scholar] [CrossRef]
- Kang, K.; Liu, B.; Yue, G.; Ren, H.; Zheng, K.; Wang, L.; Wang, Z. Preparation of carbon quantum dots from ionic liquid modified biomass for the detection of Fe3+ and Pd2+ in environmental water. Ecotoxicol. Environ. Saf. 2023, 255, 114795. [Google Scholar] [CrossRef]
- Pang, S.; Liang, Y. Studies on charge transfer properties from mixture of Schiff base and zinc complex in Langmuir–Blodgett film by UV–vis absorption and Fourier transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 435–439. [Google Scholar] [CrossRef]
- Qin, J.; Gao, X.; Chen, Q.; Liu, H.; Liu, S.; Hou, J.; Sun, T. pH sensing and bioimaging using green synthesized carbon dots from black fungus. RSC Adv. 2021, 11, 31791–31794. [Google Scholar] [CrossRef]
- Wang, X.; Guo, H.; Wu, N.; Xu, M.; Zhang, L.; Yang, W. A dual-emission fluorescence sensor constructed by encapsulating double carbon dots in zeolite imidazole frameworks for sensing Pb2+. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126218. [Google Scholar] [CrossRef]
- Bi, R.; Yin, D.; Zhang, S.; Zhang, R.; Chen, F. Efficient removal of Pb(II) and Hg(II) with eco-friendly polyaspartic acid/ layered double hydroxide by host-guest interaction. Appl. Clay Sci. 2022, 225, 106536. [Google Scholar] [CrossRef]
- Soares, E.A.; Téllez, C.; Fortes, S.A.; Coelho, A.; Versiane, O.; Ferreira, G.B.; Mondragón, M.A.; TéllezS, C.A. Fourier transform infrared and Raman spectra of the complex cation diethyldithiocarbamate Cr(III) Di-hydrate, [Cr(DDTC)2(OH2)2]+. UV-Vis spectrum, DFT:B3LYP/6-311G(d, p) structural determination, vibrational and natural bond orbital analysis. J. Mol. Struct. 2022, 1256, 132555. [Google Scholar] [CrossRef]
- Ma, H.; Liu, X.; Wang, X.; Li, X.; Yang, C.; Iqbal, A.; Liu, W.; Li, J.; Qin, W. Sensitive fluorescent light-up probe for enzymatic determination of glucose using carbon dots modified with MnO2 nanosheets. Microchim. Acta 2016, 184, 177–185. [Google Scholar] [CrossRef]
- Ren, H.; Li, M.; Liu, Y.; Zhao, T.; Zhang, R.; Duan, E. Nitrogen-rich carbon quantum dots (N-CQDs) based on natural deep eutectic solvents: Simultaneous detection and treatment of trace Co2+ under saline conditions. Sci. Total Environ. 2022, 811, 152389. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Yuan, Y.; Wu, Y. Hydrothermal synthesis of fluorescent carbon dots from sodium citrate and polyacrylamide and their highly selective detection of lead and pyrophosphate. Carbon 2017, 115, 550–560. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Wu, T.; Kong, X.; Ma, Q.; Ma, C. A graphene quantum dots-Pb2+ based fluorescent switch for selective and sensitive determination of D-penicillamine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117924. [Google Scholar] [CrossRef]
- Lalmalsawmi, J.; Zirlianngura; Tiwari, D.; Lee, S.-M.; Kim, D.-J. Indigenously synthesized nanocomposite materials: Use of nanocomposite as novel sensing platform for trace detection of Pb2+. J. Electroanal. Chem. 2021, 897, 115578. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, W.; Wang, F.; Li, T.; Nie, J.; Xiao, W.; Zhang, Q.; Zhang, Y. Ratiometric fluorescent sensing of Pb2+ and Hg2+ with two types of carbon dot nanohybrids synthesized from the same biomass. Sens. Actuators B Chem. 2019, 296, 126698. [Google Scholar] [CrossRef]
- Liu, B.; Tian, J. Investigation of Glycolic Acid Natural Deep Eutectic Solvents with Strong Proton Donors for Ammonia Capture and Separation. Ind. Eng. Chem. Res. 2021, 60, 11600–11610. [Google Scholar] [CrossRef]


| Sample | Add/μmol/L | Found/μmol/L | Recovery/% |
|---|---|---|---|
| Ground water | 5 | 5.1 | 102.2 |
| 10 | 9.9 | 99.2 | |
| 15 | 14.5 | 96.3 | |
| Lake water | 5 | 5.0 | 99.6 |
| 10 | 9.5 | 95.4 | |
| 15 | 15.1 | 100.8 |
| Fluorescent Sensors | Linear Range (μmol/L) | LOD (nmol/L) | Can It Be Recycled | Literature |
|---|---|---|---|---|
| Fluorescent carbon quantum dots | 50–50,000 | 4.78 | No | [44] |
| Fluorescent carbon quantum dots | 0.0167–1 | 4.6 | No | [45] |
| Fluorescent graphene quantum dots | 600–50,000 | 470 | Yes | [46] |
| Nanocomposite materials Au(NP)/TCODS/BN | 4.8–290 | 3.9 | No | [39] |
| Biomass carbon quantum dots | 1.3–106.7 | 3.2 | No | [47] |
| Fluorescent carbon quantum dots | 0.01–1000 | 1.55 | Yes | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, S.; Zheng, K.; Shi, L.; Kang, K.; Peng, Z.; Zhang, X.; Liu, B.; Yang, H.; Yue, G. Fluorescence Detection of Pb2+ in Environmental Water Using Biomass Carbon Quantum Dots Modified with Acetamide-Glycolic Acid Deep Eutectic Solvent. Molecules 2024, 29, 1662. https://doi.org/10.3390/molecules29071662
Xing S, Zheng K, Shi L, Kang K, Peng Z, Zhang X, Liu B, Yang H, Yue G. Fluorescence Detection of Pb2+ in Environmental Water Using Biomass Carbon Quantum Dots Modified with Acetamide-Glycolic Acid Deep Eutectic Solvent. Molecules. 2024; 29(7):1662. https://doi.org/10.3390/molecules29071662
Chicago/Turabian StyleXing, Shiwen, Keyang Zheng, Lei Shi, Kaiming Kang, Zhixiao Peng, Xiaojie Zhang, Baoyou Liu, Huilong Yang, and Gang Yue. 2024. "Fluorescence Detection of Pb2+ in Environmental Water Using Biomass Carbon Quantum Dots Modified with Acetamide-Glycolic Acid Deep Eutectic Solvent" Molecules 29, no. 7: 1662. https://doi.org/10.3390/molecules29071662