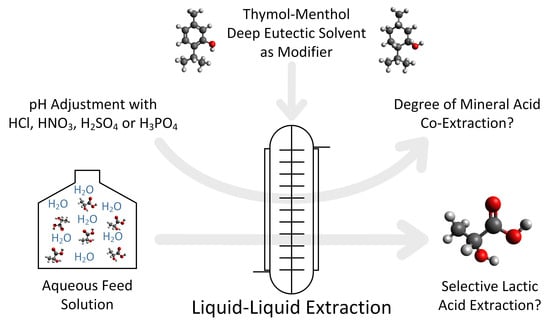
Mineral Acid Co-Extraction in Reactive Extraction of Lactic Acid Using a Thymol-Menthol Deep Eutectic Solvent as a Green Modifier
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Extraction of Mineral Acids
2.2. Reactive Extraction of Mineral Acids from Single-Acid Model Solutions
2.3. Reactive Extraction of Mineral Acids from a Multi-Acid Model Solution
2.4. Selectivity of Reactive Lactic Acid Extraction in the Presence of Mineral Acids
2.5. Back-Extraction of Lactic Acid
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Analytical Methods
3.2.1. High-Performance Liquid Chromatography
3.2.2. Ion Chromatography
3.2.3. pH Value, Density, and Water Content Measurement
3.3. Single-Stage Phase Equilibrium Measurements
3.3.1. Extraction
3.3.2. Back-Extraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Polaris Market Research. Global Bio-Based Chemicals Market Share, Size, Trends, Industry Analysis Report, By Type (Bio-Lubricants, Bio-Solvents, Bioplastics, Bio-Alcohols, Bio-Surfactants, Bio-Based Acids); By End-Use; By Region; Segment Forecast, 2023–2032, New York City, NY, USA, 2023. Available online: https://www.polarismarketresearch.com/industry-analysis/bio-based-chemicals-market (accessed on 13 November 2023).
- Kim, J.; Kim, Y.-M.; Lebaka, V.R.; Wee, Y.-J. Lactic Acid for Green Chemical Industry: Recent Advances in and Future Prospects for Production Technology, Recovery, and Applications. Fermentation 2022, 8, 609. [Google Scholar] [CrossRef]
- Ahring, B.K.; Traverso, J.J.; Murali, N.; Srinivas, K. Continuous fermentation of clarified corn stover hydrolysate for the production of lactic acid at high yield and productivity. Biochem. Eng. J. 2016, 109, 162–169. [Google Scholar] [CrossRef]
- Cizeikiene, D.; Juodeikiene, G.; Damasius, J. Use of wheat straw biomass in production of L-lactic acid applying biocatalysis and combined lactic acid bacteria strains belonging to the genus Lactobacillus. Biocatal. Agric. Biotechnol. 2018, 15, 185–191. [Google Scholar] [CrossRef]
- Demmelmayer, P.; Steiner, L.; Weber, H.; Kienberger, M. Thymol-menthol-based deep eutectic solvent as a modifier in reactive liquid-liquid extraction of carboxylic acids from pretreated sweet sorghum silage press juice. Sep. Purif. Technol. 2023, 310, 123060. [Google Scholar] [CrossRef]
- Komesu, A.; Wolf Maciel, M.R.; Maciel Filho, R. Separation and Purification Technologies for Lactic Acid—A Brief Review. BioResources 2017, 12, 6885–6901. [Google Scholar] [CrossRef]
- Kocks, C.; Görtz, J.; Holtz, A.; Gausmann, M.; Jupke, A. Electrochemical Crystallization Concept for Succinic Acid Reduces Waste Salt Production. Chemie Ingenieur Technik. Chem. Ing. Tech. 2020, 92, 221–228. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Alexandri, M.; Komesu, A.; Venus, J.; Vaz Rossell, C.E.; Maciel Filho, R. Current Advances in Separation and Purification of Second-Generation Lactic Acid. Sep. Purif. Rev. 2020, 49, 159–175. [Google Scholar] [CrossRef]
- Chakraborty, D.; Palani, S.G.; Ghangrekar, M.M.; Wong, J.W.C. Reactive extraction of lactic and acetic acids from leached bed reactor leachate and process optimization by response surface methodology. Environ. Technol. 2023, 1–16. [Google Scholar] [CrossRef]
- Thakre, N. Reactive Extraction of Citric Acid Using Different Extractants: Equilibrium, Kinetics and Modeling. Chem. Biochem. Eng. Q. 2018, 31, 437–446. [Google Scholar] [CrossRef]
- Stas, J.; Alsawaf, H. Liquid—Liquid Extraction of Hydrochloric Acid from Aqueous Solutions by Tri-n-dodecylamine and Tri-n-octylamine/diluents. Period. Polytech. Chem. Eng. 2015, 60, 130–135. [Google Scholar] [CrossRef]
- Bart, H.-J. Reactive Extraction; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 9783642074301. [Google Scholar]
- Chen, H.; Wang, L. Posttreatment Strategies for Biomass Conversion. In Technologies for Biochemical Conversion of Biomass; Chen, H., Wang, L., Eds.; Metallurgical Industry Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK; Beijing, China, 2017; pp. 197–217. ISBN 9780128024171. [Google Scholar]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; de Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Colombo Dugoni, G.; Ferro, M.; et al. Are natural deep eutectic solvents always a sustainable option? A bioassay-based study. Environ. Sci. Pollut. Res. Int. 2022, 30, 17268–17279. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are deep eutectic solvents really green?: A life-cycle perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Khorsandi, M.; Shekaari, H.; Mokhtarpour, M.; Hamishehkar, H. Cytotoxicity of some choline-based deep eutectic solvents and their effect on solubility of coumarin drug. Eur. J. Pharm. Sci. 2021, 167, 106022. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ. Sci. Pollut. Res. Int. 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Dwamena, A.K. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef]
- Novalin, S.; Zweckmair, T. Renewable resources—Green biorefinery: Separation of valuable substances from fluid-fractions by means of membrane technology. Biofuels Bioprod. Bioref. 2009, 3, 20–27. [Google Scholar] [CrossRef]
- Miller, C.; Fosmer, A.; Rush, B.; McMullin, T.; Beacom, D.; Suominen, P. 3.17—Industrial Production of Lactic Acid. In Comprehensive Biotechnology: Principles and Practices in Industry, Agcriculture, Medicine and the Environment, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands; Heidelberg, Germany, 2011; pp. 179–188. ISBN 978-0-08-088504-9. [Google Scholar]
- Gausmann, M.; Gössi, A.; Bertram, F.; Riedl, W.; Schuur, B.; Jupke, A. Electrochemical membrane-assisted pH-swing extraction and back-extraction of lactic acid. Sep. Purif. Technol. 2022, 289, 120702. [Google Scholar] [CrossRef]
- Lan, K.; Xu, S.; Li, J.; Hu, C. Recovery of Lactic Acid from Corn Stover Hemicellulose-Derived Liquor. ACS Omega 2019, 4, 10571–10579. [Google Scholar] [CrossRef] [PubMed]
- Kloetzer, L.; Tucaliuc, A.; Galaction, A.-I.; Caşcaval, D. Fractionation of dicarboxylic acids produced by Rhizopus oryzae using reactive extraction. Sci. Rep. 2022, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, T.; Blahusiak, M.; Babic, K.; Schuur, B. Reactive extraction and recovery of levulinic acid, formic acid and furfural from aqueous solutions containing sulphuric acid. Sep. Purif. Technol. 2017, 185, 186–195. [Google Scholar] [CrossRef]
- Kyuchoukov, G.; Yankov, D. Theoretical and Experimental Study of Lactic Acid Stripping from Loaded Organic Phase. Ind. Eng. Chem. Res. 2010, 49, 8238–8243. [Google Scholar] [CrossRef]
- Gausmann, M.; Wall, D.; Jupke, A. Reliable Identification of Relevant Factors for the Reactive Extraction of Succinic Acid from Electrolyte Containing Solutions. Solvent Extr. Ion Exch. 2023, 41, 826–853. [Google Scholar] [CrossRef]
- Aimer, M.; Klemm, E.; Langanke, B.; Gehrke, H.; Stubenrauch, C. Reactive Extraction of Lactic Acid by Using Tri-n-octylamine: Structure of the Ionic Phase. Chemistry 2016, 22, 3268–3272. [Google Scholar] [CrossRef] [PubMed]
- Belova, V.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Extraction of carboxylic acids with neutral extractants. Theor. Found. Chem. Eng. 2017, 51, 786–794. [Google Scholar] [CrossRef]
- Lux, S.; Siebenhofer, M. Investigation of liquid-liquid phase equilibria for reactive extraction of lactic acid with organophosphorus solvents. J. Chem. Technol. Biotechnol. 2013, 88, 462–467. [Google Scholar] [CrossRef]
- Gössi, A.; Burgener, F.; Kohler, D.; Urso, A.; Kolvenbach, B.A.; Riedl, W.; Schuur, B. In-situ recovery of carboxylic acids from fermentation broths through membrane supported reactive extraction using membrane modules with improved stability. Sep. Purif. Technol. 2020, 241, 116694. [Google Scholar] [CrossRef]
- Sprakel, L.; Schuur, B. Solvent developments for liquid-liquid extraction of carboxylic acids in perspective. Sep. Purif. Technol. 2019, 211, 935–957. [Google Scholar] [CrossRef]
- Strömvall, A.-M.; Petersson, G. Terpenes Emitted to Air from TMP and Sulphite Pulp Mills. Holzforschung 1992, 46, 99–102. [Google Scholar] [CrossRef]
- Hong, Y.K.; Hong, W.H.; Han, D.H. Application of reactive extraction to recovery of carboxylic acids. Biotechnol. Bioprocess Eng. 2001, 6, 386–394. [Google Scholar] [CrossRef]
- Liu, L.; Su, B.; Wei, Q.; Ren, X. Selective separation of lactic, malic, and tartaric acids based on the hydrophobic deep eutectic solvents of terpenes and amides. Green Chem. 2021, 23, 5866–5874. [Google Scholar] [CrossRef]
- Demmelmayer, P.; Kienberger, M. Reactive extraction of lactic acid from sweet sorghum silage press juice. Sep. Purif. Technol. 2022, 282, 120090. [Google Scholar] [CrossRef]
- Choudhury, B.; Swaminathan, T. Lactic acid extraction with trioctyl amine. Bioprocess Biosyst. Eng. 1998, 19, 317. [Google Scholar] [CrossRef]
- Brandani, S.; Brandani, V.; Vegliò, F. Extraction of Anions from Aqueous Solutions Using Secondary Amines. Ind. Eng. Chem. Res. 1998, 37, 292–295. [Google Scholar] [CrossRef]
- Genov, L.; Dukov, I. Extraktion von starken einwertigen Suren mit Trioctylamin. Monatsh. Chem. 1972, 103, 1552–1559. [Google Scholar] [CrossRef]
- de Keukeleere, K.; Coucke, S.; de Canck, E.; van der Voort, P.; Delpech, F.; Coppel, Y.; Hens, Z.; van Driessche, I.; Owen, J.S.; De Roo, J. Stabilization of Colloidal Ti, Zr, and Hf Oxide Nanocrystals by Protonated Tri- n -octylphosphine Oxide (TOPO) and Its Decomposition Products. Chem. Mater. 2017, 29, 10233–10242. [Google Scholar] [CrossRef]
- Baaden, M.; Burgard, M.; Wipff, G. TBP at the Water−Oil Interface: The Effect of TBP Concentration and Water Acidity Investigated by Molecular Dynamics Simulations. J. Phys. Chem. B 2001, 105, 11131–11141. [Google Scholar] [CrossRef]
- Lommelen, R.; Binnemans, K. Molecular thermodynamic model for solvent extraction of mineral acids by tri-n-butyl phosphate (TBP). Sep. Purif. Technol. 2023, 313, 123475. [Google Scholar] [CrossRef]
- Dhouib-Sahnoun, R.; Feki, M.; Ayedi, H.F. Liquid−Liquid Equilibria of the Ternary System Water + Phosphoric Acid + Tributyl Phosphate at 298.15 K and 323.15 K. J. Chem. Eng. Data 2002, 47, 861–866. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781498754293. [Google Scholar]
- Caşcaval, D.; Kloetzer, L.; Galaction, A.-I. Influence of Organic Phase Polarity on Interfacial Mechanism and Efficiency of Reactive Extraction of Acetic Acid with Tri- n -octylamine. J. Chem. Eng. Data 2011, 56, 2521–2526. [Google Scholar] [CrossRef]


| Acid | pHF | Solvent Phase | pHR | Dextr,mineral |
|---|---|---|---|---|
| HCl | 0.46 ± 0.01 | 1-Octanol | 0.48 ± 0.03 | 0.0359 ± 0.0042 |
| tmDES | 0.50 ± 0.01 | 0.0480 ± 0.0011 | ||
| Limonene | 0.49 ± 0.00 | 0.0670 ± 0.0026 | ||
| HNO3 | 0.46 ± 0.01 | 1-Octanol | 0.52 ± 0.00 | 0.0533 ± 0.0072 |
| tmDES | 0.50 ± 0.01 | 0.0384 ± 0.0006 | ||
| Limonene | 0.48 ± 0.00 | 0.0375 ± 0.0031 | ||
| H2SO4 | 0.47 ± 0.01 | 1-Octanol | 0.49 ± 0.01 | 0.0385 ± 0.0020 |
| tmDES | 0.48 ± 0.01 | 0.0649 ± 0.0024 | ||
| Limonene | 0.45 ± 0.01 | 0.0877 ± 0.0032 | ||
| H3PO4 | 1.15 ± 0.01 | 1-Octanol | 1.19 ± 0.00 | 0.0743 ± 0.0017 |
| tmDES | 1.18 ± 0.00 | 0.0806 ± 0.0009 | ||
| Limonene | 1.16 ± 0.01 | 0.0826 ± 0.0075 |
| HCl | HNO3 | ||||
| Solvent Phase | pHR | Dextr,mineral | pHR | Dextr,mineral | |
| TOA | 1-Octanol | 0.73 ± 0.00 | 22.5 ± 0.1 | 0.74 ± 0.00 | 22.3 ± 0.2 |
| tmDES | 0.76 ± 0.01 | 1.27 ± 0.04 | 0.75 ± 0.00 | 1.27 ± 0.03 | |
| Limonene | 0.78 ± 0.01 | 1.18 ± 0.01 | 0.76 ± 0.00 | 1.23 ± 0.01 | |
| TOPO | 1-Octanol | 0.49 ± 0.00 | 0.0520 ± 0.0018 | 0.51 ± 0.00 | 0.0794 ± 0.0050 |
| tmDES | 0.47 ± 0.00 | 0.0205 ± 0.0048 | 0.51 ± 0.00 | 0.0239 ± 0.0069 | |
| Limonene | 0.48 ± 0.00 | 0.0234 ± 0.0008 | 0.51 ± 0.00 | 0.0853 ± 0.0010 | |
| TBP | 1-Octanol | 0.47 ± 0.00 | 0.0100 ± 0.0018 | 0.50 ± 0.02 | 0.0794 ± 0.0114 |
| tmDES | 0.49 ± 0.01 | 0.0746 ± 0.0029 | 0.49 ± 0.00 | 0.0539 ± 0.0003 | |
| Limonene | 0.49 ± 0.00 | 0.0570 ± 0.0011 | 0.49 ± 0.00 | 0.0436 ± 0.0046 | |
| H2SO4 | H3PO4 | ||||
| Solvent Phase | pHR | Dextr,mineral | pHR | Dextr,mineral | |
| TOA | 1-Octanol | 0.61 ± 0.01 | 17.3 ± 0.1 | 1.37 ± 0.00 | 16.1 ± 0.0 |
| tmDES | 0.62 ± 0.00 | 0.776 ± 0.008 | 1.34 ± 0.02 | 0.402 ± 0.009 | |
| Limonene | 0.63 ± 0.00 | 0.686 ± 0.007 | 1.23 ± 0.00 | 0.386 ± 0.006 | |
| TOPO | 1-Octanol | 0.46 ± 0.01 | 0.0633 ± 0.0077 | 1.27 ± 0.00 | 0.124 ± 0.002 |
| tmDES | 0.44 ± 0.00 | 0.0888 ± 0.0016 | 1.17 ± 0.01 | 0.114 ± 0.015 | |
| Limonene | 0.44 ± 0.00 | 0.0332 ± 0.0049 | 1.17 ± 0.00 | 0.121 ± 0.001 | |
| TBP | 1-Octanol | 0.44 ± 0.00 | 0.0460 ± 0.0065 | 1.14 ± 0.00 | 0.129 ± 0.015 |
| tmDES | 0.45 ± 0.00 | 0.0737 ± 0.0030 | 1.13 ± 0.00 | 0.130 ± 0.011 | |
| Limonene | 0.46 ± 0.00 | 0.0825 ± 0.0043 | 1.17 ± 0.00 | 0.117 ± 0.001 | |
| Dextr,mineral | |||||||
|---|---|---|---|---|---|---|---|
| Solvent Phase | pHR | ztot | HCl | HNO3 | H2SO4 | H3PO4 | |
| TOA | 1-Octanol | 0.92 ± 0.01 | 1.07 ± 0.02 | 2.06 ± 0.01 | 3.26 ± 0.03 | 1.09 ± 0.01 | 0.176 ± 0.002 |
| tmDES | 0.87 ± 0.01 | 0.933 ± 0.002 | 1.82 ± 0.02 | 1.87 ± 0.02 | 0.573 ± 0.010 | 0.171 ± 0.002 | |
| Limonene | 0.92 ± 0.01 | 0.783 ± 0.008 | 1.19 ± 0.01 | 1.28 ± 0.01 | 0.392 ± 0.002 | 0.150 ± 0.019 | |
| TOPO | 1-Octanol | 0.65 ± 0.03 | 0.0780 ± 0.0134 | 0.0451 ± 0.0093 | 0.0551 ± 0.0067 | 0.0231 ± 0.0063 | 0.0383 ± 0.0060 |
| tmDES | 0.60 ± 0.01 | 0.0840 ± 0.0096 | 0.0312 ± 0.0023 | 0.0419 ± 0.0063 | 0.0487 ± 0.0056 | 0.0417 ± 0.0055 | |
| Limonene | 0.65 ± 0.01 | 0.0596 ± 0.0152 | 0.0259 ± 0.0068 | 0.0209 ± 0.0066 | 0.0364 ± 0.0085 | 0.0361 ± 0.0090 | |
| TBP | 1-Octanol | 0.63 ± 0.01 | 0.0771 ± 0.0048 | 0.0497 ± 0.0019 | 0.0407 ± 0.0026 | 0.0312 ± 0.0041 | 0.0367 ± 0.0044 |
| tmDES | 0.62 ± 0.01 | 0.0718 ± 0.0041 | 0.0450 ± 0.0031 | 0.0398 ± 0.0017 | 0.0339 ± 0.0005 | 0.0216 ± 0.0018 | |
| Limonene | 0.63 ± 0.01 | 0.0486 ± 0.0062 | 0.0118 ± 0.0037 | 0.0353 ± 0.0008 | 0.0348 ± 0.0022 | 0.0154 ± 0.0053 | |
| Mineral Acid | pHF | Solvent Phase | pHR | ztot | Dextr,LA | Dextr,mineral | Sextr,LA |
|---|---|---|---|---|---|---|---|
| HCl | 0.78 ± 0.01 | TOA:1-octanol | 1.94 ± 0.01 | 1.26 ± 0.01 | 0.429 ± 0.009 | 18.9 ± 0.2 | 0.0229 ± 0.0002 |
| TOA:tmDES | 1.86 ± 0.03 | 1.09 ± 0.01 | 0.202 ± 0.006 | 7.42 ± 0.37 | 0.0295 ± 0.0003 | ||
| TOA:limonene | 1.52 ± 0.00 | 0.979 ± 0.002 | 0.116 ± 0.003 | 5.75 ± 0.13 | 0.0201 ± 0.0002 | ||
| TOPO:tmDES | 0.79 ± 0.01 | 0.0947 ± 0.0023 | 0.0782 ± 0.0033 | 0.0207 ± 0.0004 | 3.56 ± 0.03 | ||
| HNO3 | 0.79 ± 0.01 | TOA:1-octanol | 2.05 ± 0.06 | 1.14 ± 0.01 | 0.276 ± 0.008 | 21.5 ± 0.3 | 0.0126 ± 0.0001 |
| TOA:tmDES | 1.82 ± 0.03 | 1.07 ± 0.01 | 0.158 ± 0.007 | 13.0 ± 0.1 | 0.0125 ± 0.0001 | ||
| TOA:limonene | 1.88 ± 0.04 | 1.01 ± 0.01 | 0.106 ± 0.006 | 8.97 ± 0.07 | 0.0113 ± 0.0001 | ||
| TOPO:tmDES | 0.80 ± 0.00 | 0.098 ± 0.003 | 0.0828 ± 0.0023 | 0.0229 ± 0.0005 | 3.64 ± 0.04 | ||
| H2SO4 | 0.80 ± 0.01 | TOA:1-octanol | 1.05 ± 0.00 | 0.806 ± 0.008 | 0.284 ± 0.007 | 1.56 ± 0.02 | 0.181 ± 0.002 |
| TOA:tmDES | 1.01 ± 0.00 | 0.691 ± 0.031 | 0.153 ± 0.034 | 1.27 ± 0.01 | 0.146 ± 0.001 | ||
| TOA:limonene | 1.10 ± 0.01 | 0.674 ± 0.011 | 0.145 ± 0.002 | 1.22 ± 0.04 | 0.122 ± 0.001 | ||
| TOPO:tmDES | 0.74 ± 0.00 | 0.0939 ± 0.0036 | 0.0728 ± 0.0036 | 0.0261 ± 0.0002 | 2.63 ± 0.03 | ||
| H3PO4 | 1.37 ± 0.01 | TOA:1-octanol | 1.79 ± 0.01 | 1.00 ± 0.01 | 0.877 ± 0.011 | 1.18 ± 0.04 | 0.776 ± 0.008 |
| TOA:tmDES | 1.57 ± 0.00 | 0.908 ± 0.006 | 0.790 ± 0.010 | 0.836 ± 0.008 | 0.948 ± 0.009 | ||
| TOA:limonene | 1.45 ± 0.00 | 0.296 ± 0.011 | 0.128 ± 0.012 | 0.222 ± 0.002 | 0.527 ± 0.005 | ||
| TOPO:tmDES | 1.38 ± 0.00 | 0.0933 ± 0.0009 | 0.0740 ± 0.0003 | 0.0236 ± 0.0012 | 3.29 ± 0.03 |
| Mineral Acid | Solvent Phase | pHLR | Dback,LA | Dback,mineral | Sback,LA |
|---|---|---|---|---|---|
| HCl | TOA:1-octanol | 1.90 ± 0.01 | 2.61 ± 0.15 | 0.200 ± 0.004 | 13.0 ± 0.5 |
| TOA:tmDES | 2.19 ± 0.00 | 1.45 ± 0.01 | 0.101 ± 0.002 | 14.4 ± 0.4 | |
| TOA:limonene | 1.42 ± 0.00 | 2.72 ± 0.17 | 0.668 ± 0.026 | 4.07 ± 0.10 | |
| TOPO:tmDES | 3.15 ± 0.03 | 2.22 ± 0.15 | 0.0597 ± 0.0039 | 37.6 ± 4.9 | |
| HNO3 | TOA:1-octanol | 2.02 ± 0.01 | 4.71 ± 0.14 | 0.131 ± 0.003 | 35.9 ± 0.3 |
| TOA:tmDES | 2.27 ± 0.02 | 2.48 ± 0.16 | 0.0846 ± 0.0010 | 29.3 ± 2.2 | |
| TOA:limonene | 1.69 ± 0.01 | 2.49 ± 0.20 | 0.451 ± 0.003 | 5.53 ± 0.48 | |
| TOPO:tmDES | 3.10 ± 0.03 | 2.37 ± 0.19 | 0.0463 ± 0.0019 | 51.5 ± 6.2 | |
| H2SO4 | TOA:1-octanol | 1.75 ± 0.03 | 3.79 ± 0.14 | 0.336 ± 0.018 | 11.3 ± 1.0 |
| TOA:tmDES | 2.42 ± 0.02 | 3.07 ± 0.11 | 0.140 ± 0.003 | 22.0 ± 1.3 | |
| TOA:limonene | 1.29 ± 0.00 | 2.54 ± 0.02 | 0.896 ± 0.015 | 2.84 ± 0.07 | |
| TOPO:tmDES | 3.18 ± 0.00 | 2.65 ± 0.09 | 0.0263 ± 0.0034 | 102 ± 10 | |
| H3PO4 | TOA:1-octanol | 1.74 ± 0.00 | 1.77 ± 0.04 | 15.5 ± 0.4 | 0.114 ± 0.001 |
| TOA:tmDES | 1.94 ± 0.00 | 1.10 ± 0.04 | 4.07 ± 0.07 | 0.269 ± 0.006 | |
| TOA:limonene | 2.11 ± 0.02 | 3.79 ± 0.18 | 53.1 ± 0.5 | 0.0715 ± 0.0026 | |
| TOPO:tmDES | 3.09 ± 0.01 | 2.68 ± 0.01 | 0.0624 ± 0.0040 | 43.2 ± 2.6 |
| Chemical | Shortcut | CAS | Purity | Supplier |
|---|---|---|---|---|
| Tri-n-octylamine | TOA | 1116-76-3 | 98% | Sigma Aldrich, Darmstadt, Germany |
| Cyanex® 921 (trioctylphosphine oxide) | TOPO | 78-50-2 | 91% | Solvay, Hannover, Germany |
| Tributyl phosphate | TBP | 126-73-8 | 97% | Sigma Aldrich, Darmstadt, Germany |
| 1-Octanol | 111-87-5 | ≥99% | Carl Roth, Karlsruhe, Germany | |
| Thymol | 89-83-8 | ≥99% | Carl Roth, Karlsruhe, Germany | |
| L-Menthol | 2216-51-5 | ≥99% | Sigma Aldrich, Darmstadt, Germany | |
| (R)-(+)-Limonene | 5989-27-5 | 95% | Carl Roth, Karlsruhe, Germany | |
| p-Cymene | 99-87-6 | ≥99% | Sigma Aldrich, Darmstadt, Germany | |
| Lactic acid | LA | 50-21-5 | 80% | Sigma Aldrich, Darmstadt, Germany |
| Hydrochloric acid | HCl | 7647-01-0 | 37% | Carl Roth, Karlsruhe, Germany |
| Nitric acid | HNO3 | 7697-37-2 | 65% | Carl Roth, Karlsruhe, Germany |
| Sulfuric acid | H2SO4 | 7664-93-9 | 98% | Carl Roth, Karlsruhe, Germany |
| Phosphoric acid | H3PO4 | 7664-38-2 | 85% | Merck, Darmstadt, Germany |
| Dimethyl sulfoxide | DMSO | 67-68-5 | >99.91% | ThermoFischer Scientific, Waltham, MA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demmelmayer, P.; Ćosić, M.; Kienberger, M. Mineral Acid Co-Extraction in Reactive Extraction of Lactic Acid Using a Thymol-Menthol Deep Eutectic Solvent as a Green Modifier. Molecules 2024, 29, 1722. https://doi.org/10.3390/molecules29081722
Demmelmayer P, Ćosić M, Kienberger M. Mineral Acid Co-Extraction in Reactive Extraction of Lactic Acid Using a Thymol-Menthol Deep Eutectic Solvent as a Green Modifier. Molecules. 2024; 29(8):1722. https://doi.org/10.3390/molecules29081722
Chicago/Turabian StyleDemmelmayer, Paul, Marija Ćosić, and Marlene Kienberger. 2024. "Mineral Acid Co-Extraction in Reactive Extraction of Lactic Acid Using a Thymol-Menthol Deep Eutectic Solvent as a Green Modifier" Molecules 29, no. 8: 1722. https://doi.org/10.3390/molecules29081722