On the Optimization of Carob Seed Peel Extraction Using Aqueous-Based Acidic Systems
Abstract
:1. Introduction
2. Results and Discussion
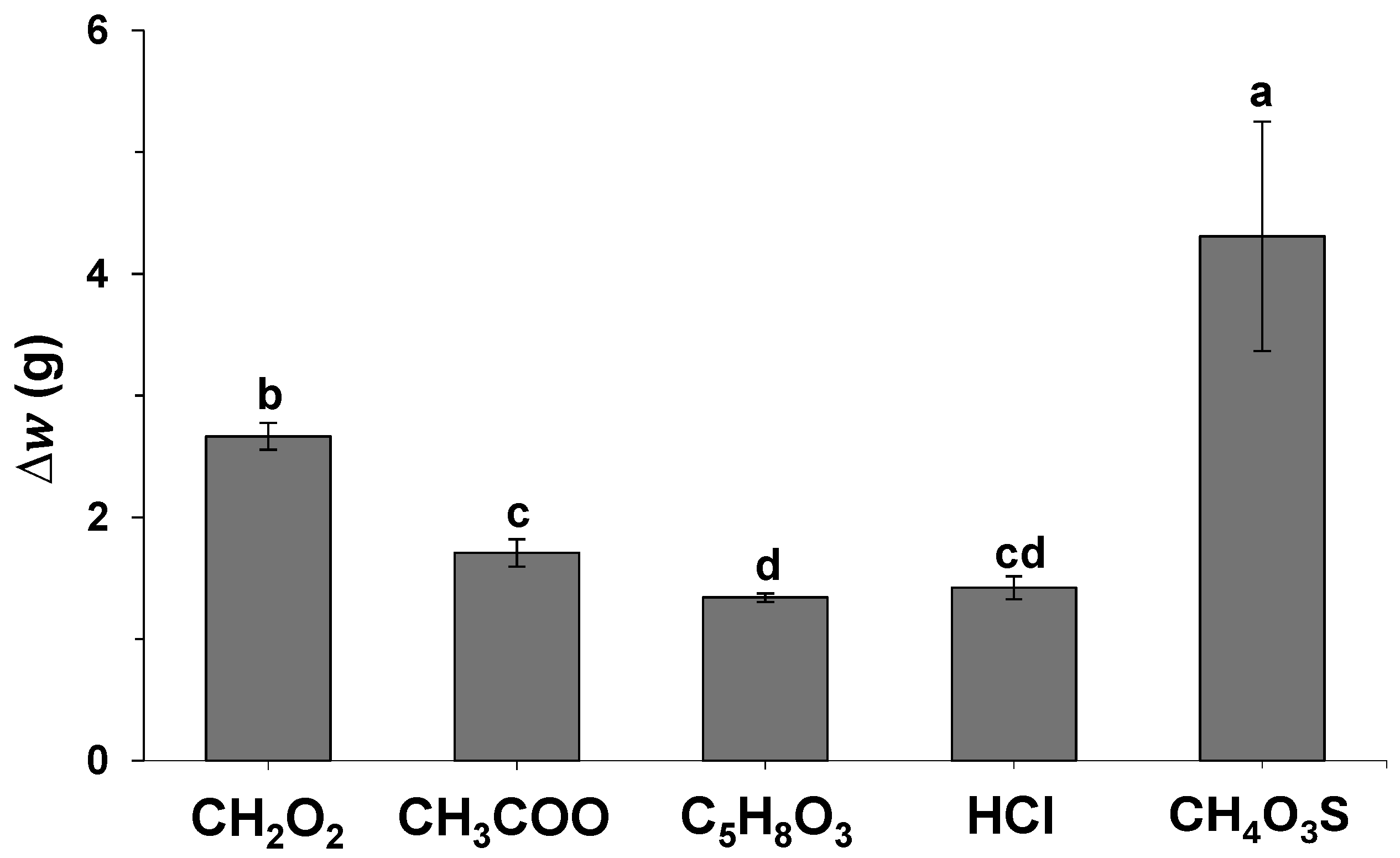
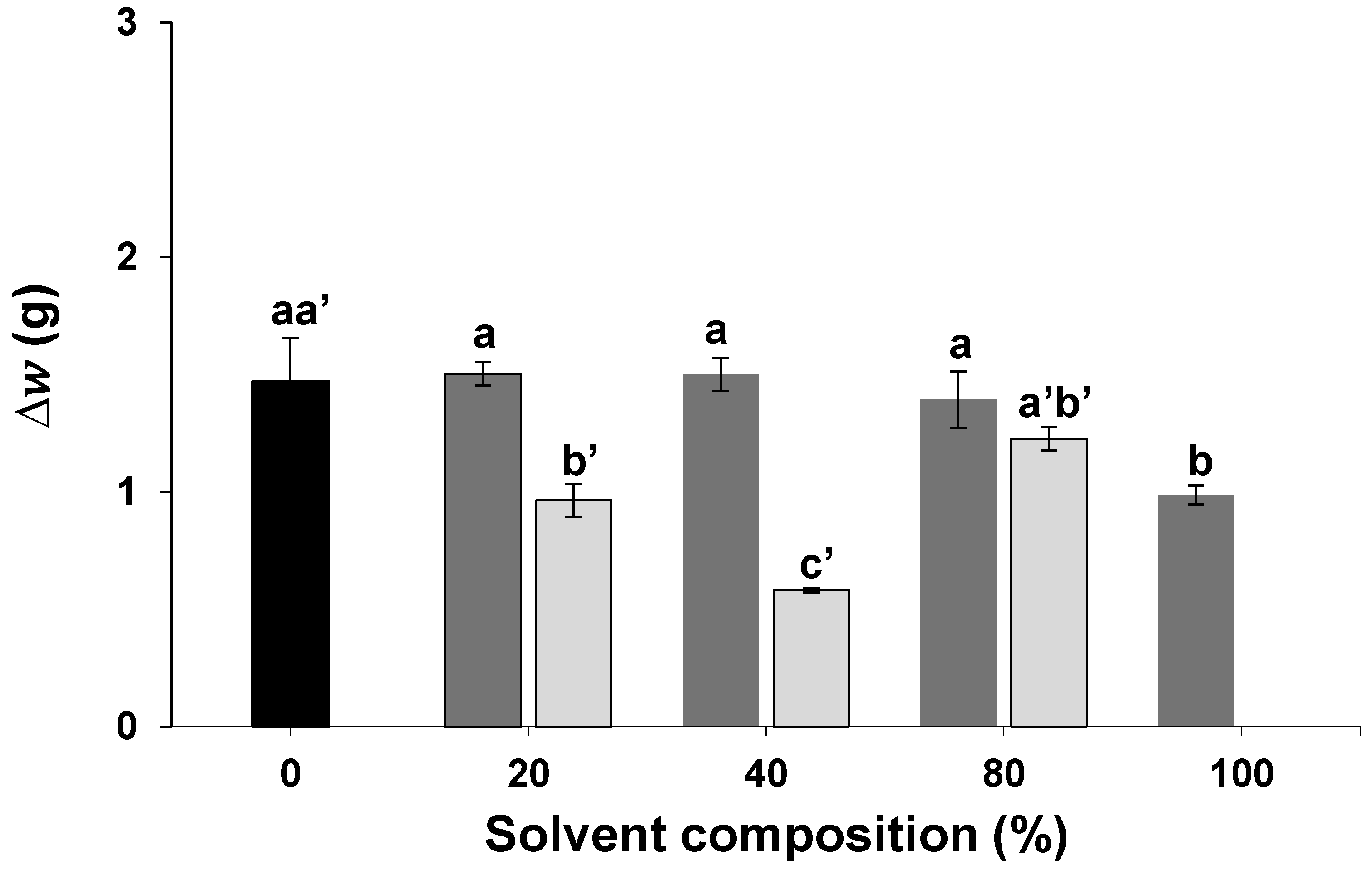
2.1. Initial Solvent Screening
2.2. Extraction Optimization with BBD-RSM
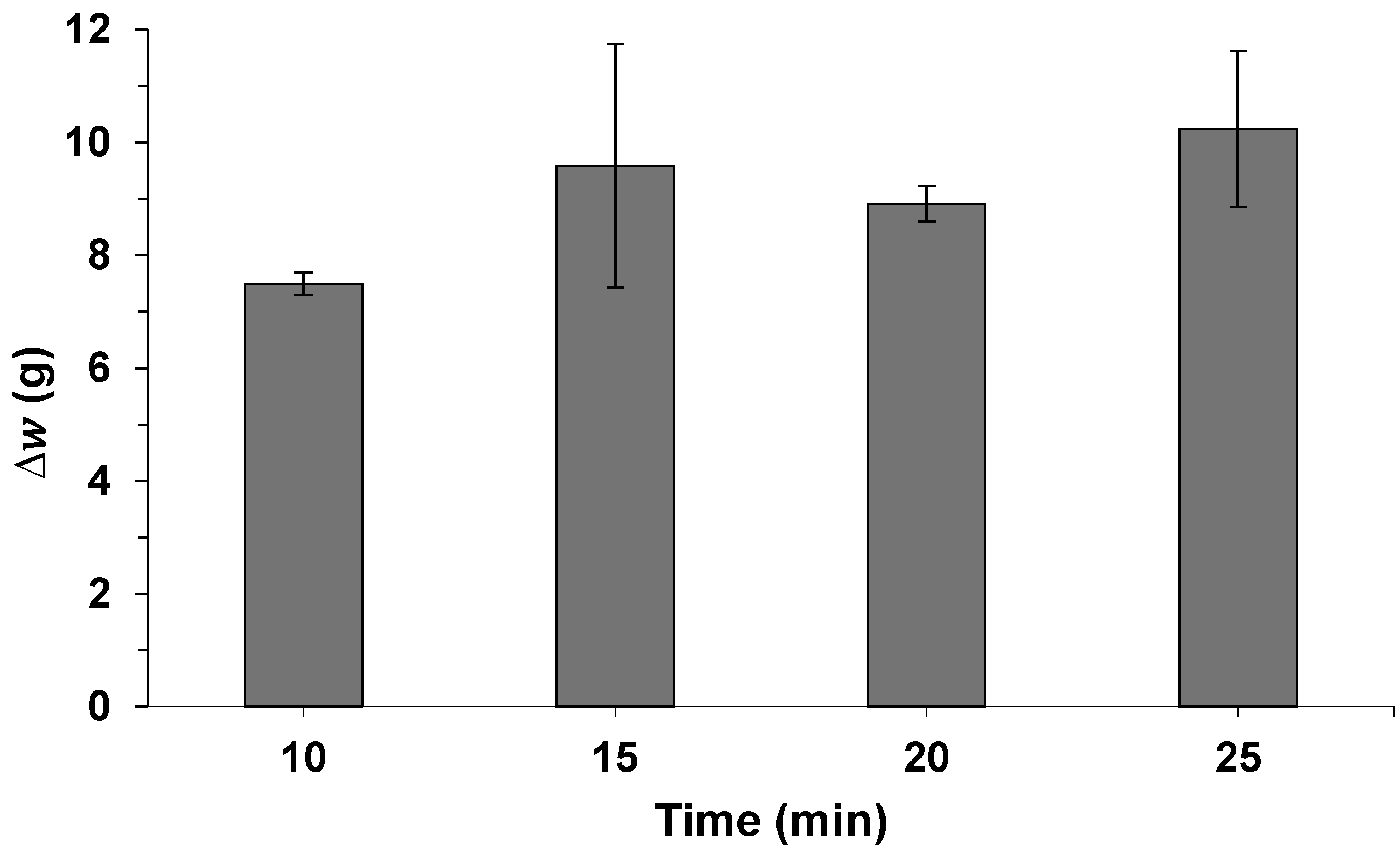
2.3. Repeatability and Intermediate Precision
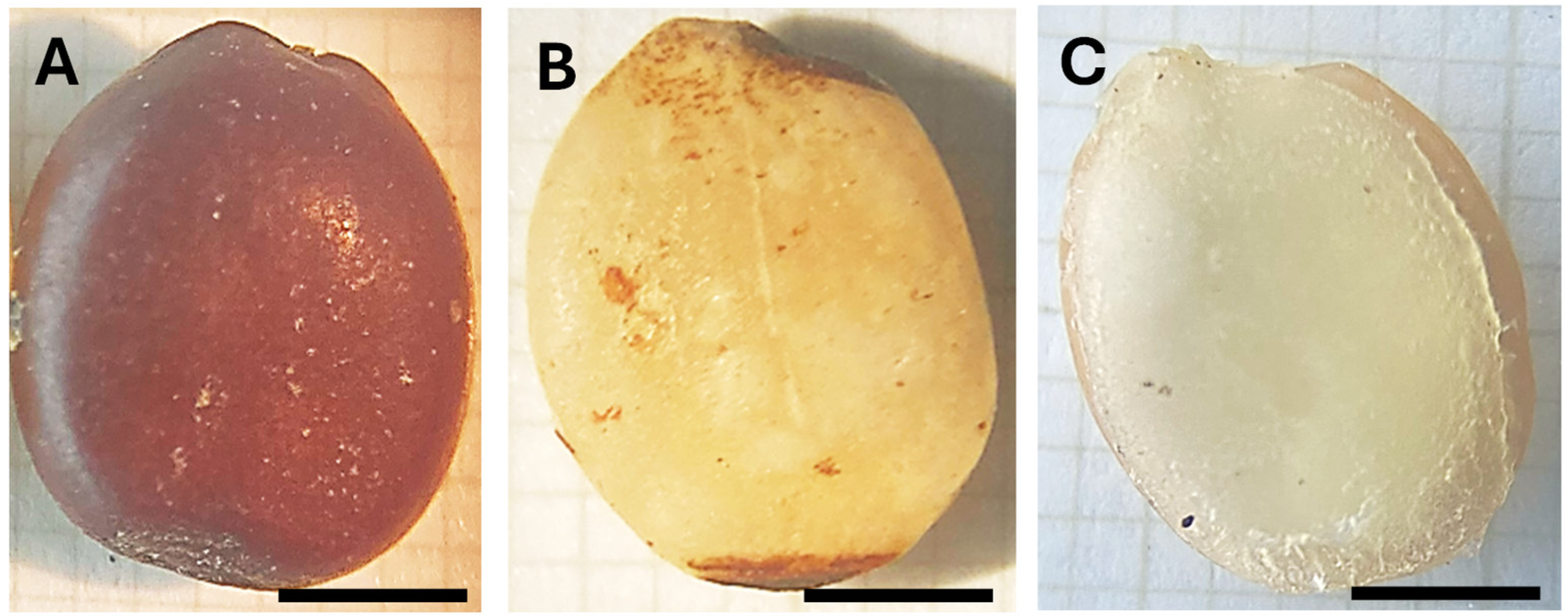
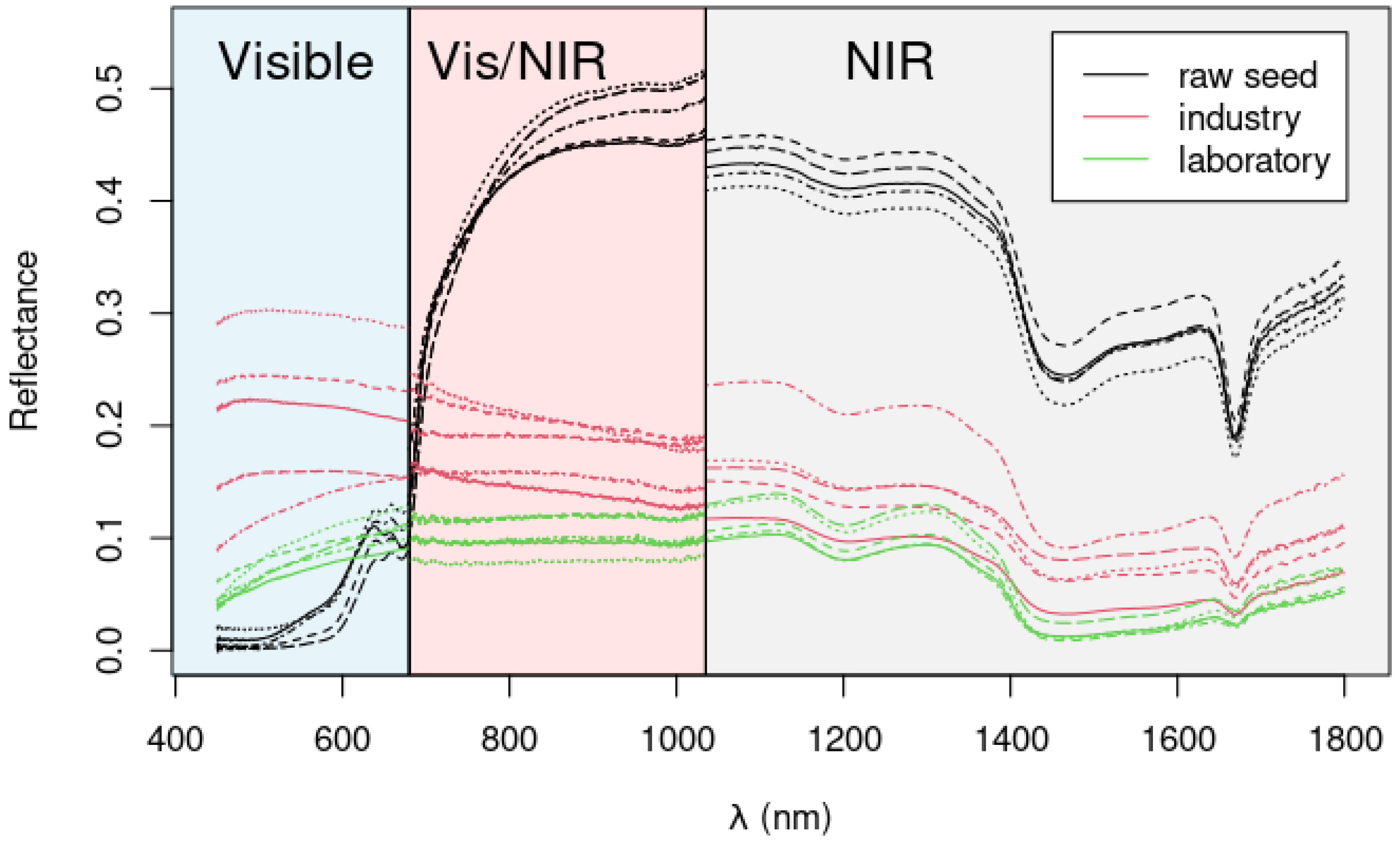
2.4. Colorimetric and Spectroscopic Analysis of the Seeds
3. Material and Methods
3.1. Plant Material and Chemicals
3.2. Methods
3.2.1. Seed Husk Extraction
3.2.2. Design of Experiments
3.2.3. Optical Microscopy
3.2.4. Colorimetric Analyzes
3.3. Diffuse Reflectance Spectroscopy
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins-Loução, M.A.; Correia, P.J.; Romano, A. Carob: A Mediterranean Resource for the Future. Plants 2024, 13, 1188. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Granata, G.; Geraci, C.; Panico, A.; Siciliano, E.A.; Raciti, G.; Puglia, C. Carob Seeds: Food Waste or Source of Bioactive Compounds? Pharmaceutics 2020, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob Pulp: A Nutritional and Functional By-Product Worldwide Spread in the Formulation of Different Food Products and Beverages. A Review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Basharat, Z.; Afzaal, M.; Saeed, F.; Islam, F.; Hussain, M.; Ikram, A.; Pervaiz, M.U.; Awuchi, C.G. Nutritional and Functional Profile of Carob Bean (Ceratonia Siliqua): A Comprehensive Review. Int. J. Food Prop. 2023, 26, 389–413. [Google Scholar] [CrossRef]
- Correia, P.J.; Pestana, M. Sugars and Phenols in Carob Tree Fruits from Different Producing Countries: A Short Review. Heliyon 2024, 10, e30922. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust Bean Gum: Processing, Properties and Food Applications—A Review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, M.; Isasi, J.R. Locust Bean Gum, a Vegetable Hydrocolloid with Industrial and Biopharmaceutical Applications. Molecules 2022, 27, 8265. [Google Scholar] [CrossRef] [PubMed]
- Brassesco, M.E.; Brandão, T.R.S.; Silva, C.L.M.; Pintado, M. Carob Bean (Ceratonia Siliqua, L.): A New Perspective for Functional Food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Dakia, P.A.; Blecker, C.; Robert, C.; Wathelet, B.; Paquot, M. Composition and Physicochemical Properties of Locust Bean Gum Extracted from Whole Seeds by Acid or Water Dehulling Pre-Treatment. Food Hydrocoll. 2008, 22, 807–818. [Google Scholar] [CrossRef]
- Wielinga, W. Seed Gums. In Food Stabilisers, Thickeners and Gelling Agents; Wiley: Hoboken, NJ, USA, 2009; pp. 275–292. [Google Scholar]
- Duarte, H.; Aliaño-González, M.J.; Cantos-Villar, E.; Faleiro, L.; Romano, A.; Medronho, B. Sustainable Extraction of Polyphenols from Vine Shoots Using Deep Eutectic Solvents: Influence of the Solvent, Vitis sp., and Extraction Technique. Talanta 2024, 267, 125135. [Google Scholar] [CrossRef] [PubMed]
- Duarte, H.; Gomes, V.; Aliano-Gonzalez, M.J.; Faleiro, L.; Romano, A.; Medronho, B. Ultrasound-Assisted Extraction of Polyphenols from Maritime Pine Residues with Deep Eutectic Solvents. Foods 2022, 11, 3754. [Google Scholar] [CrossRef] [PubMed]
- Melro, E.; Riddell, A.; Bernin, D.; da Costa, A.M.R.; Valente, A.J.M.; Antunes, F.E.; Romano, A.; Norgren, M.; Medronho, B. Levulinic Acid-Based “Green” Solvents for Lignocellulose Fractionation: On the Superior Extraction Yield and Selectivity toward Lignin. Biomacromolecules 2023, 24, 3094–3104. [Google Scholar] [CrossRef] [PubMed]
- Dapía, S.; Santos, V.; Parajó, J. Study of Formic Acid as an Agent for Biomass Fractionation. Biomass Bioenergy 2002, 22, 213–221. [Google Scholar] [CrossRef]
- Hasegawa, I.; Khoo, T.H.; Mae, K. Direct Saccharification of Lignocellulosic Biomass by Hydrolysis with Formic Acid Solution. Green Process. Synth. 2013, 2, 143–149. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Methanesulfonic Acid (MSA) in Clean Processes and Applications: A Tutorial Review. Green Chem. 2024, 26, 8583–8614. [Google Scholar] [CrossRef] [PubMed]
- Palden, T.; Onghena, B.; Regadío, M.; Binnemans, K. Methanesulfonic Acid: A Sustainable Acidic Solvent for Recovering Metals from the Jarosite Residue of the Zinc Industry. Green Chem. 2019, 21, 5394–5404. [Google Scholar] [CrossRef]
- International Commission on Illumination. CIE 015:2018 Colorimetry, 4th ed.; The International Commission on Illumination: Vienna, Austria, 2018. [Google Scholar]
- Basile, T.; Marsico, A.D.; Perniola, R. NIR Analysis of Intact Grape Berries: Chemical and Physical Properties Prediction Using Multivariate Analysis. Foods 2021, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, A.M.; Pinto, P.; Antunes, M.D.; da Silva, J.M.; Guerra, R. ‘Rocha’ Pear Firmness Predicted by a Vis/NIR Segmented Model. Postharvest Biol. Technol. 2009, 51, 311–319. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Signal: Signal Processing. Available online: https://r-forge.r-project.org/projects/signal/ (accessed on 15 January 2025).




| Experiment | Solid–Liquid Ratio (g Seeds/50 mL Solvent) | Temperature (°C) | CH4O3S (%) | Extraction Time (min) | Volume of Water (mL) | Washing Time (min) | (g) | Adjusted (g) | Error (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 75 | 70 | 7.5 | 75 | 10 | 0.34 | 0.34 | 1.81 |
| 2 | 13.75 | 60 | 80 | 7.5 | 50 | 5 | 0.52 | 0.52 | 0.45 |
| 3 | 2.5 | 75 | 80 | 5 | 50 | 7.5 | 0.22 | 0.23 | 4.00 |
| 4 | 25 | 75 | 80 | 5 | 50 | 7.5 | 0.88 | 0.84 | 4.09 |
| 5 | 13.75 | 75 | 100 | 5 | 75 | 10 | 1.07 | 0.99 | 6.85 |
| 6 | 13.75 | 85 | 80 | 7.5 | 50 | 10 | 3.14 | 3.37 | 7.46 |
| 7 | 13.75 | 60 | 100 | 7.5 | 50 | 7.5 | 2.74 | 2.76 | 0.72 |
| 8 | 13.75 | 75 | 80 | 7.5 | 75 | 7.5 | 2.16 | 2.32 | 7.42 |
| 9 | 13.75 | 90 | 80 | 7.5 | 100 | 10 | 2.28 | 2.12 | 7.07 |
| 10 | 25 | 75 | 100 | 7.5 | 75 | 10 | 1.09 | 1.18 | 8.51 |
| 11 | 13.75 | 75 | 70 | 5 | 75 | 10 | 1.56 | 1.50 | 4.07 |
| 12 | 13.75 | 60 | 70 | 7.5 | 100 | 7.5 | 0.54 | 0.53 | 0.75 |
| 13 | 13.75 | 75 | 80 | 7.5 | 75 | 7.5 | 1.98 | 1.92 | 3.19 |
| 14 | 13.75 | 75 | 70 | 10 | 75 | 5 | 2.04 | 2.01 | 1.52 |
| 15 | 2.5 | 75 | 80 | 10 | 100 | 7.5 | 0.35 | 0.35 | 0.86 |
| 16 | 13.75 | 90 | 80 | 7.5 | 50 | 5 | 2.00 | 1.92 | 3.73 |
| 17 | 2.5 | 60 | 80 | 5 | 75 | 7.5 | 0.09 | 0.10 | 5.78 |
| 18 | 25 | 60 | 80 | 10 | 75 | 7.5 | 2.53 | 2.52 | 0.19 |
| 19 | 25 | 90 | 80 | 10 | 75 | 10 | 8.86 | 8.84 | 0.18 |
| 20 | 13.75 | 60 | 80 | 7.5 | 100 | 5 | 0.67 | 0.61 | 8.70 |
| 21 | 13.75 | 90 | 100 | 7.5 | 50 | 7.5 | 2.86 | 2.70 | 5.57 |
| 22 | 13.75 | 90 | 70 | 7.5 | 50 | 7.5 | 2.07 | 2.02 | 2.11 |
| 23 | 13.75 | 75 | 70 | 5 | 75 | 5 | 1.54 | 1.60 | 3.80 |
| 24 | 13.75 | 75 | 100 | 10 | 75 | 10 | 1.12 | 1.19 | 6.00 |
| 25 | 2.5 | 60 | 80 | 10 | 75 | 7.5 | 0.26 | 0.25 | 3.69 |
| 26 | 25 | 75 | 80 | 10 | 100 | 7.5 | 3.64 | 3.38 | 7.27 |
| 27 | 2.5 | 75 | 100 | 7.5 | 75 | 5 | 0.21 | 0.21 | 2.08 |
| 28 | 13.75 | 75 | 80 | 7.5 | 75 | 7.5 | 1.93 | 2.12 | 9.93 |
| 29 | 2.5 | 75 | 80 | 5 | 100 | 7.5 | 0.27 | 0.27 | 2.36 |
| 30 | 13.75 | 75 | 80 | 7.5 | 75 | 7.5 | 2.34 | 2.32 | 0.88 |
| 31 | 25 | 90 | 100 | 5 | 75 | 7.5 | 1.83 | 1.99 | 8.70 |
| 32 | 2.5 | 75 | 100 | 7.5 | 75 | 10 | 0.30 | 0.29 | 0.76 |
| 33 | 25 | 75 | 80 | 10 | 50 | 7.5 | 4.98 | 5.02 | 0.75 |
| 34 | 13.75 | 60 | 70 | 7.5 | 50 | 7.5 | 1.67 | 1.83 | 9.94 |
| 35 | 25 | 75 | 70 | 7.5 | 75 | 10 | 2.88 | 2.95 | 2.49 |
| 36 | 25 | 60 | 80 | 5 | 75 | 7.5 | 2.22 | 2.00 | 10.10 |
| 37 | 2.5 | 75 | 70 | 7.5 | 75 | 5 | 0.52 | 0.53 | 1.03 |
| 38 | 13.75 | 75 | 80 | 7.5 | 75 | 7.5 | 2.75 | 2.32 | 15.61 |
| 39 | 13.75 | 75 | 80 | 7.5 | 75 | 7.5 | 2.76 | 2.32 | 15.92 |
| 40 | 25 | 85 | 100 | 10 | 75 | 8 | 5.86 | 5.19 | 11.39 |
| 41 | 13.75 | 75 | 100 | 10 | 75 | 5 | 2.94 | 3.08 | 4.69 |
| 42 | 13.75 | 60 | 100 | 7.5 | 100 | 7.5 | 1.96 | 1.64 | 16.32 |
| 43 | 13.75 | 90 | 70 | 7.5 | 100 | 7.5 | 3.52 | 3.56 | 1.24 |
| 44 | 13.75 | 60 | 80 | 7.5 | 100 | 10 | 1.82 | 1.76 | 3.41 |
| 45 | 13.75 | 90 | 80 | 7.5 | 100 | 5 | 3.15 | 3.35 | 6.43 |
| 46 | 25 | 75 | 70 | 7.5 | 75 | 5 | 5.39 | 4.52 | 16.20 |
| 47 | 25 | 75 | 50 | 5 | 100 | 7.5 | 3.45 | 3.47 | 0.79 |
| 48 | 13.75 | 90 | 100 | 7.5 | 100 | 7.5 | 4.12 | 3.51 | 14.63 |
| 49 | 13.75 | 90 | 80 | 7.5 | 50 | 10 | 3.47 | 3.30 | 5.13 |
| 50 | 2.5 | 90 | 80 | 10 | 75 | 7.5 | 0.75 | 0.75 | 0.70 |
| 51 | 2.5 | 75 | 80 | 10 | 50 | 7.5 | 0.53 | 0.53 | 0.44 |
| 52 | 2.5 | 90 | 80 | 5 | 75 | 7.5 | 0.46 | 0.41 | 9.94 |
| 53 | 13.75 | 75 | 100 | 5 | 75 | 5 | 2.59 | 2.17 | 16.46 |
| 54 | 13.75 | 75 | 70 | 10 | 75 | 10 | 2.47 | 2.68 | 8.13 |
| Variable | Sum of Squares | F-Value | p-Value |
|---|---|---|---|
| A: Solid–liquid ratio (g/50 mL) | 64.366 | 68.49 | 0.0000 |
| B: Temperature (°C) | 12.308 | 13.10 | 0.0013 |
| C: CH4O3S (%) | 0.222 | 0.24 | 0.6309 |
| D: Extraction time (min) | 8.519 | 9.07 | 0.0057 |
| E: Volume of water to wash (mL) | 0.021 | 0.02 | 0.8844 |
| F: Washing time (min) | 1.449 | 1.54 | 0.2255 |
| AA | 0.125 | 0.13 | 0.7184 |
| AB | 3.238 | 3.45 | 0.0748 |
| AC | 0.115 | 0.12 | 0.7290 |
| AD | 7.250 | 7.72 | 0.0100 |
| AE | 0.228 | 0.24 | 0.6267 |
| AF | 6.477 | 6.89 | 0.0143 |
| BB | 0.345 | 0.37 | 0.5498 |
| BC | 0.152 | 0.16 | 0.6909 |
| BD | 5.847 | 6.22 | 0.0193 |
| BE | 2.057 | 2.19 | 0.1510 |
| BF | 1.248 | 1.33 | 0.2597 |
| CC | 0.073 | 0.08 | 0.7833 |
| CD | 0.129 | 0.14 | 0.7144 |
| CE | 0.003 | 0.00 | 0.9573 |
| CF | 2.095 | 2.23 | 0.1475 |
| DD | 0.723 | 0.77 | 0.3884 |
| DE | 2.133 | 2.27 | 0.1440 |
| DF | 0.002 | 0.00 | 0.9659 |
| EE | 0.237 | 0.25 | 0.6199 |
| EF | 1.818 | 1.94 | 0.1760 |
| FF | 0.490 | 0.52 | 0.4768 |
| Error total | 24.433 | ||
| Total (corr.) | 146.330 |
| Average (g) | Standard Deviation (g) | C.V. (%) | |
|---|---|---|---|
| Repeatability (n = 9) | 9.14 | 0.32 | 3.49 |
| Intermediate Precision (n = 9) | 10.08 | 0.82 | 4.12 |
| Type of Seed | Color Parameters | ||
|---|---|---|---|
| L* | a* | b* | |
| A | 27.10 ± 2.57 | 4.76 ± 0.35 | 23.54 ± 2.73 |
| B | 43.16 ± 0.33 | 2.67 ± 0.87 | 14.28 ± 2.7 |
| C | 69.69 ± 2.16 | 2.08 ± 0.33 | 3.11 ± 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medronho, B.; Boutoub, O.; Duarte, H.; Aliaño-González, M.J.; Guerra, R.; Brázio, A.; Gonçalves, S.; Romano, A. On the Optimization of Carob Seed Peel Extraction Using Aqueous-Based Acidic Systems. Molecules 2025, 30, 1397. https://doi.org/10.3390/molecules30071397
Medronho B, Boutoub O, Duarte H, Aliaño-González MJ, Guerra R, Brázio A, Gonçalves S, Romano A. On the Optimization of Carob Seed Peel Extraction Using Aqueous-Based Acidic Systems. Molecules. 2025; 30(7):1397. https://doi.org/10.3390/molecules30071397
Chicago/Turabian StyleMedronho, Bruno, Oumaima Boutoub, Hugo Duarte, Maria José Aliaño-González, Rui Guerra, António Brázio, Sandra Gonçalves, and Anabela Romano. 2025. "On the Optimization of Carob Seed Peel Extraction Using Aqueous-Based Acidic Systems" Molecules 30, no. 7: 1397. https://doi.org/10.3390/molecules30071397
APA StyleMedronho, B., Boutoub, O., Duarte, H., Aliaño-González, M. J., Guerra, R., Brázio, A., Gonçalves, S., & Romano, A. (2025). On the Optimization of Carob Seed Peel Extraction Using Aqueous-Based Acidic Systems. Molecules, 30(7), 1397. https://doi.org/10.3390/molecules30071397













