Combination Treatment of Timosaponin BII and Pirfenidone Attenuated Pulmonary Fibrosis Through Anti-Inflammatory and Anti-Fibrotic Process in Rodent Pulmonary Fibrosis Model and Cellular Epithelial–Mesenchymal Transition Model
Abstract
1. Introduction
2. Results
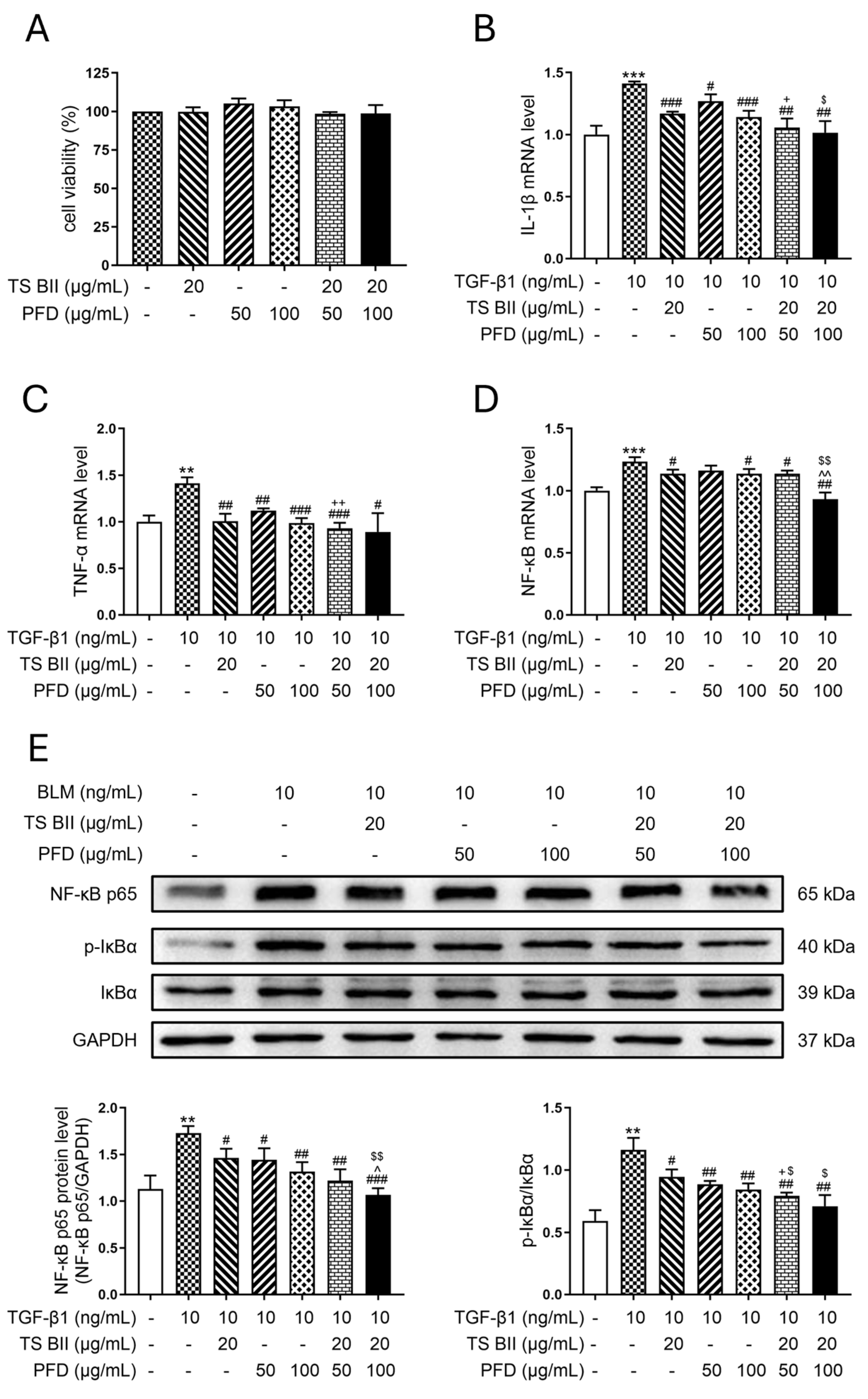
2.1. TS BII, PFD, and Their Combination Exerted Anti-Inflammatory Effects
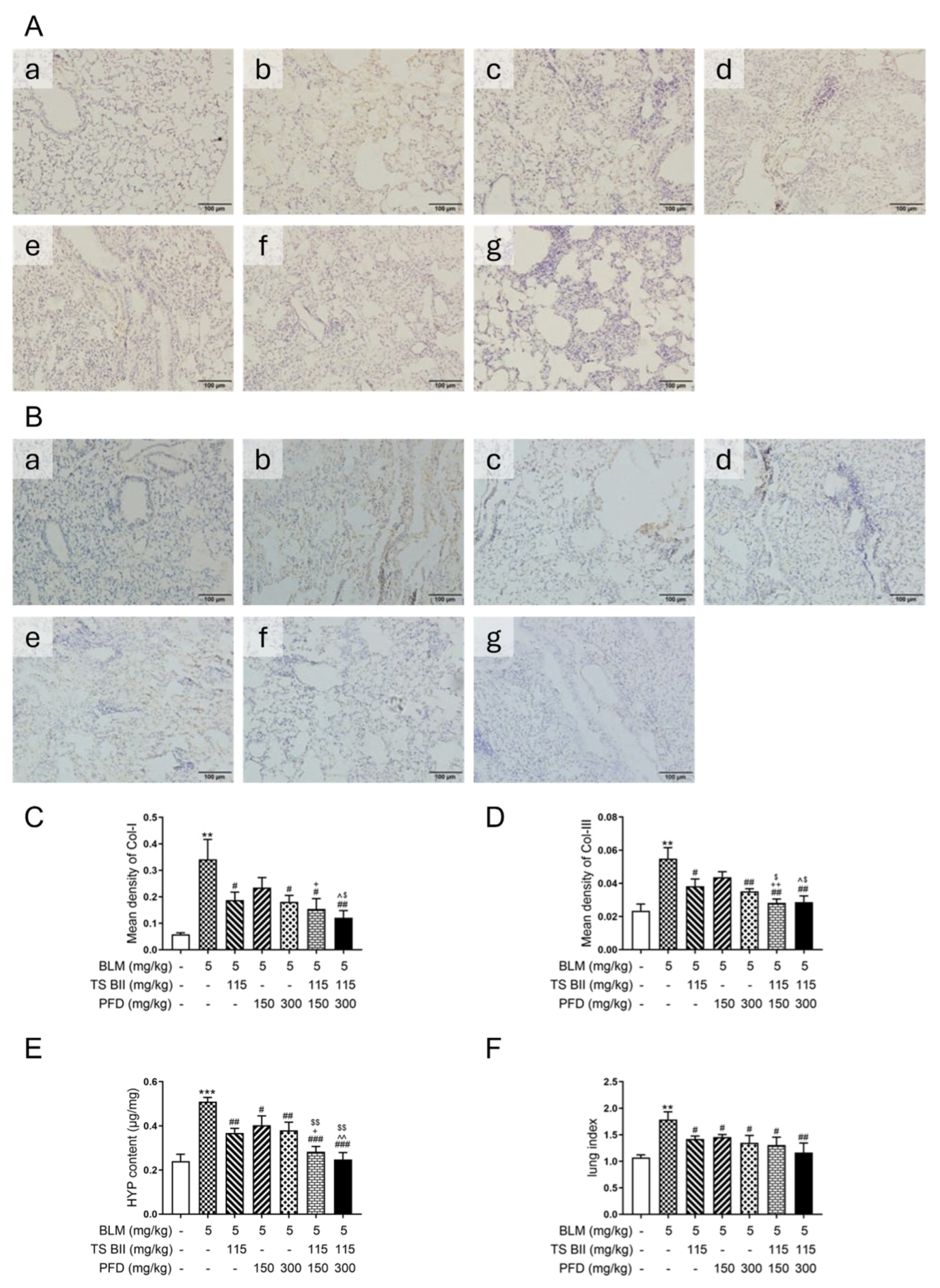
2.2. TS BII, PFD, and Their Combination Exerted Anti-Fibrotic Effects
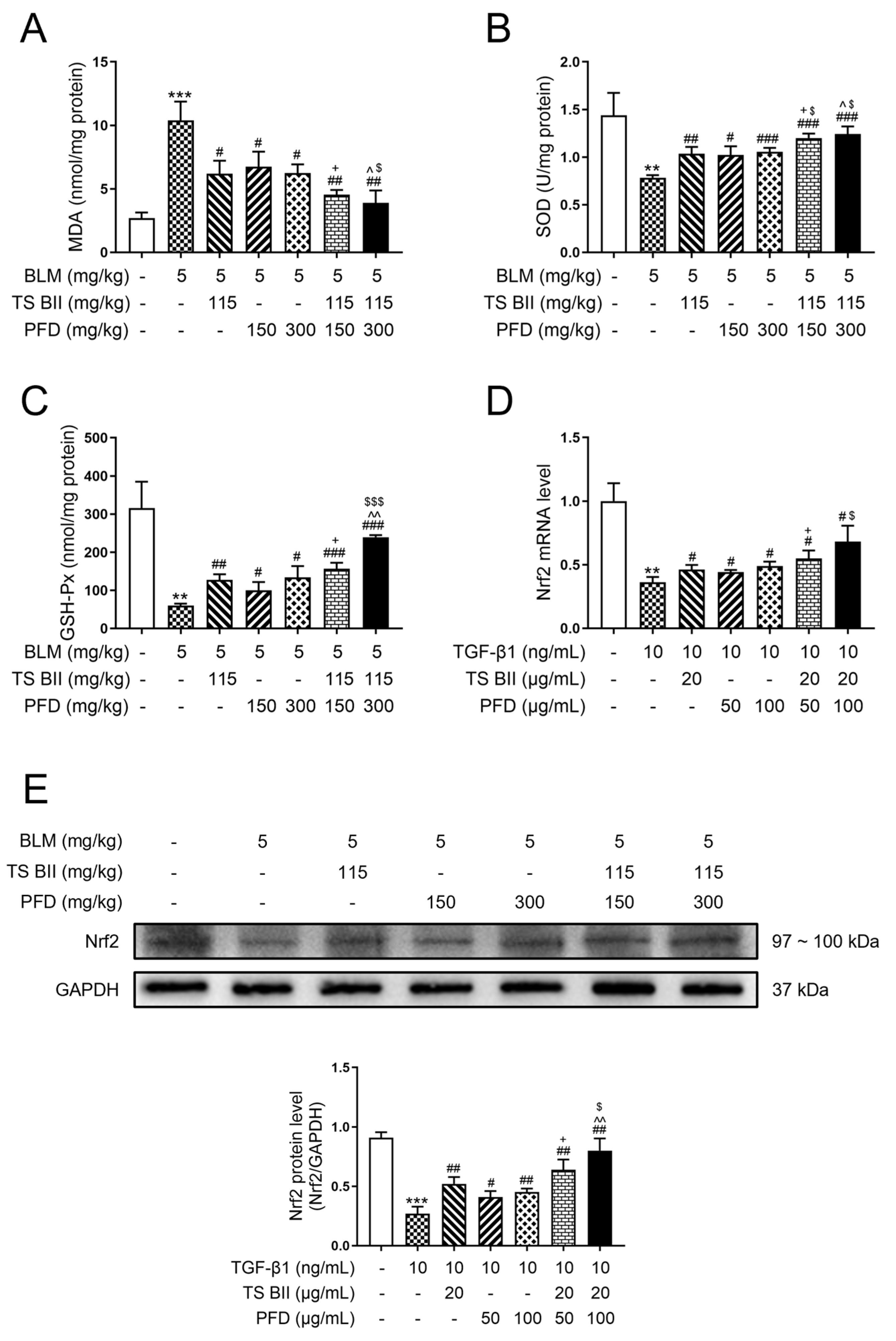
2.3. TS BII, PFD, and Their Combination Modulated Oxidative Stress
2.4. TS BII, PFD, and Their Combination Regulated Epithelial–Mesenchymal Transition (EMT)
2.5. TS BII, PFD, and Their Combination Regulated Intestinal Flora
3. Discussion
4. Materials and Methods
4.1. Material and Reagents
4.2. Animals
4.3. Establishment of Mouse PF Model and Drug Administration
4.4. Pulmonary Histopathological Examination
4.5. Biochemical Parameters Measurement
4.6. IHC Analysis
4.7. Cell Culture and Drug Administration
4.8. qRT-PCR Assay
4.9. Western Blot Analysis
4.10. IF Staining
4.11. Intestinal Flora Analysis in BLM-Induced PF Mice
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAB | Anemarrhena asphodeloides Bunge |
| AREs | antioxidant response elements |
| BLM | bleomycin |
| CMC-Na | sodium carboxymethyl cellulose |
| Col-I | collagen type I |
| Col-III | collagen type III |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| GSH-Px | glutathione peroxidase |
| H&E | hematoxylin and eosin |
| IHC | immunohistochemistry |
| HYP | hydroxyproline |
| IF | immunofluorescence |
| IL-1β | interleukin-1β |
| MDA | malondialdehyde |
| NF-κB | nuclear factor-κB |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PBS | phosphate-buffered saline |
| PF | pulmonary fibrosis |
| PFD | pirfenidone |
| qRT-PCR | quantitative real-time PCR |
| SOD | superoxide dismutase |
| TGF-β1 | transforming growth factor-β1 |
| TNF-α | tumor necrosis factor-α |
| TS BII | timosaponin BII |
References
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Xie, H.; He, X.; Zhang, Y.; Zhang, A.; Li, H. Small airway dysfunction in idiopathic pulmonary fibrosis. Front. Pharmacol. 2022, 13, 1025814. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, K.; Tu, B.; Sun, H.; Zhou, Y.; Xu, S.; Lu, D.; Sha, J.; Tao, H. New aspects of the epigenetic regulation of EMT related to pulmonary fibrosis. Eur. J. Pharmacol. 2023, 956, 175959. [Google Scholar] [CrossRef] [PubMed]
- Savin, I.A.; Zenkova, M.A.; Sen’kova, A.V. Pulmonary fibrosis as a result of acute lung inflammation: Molecular mechanisms, relevant in vivo models, prognostic and therapeutic approaches. Int. J. Mol. Sci. 2022, 23, 14959. [Google Scholar] [CrossRef]
- Veith, C.; Boots, A.W.; Idris, M.; van Schooten, F.-J.; van der Vliet, A. Redox imbalance in idiopathic pulmonary fibrosis: A role for oxidant cross-talk between NADPH oxidase enzymes and mitochondria. Antioxid. Redox Signal. 2019, 31, 1092–1115. [Google Scholar] [CrossRef]
- Sgalla, G.; Iovene, B.; Calvello, M.; Ori, M.; Varone, F.; Richeldi, L. Idiopathic pulmonary fibrosis: Pathogenesis and management. Respir. Res. 2018, 19, 32. [Google Scholar] [CrossRef]
- Podolanczuk, A.J.; Raghu, G. Idiopathic pulmonary fibrosis mortality: Update on trends in the modern treatment era. Eur. Respir. J. 2024, 64, 2401305. [Google Scholar] [CrossRef]
- Lopez-de la Mora, D.A.; Sanchez-Roque, C.; Montoya-Buelna, M.; Sanchez-Enriquez, S.; Lucano-Landeros, S.; Macias-Barragan, J.; Armendariz-Borunda, J.; Armendariz-Borunda, J. Role and new insights of pirfenidone in fibrotic diseases. Int. J. Med. Sci. 2015, 12, 840–847. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Glassberg, M.K.; Nathan, S.D.; Lin, C.-Y.; Morgenthien, E.A.; Stauffer, J.L.; Chou, W.; Noble, P.W. Cardiovascular risks, bleeding risks, and clinical events from 3 phase III trials of pirfenidone in patients with idiopathic pulmonary fibrosis. Adv. Ther. 2019, 36, 2910–2926. [Google Scholar] [CrossRef]
- Du, W.; Tang, Z.; Yang, F.; Liu, X.; Dong, J. Icariin attenuates bleomycin-induced pulmonary fibrosis by targeting Hippo/YAP pathway. Biomed. Pharmacother. 2021, 143, 112152. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Q.; Dong, B.; Qi, C.; Yang, T.; Li, M.; He, S.; Liu, B.; Wu, J. Gypenosides attenuate pulmonary fibrosis by inhibiting the AKT/mTOR/c-Myc pathway. Front. Pharmacol. 2022, 12, 806312. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lv, J.; Khan, G.J.; Duan, H.; Wang, W.; Zhai, K.; Zou, G.; Aisa, H.A. Total flavonoid extract from Dracocephalum moldavica L. improves pulmonary fibrosis by reducing inflammation and inhibiting the hedgehog signaling pathway. Phytother. Res. 2023, 37, 2745–2758. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, W.A.; Antoniou, K.M.; Borensztajn, K.; Costabel, U.; Cottin, V.; Crestani, B.; Grutters, J.C.; Maher, T.M.; Poletti, V.; Richeldi, L.; et al. Combination therapy: The future of management for idiopathic pulmonary fibrosis? Lancet Respir. Med. 2014, 2, 933–942. [Google Scholar] [CrossRef]
- Liu, C.; Cong, Z.; Wang, S.; Zhang, X.; Song, H.; Xu, T.; Kong, H.; Gao, P.; Liu, X. A review of the botany, ethnopharmacology, phytochemistry, pharmacology, toxicology and quality of Anemarrhena asphodeloides Bunge. J. Ethnopharmacol. 2023, 302, 115857. [Google Scholar] [CrossRef]
- Ding, D.; Shen, X.; Yu, L.; Zheng, Y.; Liu, Y.; Wang, W.; Liu, L.; Zhao, Z.; Nian, S.; Liu, L. Timosaponin BII inhibits TGF-β mediated epithelial-mesenchymal transition through Smad-dependent pathway during pulmonary fibrosis. Phytother. Res. 2023, 37, 2787–2799. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, R.; Moisés, S. Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am. J. Respir. Crit. Care Med. 2015, 191, 251–254. [Google Scholar] [CrossRef]
- Sun, S. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Li, H.; Zhou, T.; Zhou, A.; Zhong, Z.; Liu, Y.; Yuan, L.; Zhu, H.; Luan, D.; et al. Antidepressant-like effects of helicid on a chronic unpredictable mild stress-induced depression rat model: Inhibiting the IKK/IκBα/NF-κB pathway through NCALD to reduce inflammation. Int. Immunopharmacol. 2021, 93, 107165. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Yu, T.; Hao, W.; Wang, G. Knockout of PKC θ gene attenuates oleic acid-induced acute lung injury via reduction of inflammation and oxidative stress. Iran. J. Basic Med. Sci. 2021, 24, 986. [Google Scholar] [CrossRef]
- Shao, X.; Li, B.; Shen, J.; Wang, Q.; Chen, S.; Jiang, X.; Qiang, D. Ghrelin alleviates traumatic brain injury-induced acute lung injury through pyroptosis/NF-κB pathway. Int. Immunopharmacol. 2020, 79, 106175. [Google Scholar] [CrossRef] [PubMed]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2022, 23, 289–303. [Google Scholar] [CrossRef]
- Liu, W.; Han, X.; Li, Q.; Sun, L.; Wang, J. Iguratimod ameliorates bleomycin-induced pulmonary fibrosis by inhibiting the EMT process and NLRP3 inflammasome activation. Biomed. Pharmacother. 2022, 153, 113460. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Qin, Y.; Fu, X.; Yang, F.; Huo, F.; Liang, X.; Wang, S.; Cui, H.; Lin, P.; Zhou, G. Inhibition of ferroptosis and iron accumulation alleviates pulmonary fibrosis in a bleomycin model. Redox Biol. 2022, 57, 102509. [Google Scholar] [CrossRef]
- Kumar, V.; Kalita, J.; Bora, H.K.; Misra, U.K. Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol. Appl. Pharmacol. 2016, 293, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nie, Y.; Luo, Y.; Lin, F.; Zheng, Y.; Cheng, G.; Wu, H.; Zhang, K.; Su, W.; Shen, J.; et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem. Toxicol. 2013, 58, 133–140. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef]
- Wang, R.; Mu, J. Arbutin attenuates ethanol-induced acute hepatic injury by the modulation of oxidative stress and Nrf-2/HO-1 signaling pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22872. [Google Scholar] [CrossRef]
- Liu, M.; Xu, H.; Zhang, L.; Zhang, C.; Yang, L.; Ma, E.; Liu, L.; Li, Y. Salvianolic acid B inhibits myofibroblast transdifferentiation in experimental pulmonary fibrosis via the up-regulation of Nrf2. Biochem. Biophys. Res. Commun. 2018, 495, 325–331. [Google Scholar] [CrossRef]
- Lan, Y.; Cheng, M.; Ji, H.; Bi, Y.; Han, Y.; Yang, C.; Gu, X.; Gao, J.; Dong, H. Melatonin ameliorates bleomycin-induced pulmonary fibrosis via activating NRF2 and inhibiting galectin-3 expression. Acta Pharmacol. Sin. 2023, 44, 1029–1037. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, J.; Li, H.; Du, Y.; Li, S.; Li, A.; Suo, X.; Wang, Y.; Sun, Q. Anti-inflammatory activity of the water extract of Chloranthus serratus roots in LPS-stimulated RAW264. 7 cells mediated by the Nrf2/HO-1, MAPK and NF-κB signaling pathways. J. Ethnopharmacol. 2021, 271, 113880. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, H.; Liu, J.; Bai, W.; Wang, J.; Yang, Z.; Qiu, M.; Chang, H.; Shi, S. The intervention effect of Amygdalus mongolica oil on the metabolomics and intestinal flora in pulmonary fibrosis. Front. Pharmacol. 2022, 13, 1037563. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Simonelli, I.; Pasqualetti, P.; Sessa, R. Cervicovaginal microbiota composition in Chlamydia trachomatis infection: A systematic review and meta-analysis. Int. J. Mol. Sci. 2022, 23, 9554. [Google Scholar] [CrossRef]
- Nian, S.; Liu, E.; Fan, Y.; Alolga, R.; Li, H.; Li, P. Orthogonal separation protocol for the simultaneous preparation of four medically active compounds from Anemarrhenae Rhizoma by sequential polyamide and macroporous resin adsorbent chromatography. J. Sep. Sci. 2016, 39, 3195–3204. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Lai, D.; Huang, X. A new method for quantitative real-time polymerase chain reaction data analysis. J. Comput. Biol. 2013, 20, 703–711. [Google Scholar] [CrossRef]






| Step | Temperature | Note |
|---|---|---|
| 1 | 95 °C | |
| 2 | 95 °C | repeat steps 2 and 3 for 40 cycles |
| 3 | 60 °C |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| IL-1β | ATGATGGCTTATTACAGTGGCAA | GTCGGAGATTCGTAGCTGGA |
| TNF-α | CCTGCTGCACTTTGGAGTGA | GAGGGTTTGCTACAACATGGG |
| NF-κB | TGTAAAACGACGGCCAGT | CAGGAAACAGCTATGACC |
| Nrf2 | TCCGGGTGTGTTTGTTCCAA | CGCCCGCGAGATAAAGAGTT |
| E-cadherin | CGATTCAAAGTGGGCACAGATG | GTAGGTGGAGTCCCAGGCGTAG |
| Vimentin | TCTGGATTCACTCCCTCTGGTT | ATCGTGATGCTGAGAAGAGTCTC |
| α-SMA | ATGCTCCCAGGGCTGTTTTC | CTTTTGCTCTGTGCTTCGGTC |
| GAPDH | CTTTGGTATCGTGGAAGGACTC | GTAGAGGCAGGGATGATGTTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Zheng, Y.; Yang, H.; Liu, L.; Yu, L.; Zhang, Y.; Song, X.; He, Y.; Jin, R.; Jiao, J.; et al. Combination Treatment of Timosaponin BII and Pirfenidone Attenuated Pulmonary Fibrosis Through Anti-Inflammatory and Anti-Fibrotic Process in Rodent Pulmonary Fibrosis Model and Cellular Epithelial–Mesenchymal Transition Model. Molecules 2025, 30, 1821. https://doi.org/10.3390/molecules30081821
Shen X, Zheng Y, Yang H, Liu L, Yu L, Zhang Y, Song X, He Y, Jin R, Jiao J, et al. Combination Treatment of Timosaponin BII and Pirfenidone Attenuated Pulmonary Fibrosis Through Anti-Inflammatory and Anti-Fibrotic Process in Rodent Pulmonary Fibrosis Model and Cellular Epithelial–Mesenchymal Transition Model. Molecules. 2025; 30(8):1821. https://doi.org/10.3390/molecules30081821
Chicago/Turabian StyleShen, Xuebin, Yueyue Zheng, Hui Yang, Li Liu, Lizhen Yu, Yuanxiang Zhang, Xiaojun Song, Yuqing He, Runze Jin, Jianhao Jiao, and et al. 2025. "Combination Treatment of Timosaponin BII and Pirfenidone Attenuated Pulmonary Fibrosis Through Anti-Inflammatory and Anti-Fibrotic Process in Rodent Pulmonary Fibrosis Model and Cellular Epithelial–Mesenchymal Transition Model" Molecules 30, no. 8: 1821. https://doi.org/10.3390/molecules30081821
APA StyleShen, X., Zheng, Y., Yang, H., Liu, L., Yu, L., Zhang, Y., Song, X., He, Y., Jin, R., Jiao, J., Gu, Z., Zhai, K., Nian, S., & Liu, L. (2025). Combination Treatment of Timosaponin BII and Pirfenidone Attenuated Pulmonary Fibrosis Through Anti-Inflammatory and Anti-Fibrotic Process in Rodent Pulmonary Fibrosis Model and Cellular Epithelial–Mesenchymal Transition Model. Molecules, 30(8), 1821. https://doi.org/10.3390/molecules30081821







