Chemical Characterization, Lipid Profile, and Volatile Compounds in Chlorella sp. and Spirulina platensis: A Promising Feedstock for Various Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Raw Material
2.2. Volatile Compounds from Microalgae
2.3. Extraction of Lipids from Microalgae
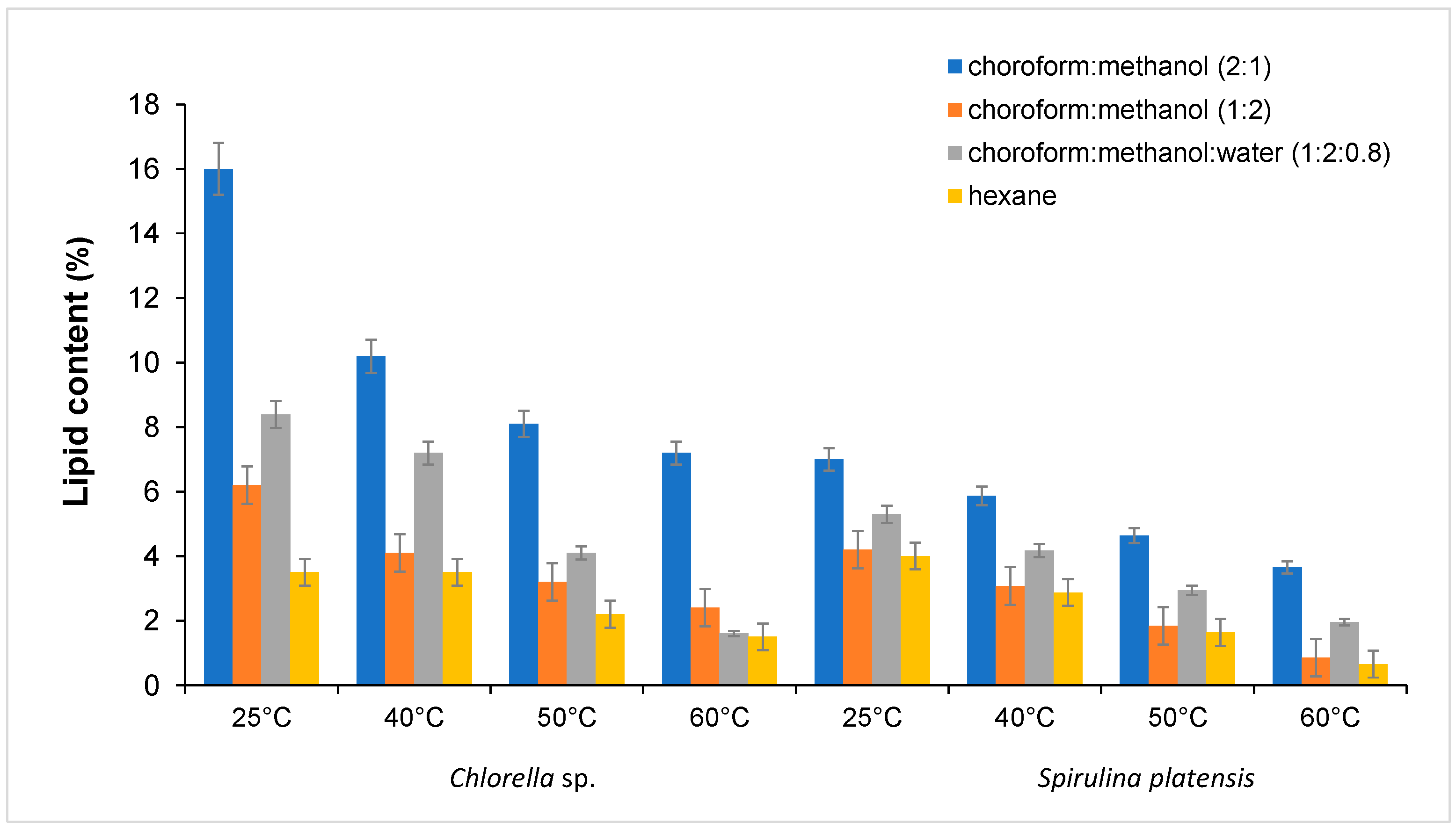
2.3.1. Effects of Solvent Mixtures
2.3.2. Effects of Extraction Temperature
2.3.3. Effects of Solid-to-Solvent Ratio
2.4. Fatty Acids Composition in Oil Extracted from Microalgae
2.5. Lipid Quality Indices
3. Materials and Methods
3.1. Chemicals and Raw Material
3.2. Physico-Chemical Characterization of the Raw Material
3.2.1. Cellulose and Hemicellulose Analysis
3.2.2. Moisture, Ash, Protein, and Nitrogen Content
3.2.3. Calorific Value
3.2.4. Determination of Volatile Compounds from Microalgae
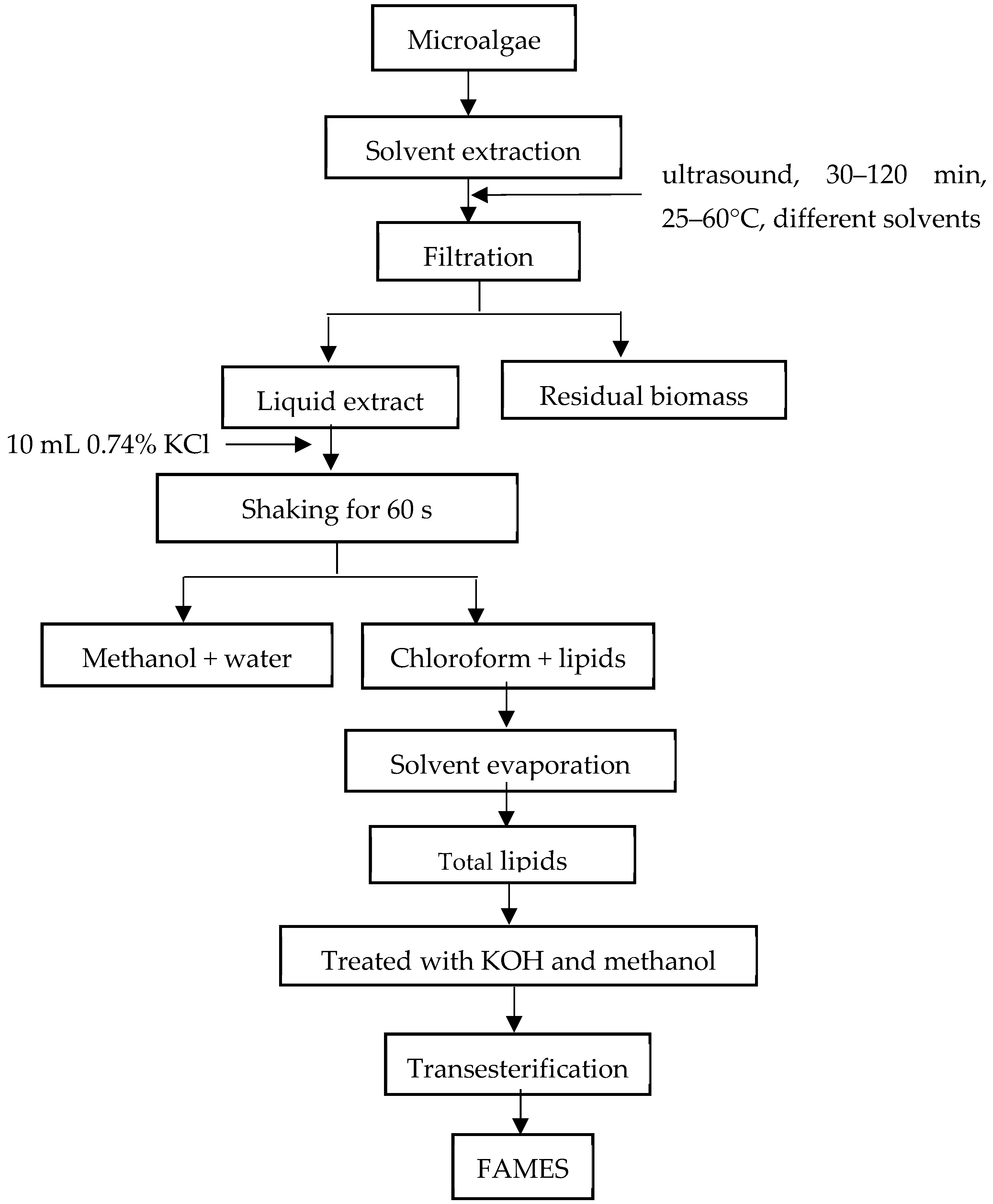
3.3. Extraction of Lipids from Samples
3.3.1. Fatty Acid Methyl Esters (FAMEs)
3.3.2. Determination of FAMEs Content Using GC-FID
3.3.3. Free Fatty Acid (FFA) Content from Extracted Oils
3.4. Lipid Nutritional Indices
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2009, 14, 217–232. [Google Scholar] [CrossRef]
- Qari, H.; Rehan, M.; Nizami, A.-S. Key issues in microalgae Biofuels: A Short review. Energy Procedia 2017, 142, 898–903. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.-M.; Jung, S.; Park, Y.-K.; Tsang, Y.F.; Lin, K.-Y.A.; Choi, Y.-E.; Kwon, E.E. Biodiesel from microalgae: Recent progress and key challenges. Prog. Energy Combust. Sci. 2022, 93, 101020. [Google Scholar] [CrossRef]
- Polat, E.; Altınbaş, M. A review on microalgal growth stress parameters for sustainable biofuel production. Clean Technol. Environ. 2023, 25, 2469–2487. [Google Scholar] [CrossRef]
- Cervera, R.; Villalba, M.R.; Sánchez, J. The Artificial Tree: Integrating Microalgae into Sustainable Architecture for CO2 Capture and Urban Efficiency—A Comprehensive Analysis. Buildings 2024, 14, 4045. [Google Scholar] [CrossRef]
- Bora, A.; Rajan, A.S.T.; Ponnuchamy, K.; Muthusamy, G.; Alagarsamy, A. Microalgae to bioenergy production: Recent advances, influencing parameters, utilization of wastewater—A critical review. Sci. Total Environ. 2024, 946, 174230. [Google Scholar] [CrossRef]
- Filipe, H.A.L.; Moreira, A.F.; Miguel, S.P.; Coutinho, P. Microalgae lipid membrane models: A computational biophysics characterization. Algal Res. 2024, 85, 103884. [Google Scholar] [CrossRef]
- Gaurav, K.; Neeti, K.; Singh, R. Microalgae-based biodiesel production and its challenges and future opportunities: A review. Green Technol. Sustain. 2023, 2, 100060. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Araujo, G.S.; Matos, L.J.B.L.; Fernandes, J.O.; Cartaxo, S.J.M.; Gonçalves, L.R.B.; Fernandes, F.A.N.; Farias, W.R.L. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2012, 20, 95–98. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Barczak, Ł.; Rusanowska, P.; Dudek, M.; Zieliński, M. Characterization of Lipid Production in Chlorella sp. Cultivated in Different Plant Fertilizers. Energies 2024, 17, 6193. [Google Scholar] [CrossRef]
- Viena, V.; Elvitriana, N.; Juwandi, N.; Suhendrayatna, N. Effect of lighting cycles and wastewater concentrations on biomass growth and lipid content of green microalgae in laundry wastewater treatment. Results Eng. 2025, 25, 104316. [Google Scholar] [CrossRef]
- Sharma, S.G.; Singla, P.; Kocher, G.S. Development of Microalgae-Bacteria Consortium for the treatment of domestic waste water. Water Air Soil Pollut. 2024, 235, 347. [Google Scholar]
- Torres, M.J.; Bellido-Pedraza, C.M.; Llamas, A. Applications of the microalgae chlamydomonas and its bacterial consortia in detoxification and bioproduction. Life 2024, 14, 940. [Google Scholar] [CrossRef]
- Calatrava, V.; Ballester, D.G.; Dubini, A. Microalgae for bioremediation: Advances, challenges, and public perception on genetic engineering. BMC Plant Biol. 2024, 24, 1261. [Google Scholar]
- Diankristanti, P.A.; Ng, I.-S. Marine microalgae for bioremediation and waste-to-worth valorization: Recent progress and future prospects. Blue Biotechnol. 2024, 1, 10. [Google Scholar] [CrossRef]
- Andrade, L.M. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; An overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar]
- Su, M.; Bastiaens, L.; Verspreet, J.; Hayes, M. Applications of microalgae in foods, pharma and feeds and their use as fertilizers and biostimulants: Legislation and regulatory aspects for consideration. Foods 2023, 12, 3878. [Google Scholar] [CrossRef]
- De Oliveira, A.P.F.; Bragotto, A.P.A. Microalgae-based products: Food and public health. Future Foods 2022, 6, 100157. [Google Scholar]
- U.S. Food & Drug Administration. Generally Recognized as Safe (GRAS)|FDA. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 17 March 2025).
- Esipovich, A.L.; Kanakov, E.A.; Charykova, T.A.; Otopkova, K.V.; Mityukova, Y.A.; Belousov, A.S. Processing of lipid-enriched microalgae Chlorella biomass into biofuels and value-added chemicals. Fuel 2025, 381, 133484. [Google Scholar]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2008, 36, 269–274. [Google Scholar] [PubMed]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Psychol. 2009, 21, 493–507. [Google Scholar]
- James, J.; Singh, M.; Sharma, N. Exploration of marine microalgae as sustainable feedstock for lipid extraction and biofuel production. In Microalgal Biofuels; Jaiswal, K.K., Singh, B., Jaiswal, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 551–570. [Google Scholar]
- Tavakoli, Z.; Kavoosi, G.; Siahbalaei, R.; Karimi, J. Spirulina maxima as a valuable ingredient: Determination of broad fatty acid and amino acid profiles and nutritional quality and anti-amylase capacity. Appl. Food Res. 2025, 5, 100741. [Google Scholar]
- Ladjal-Ettoumi, Y.; Hamadi, M.; Douik, L.H.; Cherifi, Z.; Nazir, A. Physicochemical, functional, and nutraceutical properties of Spirulina and Chlorella biomass: A comparative study. Algal Res. 2024, 81, 103561. [Google Scholar]
- Gallego, I.; Medic, N.; Pedersen, J.S.; Ramasamy, P.K.; Robbens, J.; Vereecke, E.; Romeis, J. The microalgal sector in Europe: Towards a sustainable bioeconomy. New Biotechnol. 2025, 86, 1–13. [Google Scholar]
- Paraskevopoulou, A.; Kaloudis, T.; Hiskia, A.; Steinhaus, M.; Dimotikali, D.; Triantis, T.M. Volatile profiling of spirulina food supplements. Foods 2024, 13, 1257. [Google Scholar] [CrossRef]
- Shahid, A.; Fan, Z.; Su, K.; Zhao, A.; Mehmood, M.A.; Chang, J.-S.; Solovchenko, A.E.; Alam, M.A.; Xu, J. Sensory chemistry of Spirulina: Unveiling trends and innovations in aromatic volatile organic compound biosynthesis in off-flavors and odor mitigation strategies. Trends Food Sci. Technol. 2025, 156, 104886. [Google Scholar]
- Nunes, M.C.; Ferreira, J.; Raymundo, A. Volatile fingerprint impact on the sensory properties of microalgae and development of mitigation strategies. Curr. Opin. Food Sci. 2023, 51, 101040. [Google Scholar]
- Grácio, M.; Ferreira, J.; Steinrücken, P.; Kleinegris, D.M.M.; Sousa, I.; Nunes, M.C.; Raymundo, A. The volatile composition and the potential health benefits of different microalgae strains. Foods 2024, 13, 2174. [Google Scholar] [CrossRef]
- Chamkalani, A.; Zendehboudi, S.; Rezaei, N.; Hawboldt, K. A critical review on life cycle analysis of algae biodiesel: Current challenges and future prospects. Renew. Sustain. Energy Rev. 2020, 134, 110143. [Google Scholar]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: A review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar]
- Santillan-Jimenez, E.; Pace, R.; Marques, S.; Morgan, T.; McKelphin, C.; Mobley, J.; Crocker, M. Extraction, characterization, purification and catalytic upgrading of algae lipids to fuel-like hydrocarbons. Fuel 2016, 180, 668–678. [Google Scholar]
- Ambrozova, J.; Misurcova, L.; Vicha, R.; Machu, L.; Samek, D.; Baron, M.; Mlcek, J.; Sochor, J.; Jurikova, T. Influence of Extractive Solvents on Lipid and Fatty Acids Content of Edible Freshwater Algal and Seaweed Products, the Green Microalga Chlorella kessleri and the Cyanobacterium Spirulina platensis. Molecules 2014, 19, 2344–2360. [Google Scholar] [CrossRef]
- Baba, H.S.; Baba, H.M.B.; Kassouar, S.E.H.E.R.; Abi, A.S.N.M.E.A. Physicochemical analysis of cellulose from microalgae Nannochloropsis gaditana. Afr. J. Biotechnol. 2016, 15, 1201–1207. [Google Scholar]
- Kavitha, S.; Gajendran, T.; Saranya, K.; Selvakumar, P.; Manivasagan, V. Study on consolidated bioprocessing of pre-treated Nannochloropsis gaditana biomass into ethanol under optimal strategy. Renew. Energy 2021, 172, 440–452. [Google Scholar]
- Silva, M.; Geada, P.; Pereira, R.N.; Teixeira, J.A. Microalgae biomass—A source of sustainable dietary bioactive compounds towards improved health and well-being. Food Chem. Adv. 2025, 6, 100926. [Google Scholar]
- Demirbaş, A. Production of Biodiesel from Algae Oils. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 31, 163–168. [Google Scholar] [CrossRef]
- Gu, S.-Q.; Wang, X.-C.; Tao, N.-P.; Wu, N. Characterization of volatile compounds in different edible parts of steamed Chinese mitten crab (Eriocheir sinensis). Food Res. Int. 2013, 54, 81–92. [Google Scholar]
- Chua, W.C.L.; Yeo, A.Y.Y.; Yuan, W.; Lee, Y.Y.; Ikasari, L.; Dharmawan, J.; Delahunty, C.M. Flavour characterization of twelve species of edible algae. Algal Res. 2024, 80, 103540. [Google Scholar]
- Urlass, S.; Wu, Y.; Nguyen, T.T.L.; Winberg, P.; Turner, M.S.; Smyth, H. Unravelling the aroma and flavour of algae for future food applications. Trends Food Sci. Technol. 2023, 138, 370–381. [Google Scholar]
- Villaró, S.; García-Vaquero, M.; Morán, L.; Álvarez, C.; Cabral, E.M.; Lafarga, T. Effect of seawater on the biomass composition of Spirulina produced at a pilot-scale. New Biotechnol. 2023, 78, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bai, M.; Qiu, D.; Zhang, J.; Zhao, N.; Feng, G.; Wu, H.; Zeng, M.; Obadina, A.O. Comparative evaluation of sensory and instrumental flavor profiles of four edible microalgae: Spirulina platensis, Chlorella pyrenoidosa, Chlamydomonas reinhardtii, and Haematococcus pluvialis. Algal Res. 2024, 82, 103628. [Google Scholar] [CrossRef]
- Braga-Souto, R.N.; Bürck, M.; Nakamoto, M.M.; Braga, A.R.C. Cracking Spirulina flavor: Compounds, sensory evaluations, and solutions. Trends Food Sci. Technol. 2024, 156, 104847. [Google Scholar] [CrossRef]
- Moran, L.; Bou, G.; Aldai, N.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Barron, L.J.R.; Lafarga, T. Characterisation of the volatile profile of microalgae and cyanobacteria using solid-phase microextraction followed by gas chromatography coupled to mass spectrometry. Sci. Rep. 2022, 12, 3661. [Google Scholar]
- Chaudry, S.; Hurtado-McCormick, V.; Cheng, K.Y.; Willis, A.; Speight, R.; Kaksonen, A.H. Microalgae to bioplastics—Routes and challenges. Clean. Eng. Technol. 2025, 25, 100922. [Google Scholar] [CrossRef]
- Ugya, A.Y.; Sheng, Y.; Chen, H.; Wang, Q. Microalgal bioengineering: A futuristic tool for carbon capture. Results Eng. 2024, 24, 102990. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Sousa, V.; Pereira, R.N.; Vicente, A.A.; Dias, O.; Geada, P. Microalgae biomass as an alternative source of biocompounds: New insights and future perspectives of extraction methodologies. Food Res. Int. 2023, 173, 113282. [Google Scholar]
- Ren, X.; Zhao, X.; Turcotte, F.; Deschênes, J.; Tremblay, R. Current lipid extraction methods are significantly enhanced adding a water treatment step in Chlorella protothecoides. Microb. Cell Factories 2017, 16, 26. [Google Scholar] [CrossRef]
- Getachew, A.T.; Jacobsen, C.; Sørensen, A.-D.M. Supercritical CO2 for efficient extraction of high-quality starfish (Asterias rubens) oil. J. Supercrit. Fluids 2023, 206, 106161. [Google Scholar]
- Deng, Y.; Wang, W.; Zhao, S.; Yang, X.; Xu, W.; Guo, M.; Xu, E.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted extraction of lipids as food components: Mechanism, solvent, feedstock, quality evaluation and coupled technologies—A review. Trends Food Sci Technol. 2022, 122, 83–96. [Google Scholar]
- Marques, F.; Pinho, M.; Guerra, I.M.S.; Conde, T.A.; Silva, J.; Martins, H.M.; Abreu, M.H.; Cerqueira, Â.; Domingues, M.R. Unlocking functional lipid ingredients from algae by food-grade biosolvents and ultrasound-assisted extraction for nutritional applications. LWT 2024, 200, 116136. [Google Scholar]
- Mienis, E.; Vandamme, D.; Foubert, I. Ultrasound assisted extraction of Nannochloropsis: Effects on lipid extraction efficiency and lipid stability. Algal Res. 2024, 80, 103520. [Google Scholar]
- Krishnamoorthy, A.; Rodriguez, C.; Durrant, A. Optimisation of ultrasonication pretreatment on microalgae Chlorella vulgaris & Nannochloropsis oculata for lipid extraction in biodiesel production. Energy 2023, 278, 128026. [Google Scholar]
- Rezvani, S.; Saadaoui, I.; Jabri, H.A.; Moheimani, N.R. Techno-economic modelling of high-value metabolites and secondary products from microalgae cultivated in closed photobioreactors with supplementary lighting. Algal Res. 2022, 65, 102733. [Google Scholar]
- De Souza, M.P.; Hoeltz, M.; Gressler, P.D.; Benitez, L.B.; Schneider, R.C.S. Potential of microalgal Bioproducts: General perspectives and main challenges. Waste Biomass Valorization 2018, 10, 2139–2156. [Google Scholar]
- Ferreira, G.F.; Pinto, L.F.R.; Carvalho, P.O.; Coelho, M.B.; Eberlin, M.N.; Filho, R.M.; Fregolente, L.V. Biomass and lipid characterization of microalgae genera Botryococcus, Chlorella, and Desmodesmus aiming high-value fatty acid production. Biomass Convers. Biorefin. 2019, 11, 1675–1689. [Google Scholar]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar]
- Kumari, A.; Chakraborty, S.; Sirotiya, V.; Kalita, D.; Rai, A.; Yadav, K.K.; Bhutto, J.K.; Vinayak, V. A review on economical and impact of bioethanol production from microalgae: Current scenario and future prospect. Ind. Crops Prod. 2024, 222, 119927. [Google Scholar]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Saraiva, J.A.; Martins, A.P.; Pinto, C.A.; Prieto, M.A.; Simal-Gandara, J.; Cao, H.; Xiao, J.; Barba, F.J. Extraction of lipids from microalgae using classical and innovative approaches. Food Chem. 2022, 384, 132236. [Google Scholar] [PubMed]
- ISO 18125: 2017; Solid Biofuels—Determination of Calorific Value. ISO: Geneve, Switzerland, 2017.
- Boffito, D.C.; Pirola, C.; Galli, F.; Michele, A.D.; Bianchi, C.L. Free fatty acids esterification of waste cooking oil and its mixtures with rapeseed oil and diesel. Fuel 2013, 108, 612–619. [Google Scholar]
- Senila, M.; Kovacs, E.; Senila, L. Essential and nonessential elements, lipids and volatile compounds in coffee and transfer to coffee brews: Assessment of the benefits and potential risks for human health. Food Sci. Nutr. 2025, 13, e4640. [Google Scholar] [PubMed]


| Parameter | Chlorella sp. | Spirulina platensis |
|---|---|---|
| Moisture (%) | 0.7 ± 0.03 b | 1.1 ± 0.1 a |
| Carbohydrates (%) | 10.2 ± 1.01 b | 19.4 ± 1.13 a |
| Lipid content (% dry wt) | 17.4 ± 1.2 a | 7.2 ± 0.62 b |
| Ash (%) | 7.2 ± 0.52 a | 6.5 ± 0.41 a |
| Protein (%) | 59.3 ± 3.2 a | 65.6 ± 2.5 a |
| Nitrogen (%) | 9.5 ± 0.52 a | 10.5 ± 1.01 a |
| Calorific value (kJ/g) | 21.7 ± 1.81 a | 20.1 ± 1.8 a |
| Cellulose (%) | 13.2 ± 1.1 a | 15.4 ± 1.1 a |
| Hemicellulose (%) | 3.2 ± 0.20 a | 1.7 ± 0.15 b |
| VOC | Molecular Formula | Chlorella sp. | Spirulina platensis | tR (min) | Odor Descriptors * |
|---|---|---|---|---|---|
| Amines | |||||
| 2-Propyn-1-amine | C3H5N | 0.12 ± 0.01 a | nd | 4.689 | Pungent, slightly fishy |
| N-Methylallylamine | C4H9N | nd | 0.11 ± 0.01 a | 8.085 | Strong, fishy, ammonia-like |
| 3,4-Pyridinediamine | C6H6N2 | 0.05 ± 0.002 a | nd | 8.917 | Amine-like, medicinal |
| 2-Propen-1-amine | C3H7N | 0.20 ± 0.01 b | 0.31 ± 0.02 a | 16.718 | Strong, fishy |
| Ethylmethylamine | C3H9N | nd | 0.59 ± 0.04 a | 18.450 | Fishy, ammonia-like |
| N,N-2-trimethylpyridin-4-amine | C6H11N2 | nd | 3.50 ± 0.18 a | 20.49 | Pungent, fishy |
| 2-Methyl-2-propen-1-amine | C5H11N | 0.36 ± 0.02 a | 0.35 ± 002 a | 25.112 | Strong, amine-like odor |
| 1H-Tetrazol-5-amine | C2H3N3 | 2.68 ± 0.02 a | nd | 40.15 | Not found |
| 5H-Tetrazol-5-amine | C2H3N3 | 1.01 ± 0.10 a | 0.41 ± 0.03 b | 17.537 | Slightly pungent, medicinal |
| Aldehydes | |||||
| 2-Methyl-2-butenal | C5H10O | 0.33 ± 0.02 a | nd | 3.919 | Pungent, strong, fruity |
| Hexanal | C6H12O | 9.48 ± 0.61 b | 19.76 ± 1.52 a | 5.765 | Green, grassy |
| 4-Methylbenzaldehyde | C6H7NO | 0.11 ± 0.01 a | nd | 21.384 | Sweet, almond-like |
| Alcohols | |||||
| cis-(3,3,5)-trimethylcyclohexanol | C9H18O | nd | 12.43 ± 0.98 a | 8.917 | Woody, camphoraceous |
| 6-Methyl-1-heptanol | C8H18O | nd | 5.04 ± 0.21 a | 15.41 | Floral, fruity, citrus |
| 3,5-Dimethylcyclohexanol | C8H14O | 5.13 ± 0.31 a | nd | 21.628 | Sweet, floral |
| Ketones | |||||
| 2,2,6-Trimethylcyclohexanone | C9H16O | nd | 11.32 ± 1.1 a | 18.797 | Sweet, woody |
| 6-Methyl-2-azabicyclo[2.2.0]hex-5-en-3-one | C7H11NO | 0.29 ± 0.01 a | nd | 23.123 | Earthy |
| Acetone oxime | C3H7NO | 0.62 ± 0.03 a | nd | 29.253 | Slightly sweet, pungent |
| Hydrocarbon | |||||
| 4,4-Dimethylcyclopentene | C8H14 | 7.24 ± 0.5 a | nd | 2.987 | Slightly sweet, petroleum-like |
| Methoxyethylene | C3H6O | nd | 1.85 ± 0.011 a | 3.000 | Sweet, ether-like flavor |
| 2-methyl-1,5-hexadien-3-yne | C7H8 | 1.02 ± 0.1 b | 2.18 ± 0.2 a | 4.463 | Slightly sweet |
| 1,2-Dodecane oxide | C12H26O | nd | 6.33 ± 0.31 a | 21.622 | Waxy, fatty, slightly floral |
| 4-Ethylguaiacol | C9H12O2 | nd | 0.15 ± 0.01 a | 25.769 | Sweet, smoky, spicy |
| Heterocycle | |||||
| 6-Azaspiro[2.5]octa-4,7-diene-6-carboxylic acid | C8H13NO2 | 3.26 ± 0.18 a | nd | 6.640 | Pungent |
| 1,2,3,6-Tetrahydropyridine | C5H9N | nd | 0.94 ± 0.06 a | 17.181 | Musty, strong |
| Azetidine | C3H7N | 2.59 ± 0.18 a | nd | 18.807 | Slightly pungent |
| 5-formamidopyrimidine | C5H5N3O | nd | 0.52 ± 0.02 a | 19.357 | Odorless |
| 9-Aza-10-boradecalin | C10H14BN | 7.82 ± 0.51 a | nd | 20.759 | Not found |
| 1-Oxaspiro[2.5]oct-5-ene, 8,8-dimethyl-4-methylene | C12H14O | nd | 7.47 ± 0.42 a | 21.378 | Slightly sweet, woody, herbal |
| Tetrahydropyridine | C6H11N | 3.54 ± 0.21 a | nd | 22.835 | Amine-like odor |
| Acids | |||||
| Aminooxyacetic acid | C4H9NO3 | 0.11 ± 0.01 a | nd | 9.630 | Slightly sweet, amine-like |
| 3-Chloropropionamide | C3H6ClNO | 0.40 ± 0.02 a | nd | 24.90 | Odorless |
| Ether | |||||
| Propylene oxide | C3H6O | 0.09 ± 0.004 a | nd | 11.557 | Slightly sweet, ether-like |
| Furans | |||||
| Furan | C6H9N | 0.48 ± 0.02 a | nd | 16.211 | Sweet, medicinal |
| 2-Pentyl-furan | C9H14O | 16.33 ± 1.2 a | 6.08 ± 0.30 b | 17.018 | Pleasant, slightly sweet, nutty |
| Nitrogenous compounds | |||||
| N-methylaziridine | C3H7N | 13.13 ± 0.1 a | nd | 2.768 | Strong, pungent, amine-like |
| 2,4-Hexadienenitrile | C3H6O | 0.15 ± 0.01 a | nd | 16.155 | Pungent, sharp, and acrid |
| 3-Fluoro-2-propynenitrile | C3HFN | nd | 0.13 ± 0.01 a | 23.254 | Not found |
| Cyclopentaneacetonitrile | C6H9N | 0.10 ± 0.01 a | nd | 7.923 | Sweet, almonds |
| Sulfur compounds | |||||
| Methanesulfonyl fluoride | CH3SO2F | 0.45 ± 0.03 a | nd | 19.758 | Pungent, acrid |
| Esters | |||||
| Propyl cyanate | C4H9NO | 8.57 ± 0.41 a | nd | 16.905 | Pungent |
| Methyl sulfocyanate | CH3SCN | 7.25 ± 0.53 a | nd | 17.13 | Acrid, mustard-like odor, pungent |
| Fatty Acids | Formula | Chlorella sp. | Spirulina platensis |
|---|---|---|---|
| Capric acid | C10:0 | 0.12 ± 0.01 a | 0.08 ± 0.02 b |
| Undecanoic acid | C11:0 | 0.10 ± 0.01 b | 5.32 ± 0.21 a |
| Myristic acid | C14:0 | 0.81 ± 002 a | 0.28 ± 0.02 b |
| Myristoleic | C14:1(n-5) | nd | 0.22 ± 0.01 a |
| Pentadecanoic | C15:0 | 0.29 ± 0.01 a | nd |
| Palmitic acid | C16:0 | 26.53 ± 1.8 b | 42.85 ± 2.3 a |
| Palmitoleic acid | C16:1(n-7) | 1.26 ± 0.12 b | 3.16 ± 0.18 a |
| Margaric acid | C17:0 | 2.80 ± 0.2 a | 0.01 ± 0.001 b |
| Heptadecenoic acid | C17:1(n-7) | nd | 0.77 ± 0.03 a |
| Stearic acid | C18:0 | 3.42 ± 0.2 a | 1.00 ± 0.11 b |
| Oleic acid | C18:1(c+t)(n-9) | 21.90 ± 1.8 a | 3.53 ± 0.21 b |
| Linoleic acid | C18:2(c+t)(n-6) | 25.32 ± 1.5 a | 14.43 ± 1.1 b |
| γ-linolenic acid | C18:3(n-6) | 0.14 ± 0.01 b | 22.45 ± 2.1 a |
| α-Linolenic acid | C18:3(n-3) | 10.23 ± 1.0 a | 0.47 ± 0.02 b |
| Arachidic acid | C20:0 | 1.85 ± 0.1 a | nd |
| Gondoic acid/cis-11-Eicosaenoic Acid | C20:1(n-9) | nd | 0.72 ± 0.04 a |
| cis-11,14-Eicosadienoic Acid | C20:2(n-6) | 4.46 ± 0.3 a | 0.80 ± 0.03 b |
| Heneicosylic acid | C21:0 | nd | 0.58 ± 0.02 a |
| Eicosatrienoic acid | C20:3(n-3) | 0.46 ± 002 b | 1.31 ± 0.09 a |
| Eicosapentaenoic acid | C20:5(n-3) | 0.02 ± 0.001 b | 1.16 ± 0.08 a |
| Erucic acid | C22:1(n-9) | nd | 0.12 ± 0.01 a |
| Docosahexaenoic acid | C22:6(n-3) | 0.42 ± 0.02 a | nd |
| Free fatty acids (mg KOH/g) | FFAs | 1.4 ± 0.1 a | 0.52 ± 0.02 b |
| ∑SFA | 35.9 ± 2.1 b | 50.1 ± 2.2 a | |
| ∑MUFA | 23.2 ± 1.5 a | 8.5 ± 0.61 b | |
| ∑PUFA | 41.0 ± 1.8 a | 40.6 ± 2.3 a | |
| ∑UFA | 64.2 ± 2.2 a | 49.2 ± 2.8 b | |
| Omega-6 | 30.3 ± 2.6 b | 37.7 ± 2.6 a | |
| Omega-3 | 10.7 ± 0.9 a | 2.9 ± 0.17 b | |
| Lipid Nutritional Indices | Chlorella sp. | Spirulina platensis |
|---|---|---|
| MUFA/SFA | 0.64 | 0.17 |
| PUFA/SFA | 1.14 | 0.81 |
| Omega-6 (%) | 30.3 | 37.7 |
| Omega-3 (%) | 10.7 | 2.9 |
| Omega-6/omega-3 | 2.83 | 13.0 |
| Nutritive value index (NVI) | 20.10 | 1.43 |
| Atherogenicity index (AI) | 0.46 | 0.89 |
| Thrombogenicity index (TI) | 0.22 | 0.28 |
| Hypocholesterolemic/hypercholesterolemic ratio (h/H) | 2.3 | 1.0 |
| Health promoting index (HPI) | 46.4 | 86.7 |
| Linoleic acid/linolenic acid | 0.014 | 47.52 |
| LA/ALA (linoleic acid/α-linoleic acid ratio) | 2.5 | 30.5 |
| EPA + DHA (%) | 0.44 | 1.16 |
| Index | Calculation Formulas |
|---|---|
| NVI | |
| AI | |
| TI | |
| h/H | |
| HPI | |
| EPA + DHA | C22:6(n-3) + C20:5(n-3) |
| LA/ALA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, L.; Kovacs, E.; Roman, C. Chemical Characterization, Lipid Profile, and Volatile Compounds in Chlorella sp. and Spirulina platensis: A Promising Feedstock for Various Applications. Molecules 2025, 30, 1499. https://doi.org/10.3390/molecules30071499
Senila L, Kovacs E, Roman C. Chemical Characterization, Lipid Profile, and Volatile Compounds in Chlorella sp. and Spirulina platensis: A Promising Feedstock for Various Applications. Molecules. 2025; 30(7):1499. https://doi.org/10.3390/molecules30071499
Chicago/Turabian StyleSenila, Lacrimioara, Eniko Kovacs, and Cecilia Roman. 2025. "Chemical Characterization, Lipid Profile, and Volatile Compounds in Chlorella sp. and Spirulina platensis: A Promising Feedstock for Various Applications" Molecules 30, no. 7: 1499. https://doi.org/10.3390/molecules30071499
APA StyleSenila, L., Kovacs, E., & Roman, C. (2025). Chemical Characterization, Lipid Profile, and Volatile Compounds in Chlorella sp. and Spirulina platensis: A Promising Feedstock for Various Applications. Molecules, 30(7), 1499. https://doi.org/10.3390/molecules30071499







