Visible Light Photo-Fenton with Hybrid Activated Carbon and Metal Ferrites for Efficient Treatment of Methyl Orange (Azo Dye)
Abstract
:1. Introduction
2. Results and Discussion
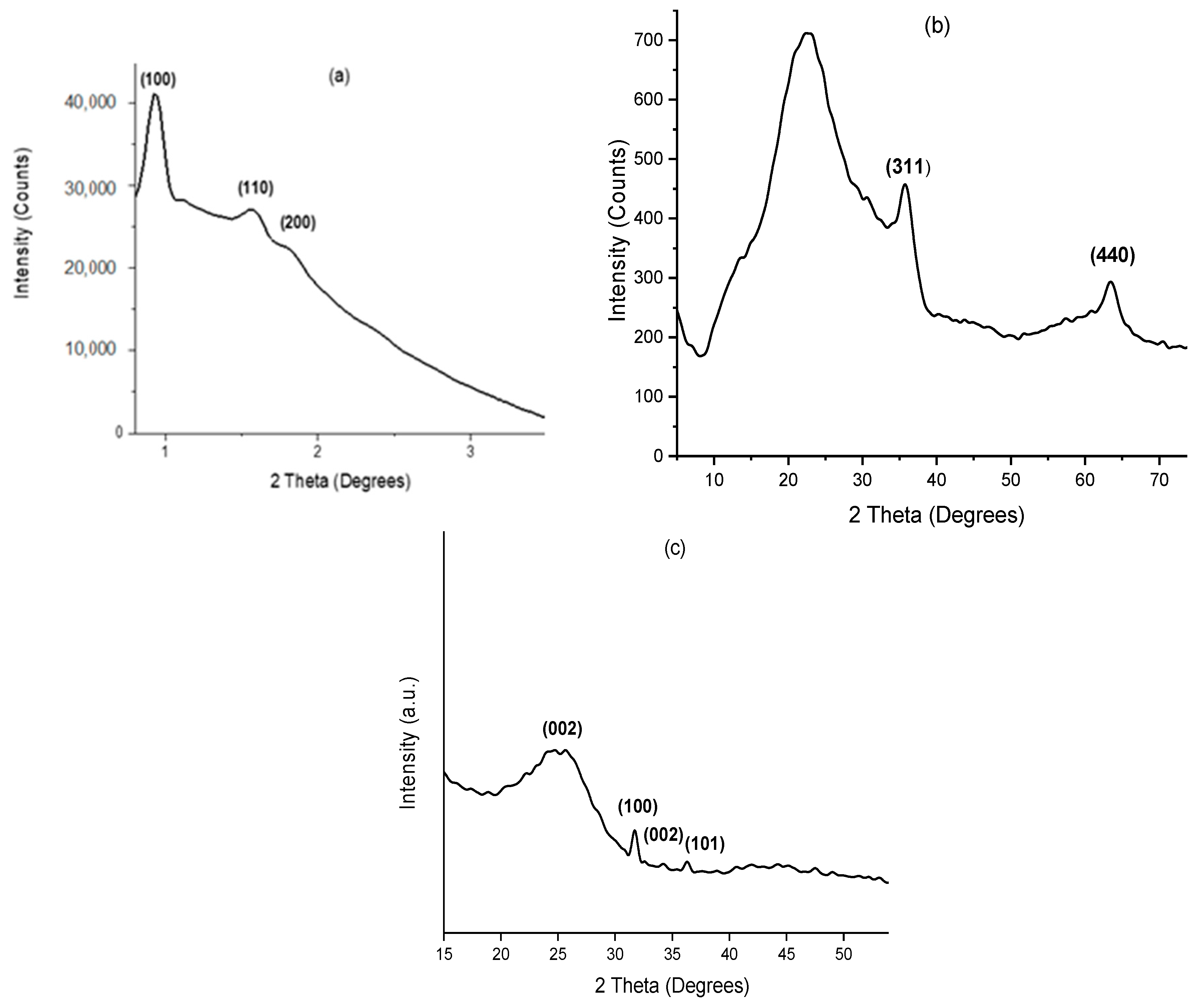
2.1. X-Ray Diffraction (XRD)
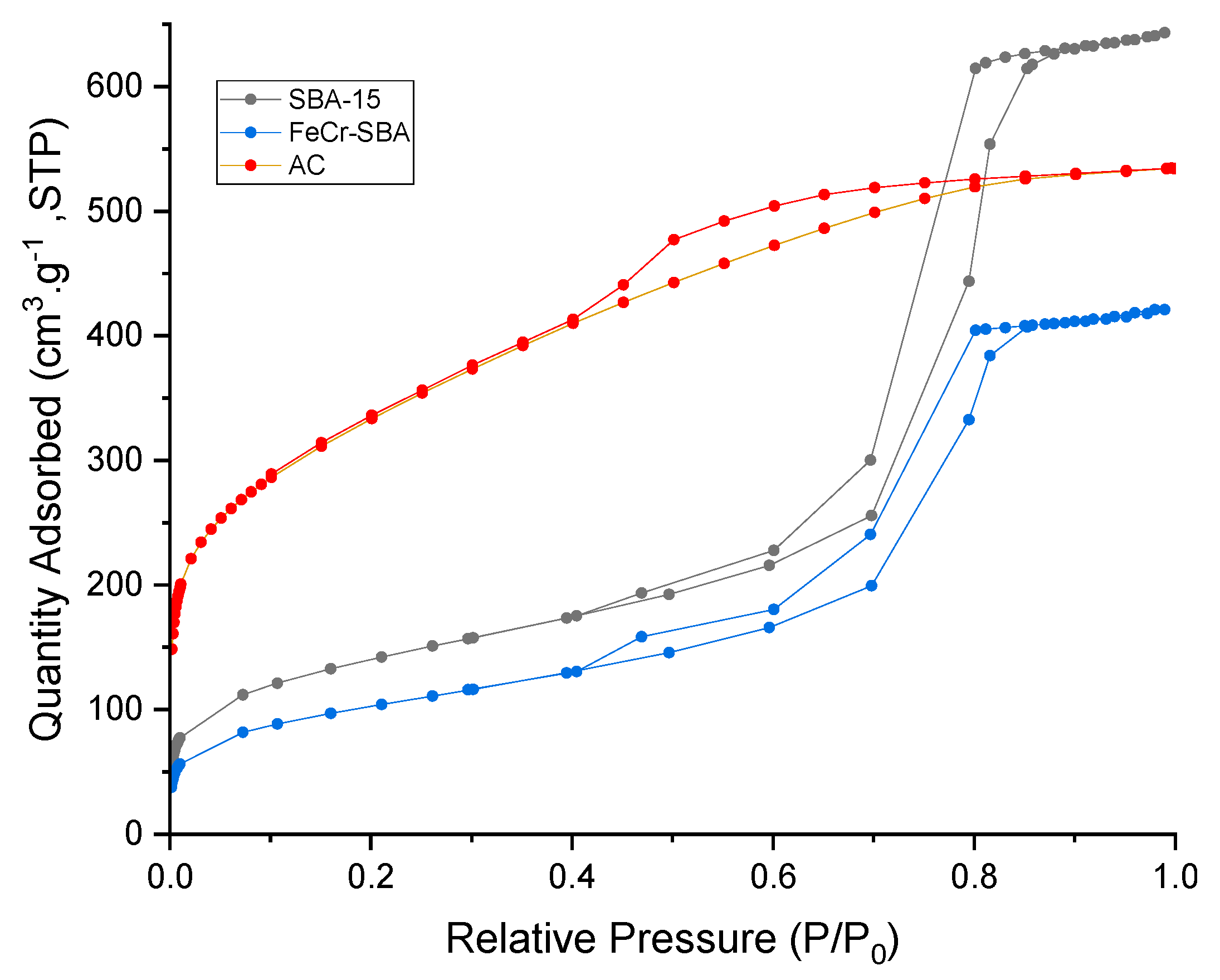
2.2. Nitrogen Adsorption–Desorption Isotherms
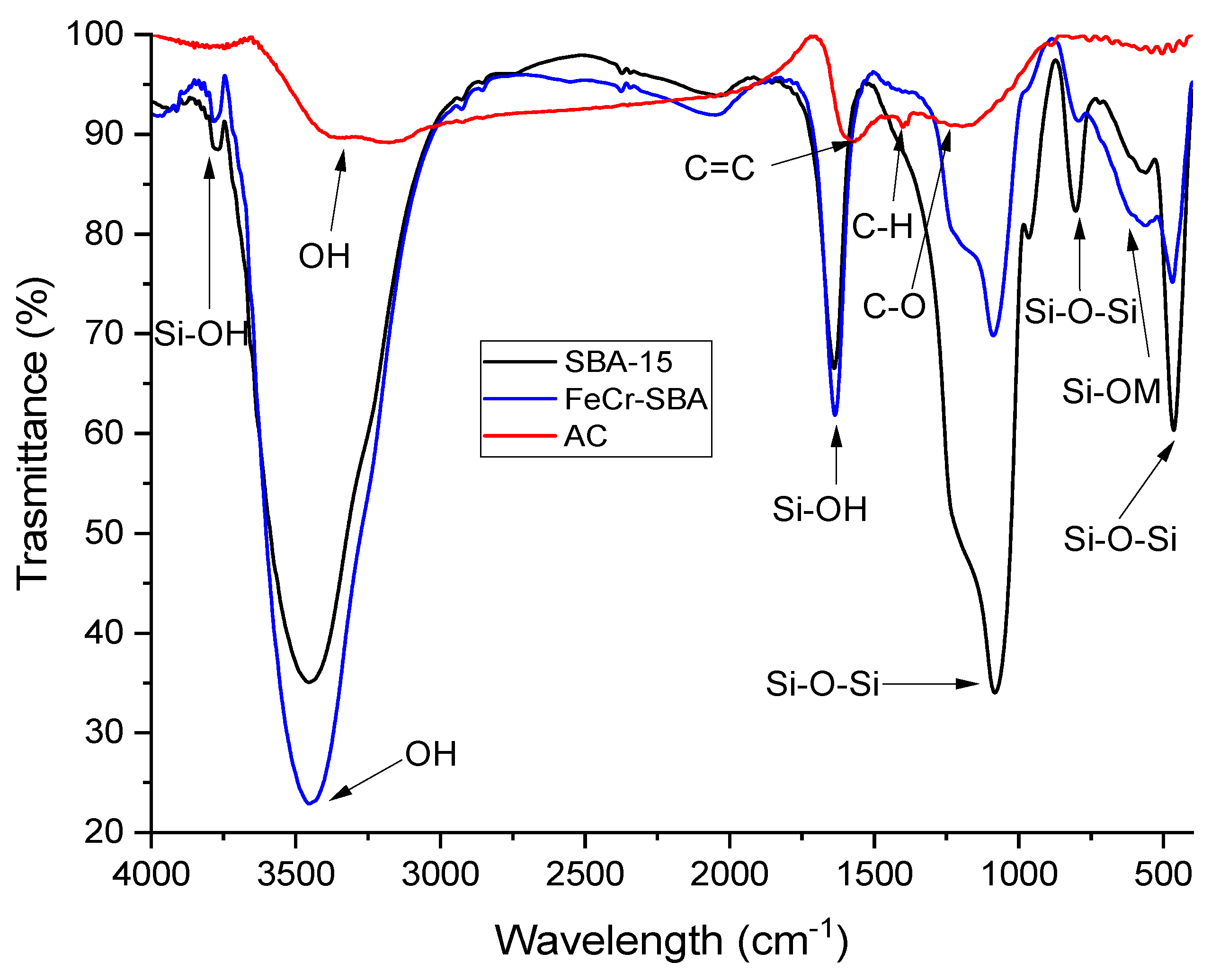
2.3. Fourier Transform Infrared (FT-IR)
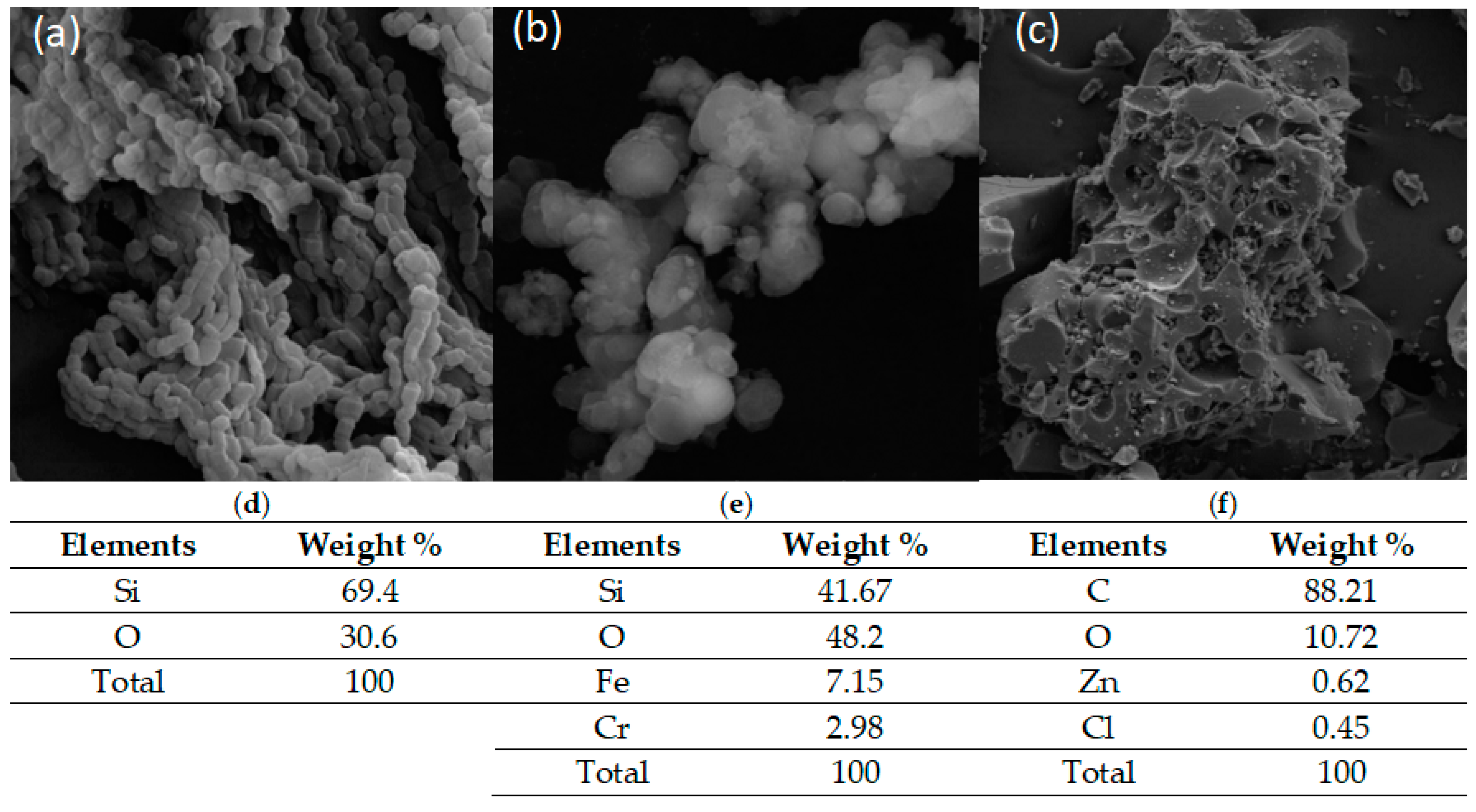
2.4. Scanning Electron Microscope (SEM)–Energy Dispersive Spectroscopy (EDS)
2.5. Transmission Electron Microscope (TEM)
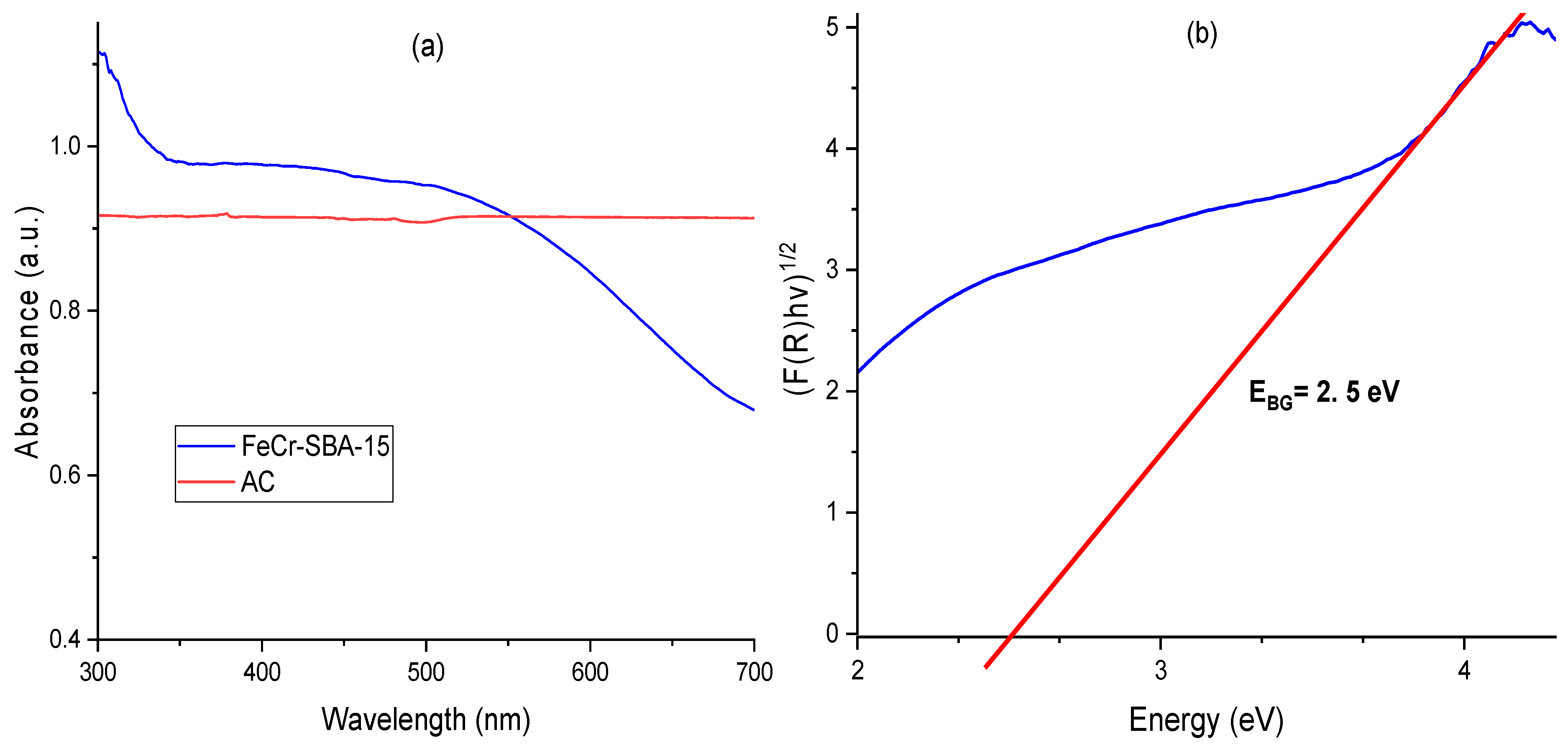
2.6. UV-Vis Spectroscopy
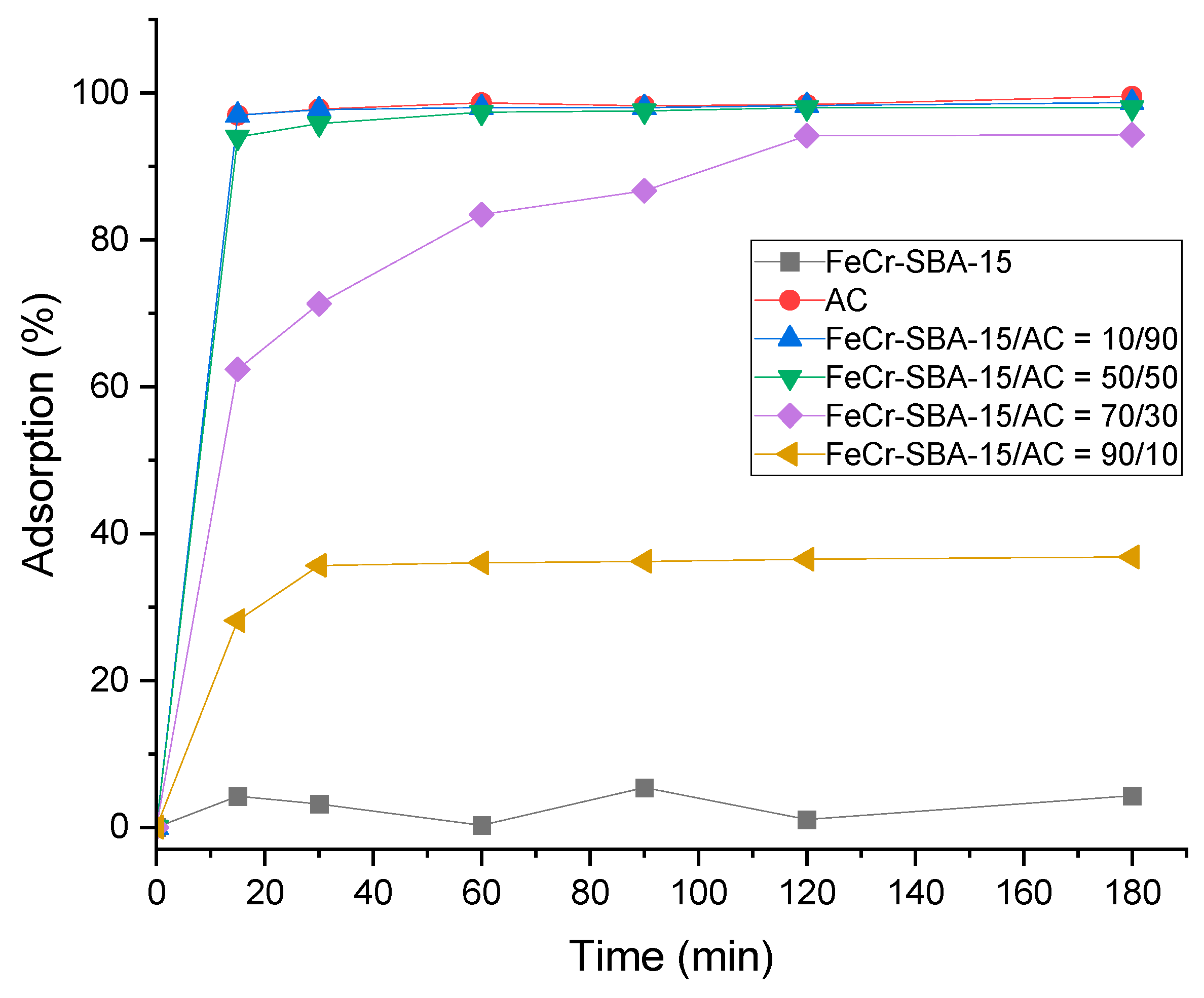
2.7. Adsorption of MO over Hybrid Mixture
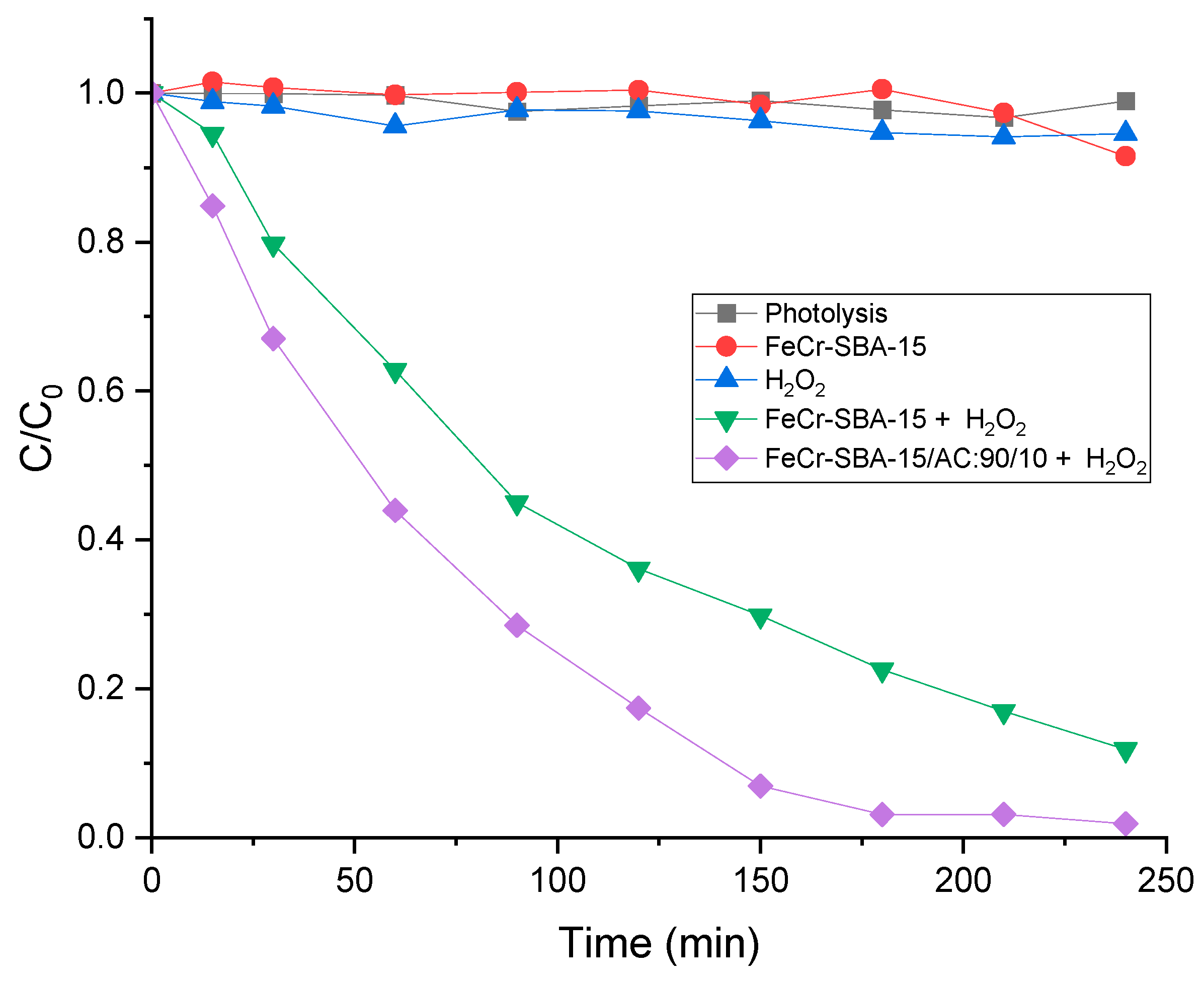
2.8. Photo-Fenton Degradation of MO
2.9. Influence of H2O2 Concentration
2.10. Influence of Catalyst Dose on the Degradation of MO
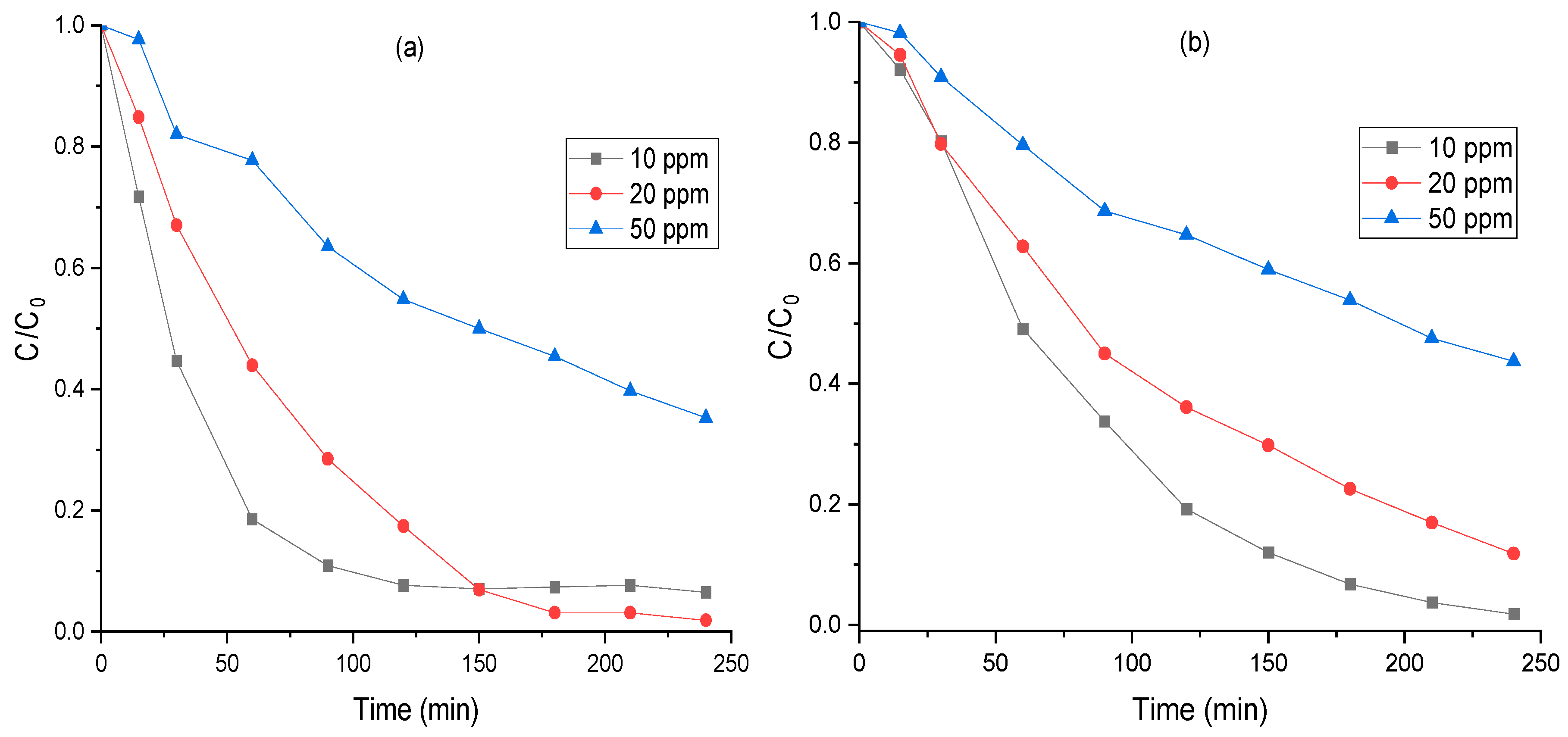
2.11. Influence of Initial MO Concentration
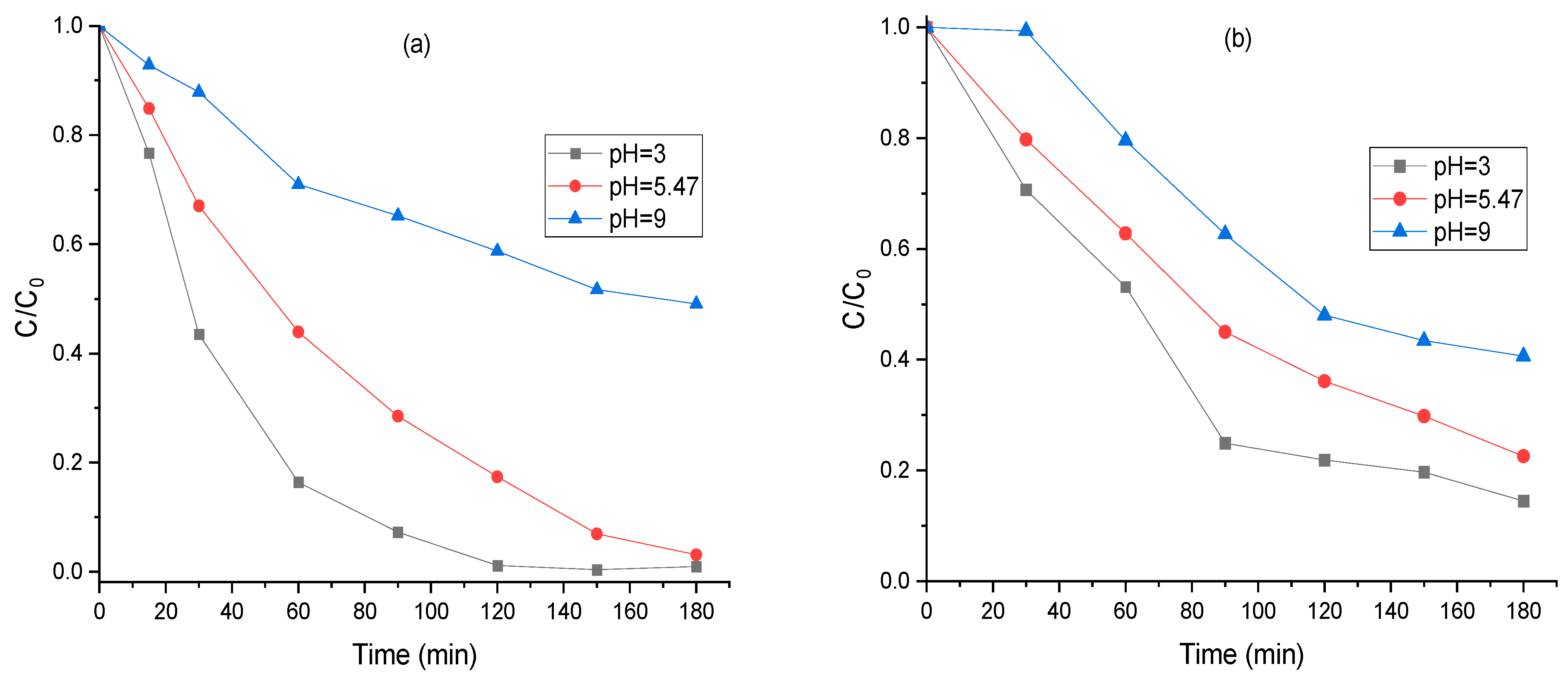
2.12. Influence of Initial pH
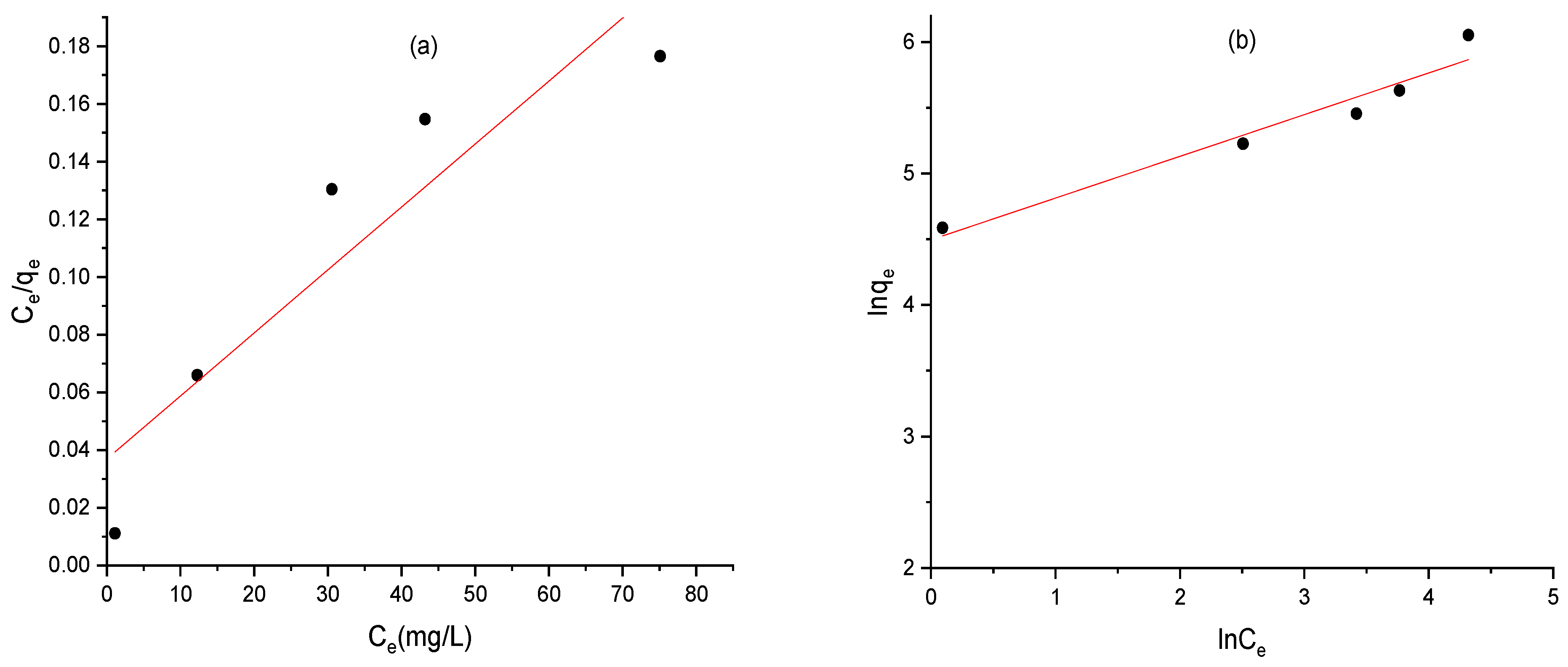
2.13. Adsorption Isotherms of AC
2.14. Kinetic Study of MO
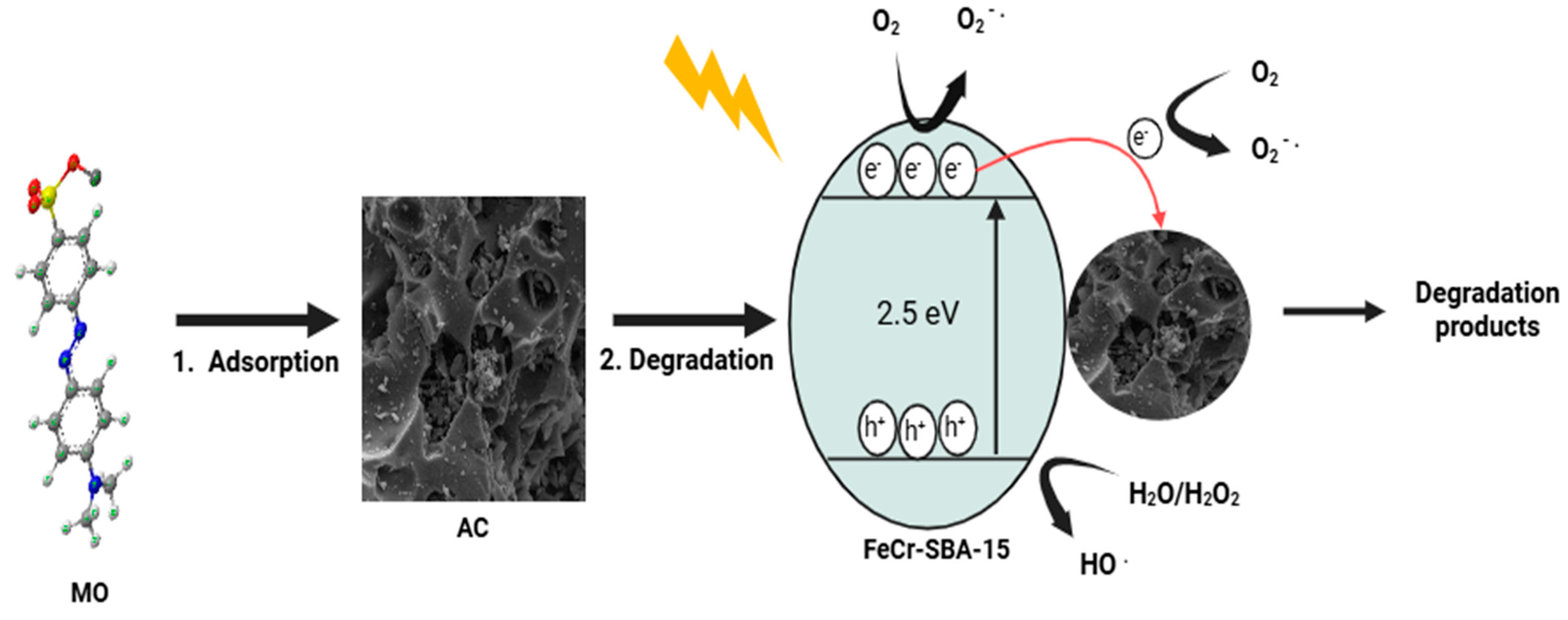
2.15. Possible Degradation Mechanism of MO by Hybrid Mixture
2.16. Comparison with Other Studies
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of SBA-15 Support
3.3. Synthesis of Chromium Ferrite Supported on the Mesoporous SBA-15
3.4. Preparation of Activated Carbon
3.5. Preparation of Hybrid Mixture (FeCr-SBA/AC)
3.6. Technique Characterization
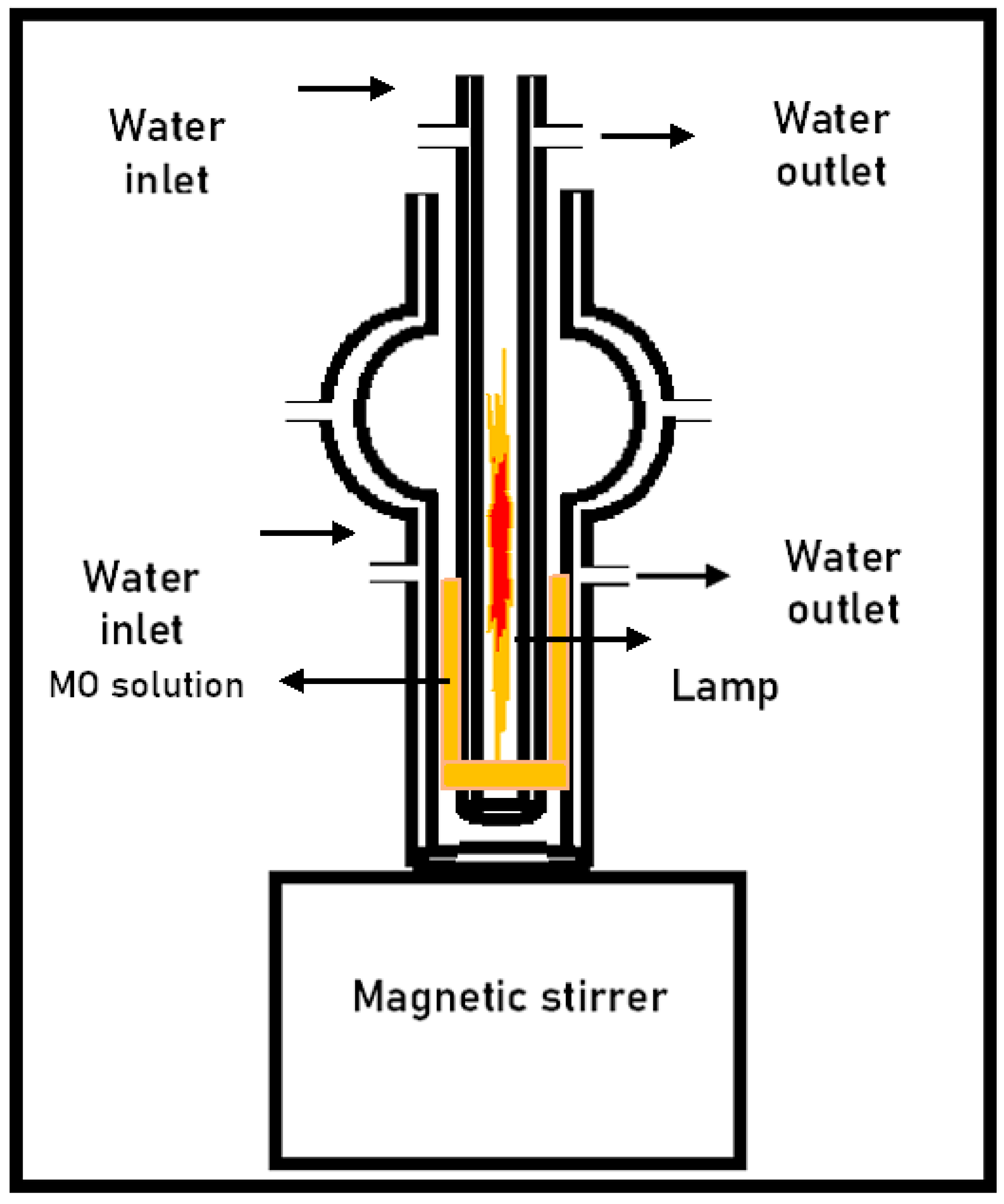
3.7. Photo-Fenton Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, Selective Heavy Metal Removal from Water by a Metal-Organic Framework/Polydopamine Composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Ali, A.; Abbood, N.K.; Panchal, S.; Akram, N.; Saeed, M.; Doshi, O.P.; Ali, F.; Muhammad, S.; Sameeh, M.Y. Enhanced Photocatalytic Activity of the Bi2O3-NiO Heterojunction for the Degradation of Methyl Orange under Irradiation of Sunlight. Water 2023, 15, 3182. [Google Scholar] [CrossRef]
- Ogugbue, C.J.; Sawidis, T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by Aeromonas hydrophila Isolated from Industrial Effluent. Biotechnol. Res. Int. 2011, 2011, 967925. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.B.; Vakili, M.; Amini Horri, B.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B.K. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Gao, J.; Qin, Y.; Zhou, T.; Cao, D.; Xu, P.; Hochstetter, D.; Wang, Y. Adsorption of methylene blue onto activated carbon produced from tea (Camellia sinensis L.) seed shells: Kinetics, equilibrium, and thermodynamics studies. J. Zhejiang Univ. Sci. 2013, 14, 650–658. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Fiyadh, S.S.; Salman, A.D.; Adelikhah, M. Prediction of methyl orange dye (MO) adsorption using activated carbon with an artificial neural network optimization modeling. Heliyon 2023, 9, e12888. [Google Scholar] [CrossRef]
- Shah, S.S.; Sharma, T.; Dar, B.A.; Bamezai, R.K. Adsorptive removal of methyl orange dye from aqueous solution using populous leaves: Insights from kinetics, thermodynamics and computational studies. Environ. Chem. Ecotoxicol. 2021, 3, 172–181. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Umembamalu, C.J. Electrocoagulation-flocculation of aquaculture effluent using hybrid iron and aluminium electrodes: A comparative study. Chem. Eng. J. Adv. 2021, 6, 100107. [Google Scholar] [CrossRef]
- Golob, V.; Vinder, A.; Simonič, M. Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dye. Pigment. 2005, 67, 93–97. [Google Scholar] [CrossRef]
- Joseph, J.; Radhakrishnan, R.C.; Johnson, J.K.; Joy, S.P.; Thomas, J. Ion-exchange mediated removal of cationic dye-stuffs from water using ammonium phosphomolybdate. Mater. Chem. Phys. 2020, 242, 122488. [Google Scholar] [CrossRef]
- Maruthanayagam, A.; Mani, P.; Kaliappan, K.; Chinnappan, S. In vitro and In silico Studies on the Removal of Methyl Orange from Aqueous Solution Using Oedogonium subplagiostomum AP1. Water. Air. Soil Pollut. 2020, 231, 232. [Google Scholar] [CrossRef]
- Ben Fradj, A.; Boubakri, A.; Hafiane, A.; Ben Hamouda, S. Removal of azoic dyes from aqueous solutions by chitosan enhanced ultrafiltration. Results Chem. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Haji, S.; Benstaali, B.; Al-Bastaki, N. Degradation of methyl orange by UV/H2O2 advanced oxidation process. Chem. Eng. J. 2011, 168, 134–139. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.; Zhang, T.; Wang, Y.; Wu, J.; Ran, F. Enhanced photodegradation activity of methyl orange over Ag2CrO4/SnS2 composites under visible light irradiation. Mater. Res. Bull. 2016, 77, 291–299. [Google Scholar] [CrossRef]
- Üner, O.; Bayrak, Y. The effect of carbonization temperature, carbonization time and impregnation ratio on the properties of activated carbon produced from Arundo dona. Microporous Mesoporous Mater. 2018, 268, 225–234. [Google Scholar] [CrossRef]
- Chandraboss, V.L.; Kamalakkannan, J.; Prabha, S.; Senthilvelan, S. An efficient removal of methyl violet from aqueous solution by an AC-Bi/ZnO nanocomposite material. RSC Adv. 2015, 5, 25857–25869. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Celorrio, V.; Iniesta, J.; Fermin, D.J.; Ania, C.O. Nanoporous carbon/WO3 anodes for an enhanced water photooxidation. Carbon 2016, 108, 471–479. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, B.; Lou, Z.; Wang, Z.; Meng, X.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Immobilization of BiOX (X=Cl, Br) on activated carbon fibers as recycled photocatalysts. Dalt. Trans. 2014, 43, 8170–8173. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Li, J.; Yin, J. Photocatalytic degradation of methyl orange by TiO2-coated activated carbon and kinetic study. Water Res. 2006, 40, 1119–1126. [Google Scholar] [CrossRef]
- Matos, J.; Laine, J.; Herrmann, J.M. Effect of the type of activated carbons on the photocatalytic degradation of aqueous organic pollutants by UV-irradiated titania. J. Catal. 2001, 200, 10–20. [Google Scholar] [CrossRef]
- Kadirova, Z.C.; Katsumata, K.I.; Isobe, T.; Matsushita, N.; Nakajima, A.; Okada, K. Adsorption and photodegradation of methylene blue by iron oxide impregnated on granular activated carbons in an oxalate solution. Appl. Surf. Sci. 2013, 284, 72–79. [Google Scholar] [CrossRef]
- Seleš, P.; Vengust, D.; Radošević, T.; Kocijan, M.; Einfalt, L.; Kurtjak, M.; Shvalya, V.; Knafelc, T.; Bernik, S.; Omerzu, A.; et al. Altering defect population during the solvothermal growth of ZnO nanorods for photocatalytic applications. Ceram. Int. 2024, 50, 26819–26828. [Google Scholar] [CrossRef]
- Parastar Gharehlar, M.; Sheshmani, S.; Nikmaram, F.R.; Doroudi, Z. Synergistic potential in spinel ferrite MFe2O4 (M = Co, Ni) nanoparticles-mediated graphene oxide: Structural aspects, photocatalytic, and kinetic studies. Sci. Rep. 2024, 14, 4625. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Spinel ferrites nanoparticles: Synthesis methods and application in heterogeneous Fenton oxidation of organic pollutants—A review. Appl. Surf. Sci. Adv. 2021, 6, 100145. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Akhavan, O.; Azimirad, R. Photocatalytic property of Fe2O3 nanograin chains coated by TiO2 nanolayer in visible light irradiation. Appl. Catal. A Gen. 2009, 369, 77–82. [Google Scholar] [CrossRef]
- Tabaja, N.; Brouri, D.; Casale, S.; Zein, S.; Jaafar, M.; Selmane, M.; Toufaily, J.; Davidson, A.; Hamieh, T. Use of SBA-15 silica grains for engineering mixtures of oxides CoFe and NiFe for Advanced Oxidation Reactions under visible and NIR. Appl. Catal. B Environ. 2019, 253, 369–378. [Google Scholar] [CrossRef]
- Wang, J.; Ge, H.; Bao, W. Synthesis and characteristics of SBA-15 with thick pore wall and high hydrothermal stability. Mater. Lett. 2015, 145, 312–315. [Google Scholar] [CrossRef]
- Larki, A.; Saghanezhad, S.J.; Ghomi, M. Recent advances of functionalized SBA-15 in the separation/preconcentration of various analytes: A review. Microchem. J. 2021, 169, 106601. [Google Scholar] [CrossRef]
- Hachemaoui, M.; Boudjadi, N.; Al-Naqbi, M.M.; Benhammou, A.; Daoui, M. CuNPs-loaded amines-functionalized-SBA-15 as effective catalysts for catalytic reduction of cationic and anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126729. [Google Scholar] [CrossRef]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef]
- Yousif, M.; Ibrahim, A.H.; Al-Rawi, S.S.; Majeed, A.; Iqbal, M.A.; Kashif, M.; Abidin, Z.U.; Arbaz, M.; Ali, S.; Hussain, S.A.; et al. Visible light assisted photooxidative facile degradation of azo dyes in water using a green method. RSC Adv. 2024, 14, 16138–16149. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Hashim, M.; Alimuddin; Shirsath, S.E.; Kumar, S.; Kumar, R.; Roy, A.S.; Shah, J.; Kotnala, R.K. Preparation and characterization chemistry of nano-crystalline Ni-Cu-Zn ferrite. J. Alloys Comp. 2013, 549, 11–18. [Google Scholar] [CrossRef]
- Chauhan, J.; Shrivastav, N.; Dugaya, A.; Pandey, D. Synthesis and characterization of Ni and Cu doped Zno. MOJ Polym. Sci. 2019, 1, 26–34. [Google Scholar] [CrossRef]
- Van Grieken, R.; Escola, J.M.; Moreno, J.; Rodríguez, R. Direct synthesis of mesoporous M-SBA-15 (M = Al, Fe, B, Cr) and application to 1-hexene oligomerization. Chem. Eng. J. 2009, 155, 442–450. [Google Scholar] [CrossRef]
- Poh, N.E.; Nur, H.; Muhid, M.N.M.; Hamdan, H. Sulphated AlMCM-41: Mesoporous solid Brønsted acid catalyst for dibenzoylation of biphenyl. Catal. Today 2006, 114, 257–262. [Google Scholar] [CrossRef]
- Jin, Q.; Qu, F.; Jiang, J.; Dong, Y.; Guo, W.; Lin, H. A pH-sensitive controlled dual-drug release from meso-macroporous silica/multilayer-polyelectrolytes coated SBA-15 composites. J. Sol-Gel Sci. Technol. 2013, 66, 466–471. [Google Scholar] [CrossRef]
- Van Der Meer, J.; Bardez-Giboire, I.; Mercier, C.; Revel, B.; Davidson, A.; Denoyel, R. Mechanism of metal oxide nanoparticle loading in SBA-15 by the double solvent technique. J. Phys. Chem. C 2010, 114, 3507–3515. [Google Scholar] [CrossRef]
- Akbar, N.A.; Aziz, H.A.; Adlan, M.N. The characteristics of limestone and anthracite coal as filter media in treating pollutants from groundwater. Int. J. Environ. Sci. Dev. 2021, 12, 58–62. [Google Scholar] [CrossRef]
- Miyamori, Y.; Kong, Y.; Nabae, Y.; Hatakeyama-Sato, K.; Hayakawa, T. Highly Ordered Bimodal Mesoporous Carbon from ABC Triblock Terpolymers with Phenolic Resol. ACS Macro Lett. 2024, 13, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Xin, Z.; Meng, X.; Lv, Y.; Bian, Z. Impact of double-solvent impregnation on the Ni dispersion of Ni/SBA-15 catalysts and catalytic performance for the syngas methanation reaction. RSC Adv. 2016, 6, 35875–35883. [Google Scholar] [CrossRef]
- Guillet-Nicolas, R.; Bérubé, F.; Thommes, M.; Janicke, M.T.; Kleitz, F. Selectively Tuned Pore Condensation and Hysteresis Behavior in Mesoporous SBA-15 Silica: Correlating Material Synthesis to Advanced Gas Adsorption Analysis. J. Phys. Chem. C 2017, 121, 24505–24526. [Google Scholar] [CrossRef]
- Guayaquil-Sosa, J.F.; Serrano-Rosales, B.; Valadés-Pelayo, P.J.; de Lasa, H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B Environ. 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Holinsworth, B.S.; Mazumdar, D.; Sims, H.; Sun, Q.-C.; Yurtisigi, M.K.; Sarker, S.K.; Gupta, A.; Butler, W.H.; Musfeldt, J.L. Chemical tuning of the optical band gap in spinel ferrites: CoFe2O4 vs NiFe2O4. Appl. Phys. Lett. 2013, 103, 2–5. [Google Scholar] [CrossRef]
- Ghaly, M.Y.; Farah, J.Y.; Fathy, A.M. Enhancement of decolorization rate and COD removal from dyes containing wastewater by the addition of hydrogen peroxide under solar photocatalytic oxidation. Desalination 2007, 217, 74–84. [Google Scholar] [CrossRef]
- Du, J.; Bao, J.; Fu, X.; Lu, C.; Kim, S.H. Mesoporous sulfur-modified iron oxide as an effective Fenton-like catalyst for degradation of bisphenol A. Appl. Catal. B Environ. 2016, 184, 132–141. [Google Scholar] [CrossRef]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef]
- So, C.M.; Cheng, M.Y.; Yu, J.C.; Wong, P.K. Degradation of azo dye Procion Red MX-5B by photocatalytic oxidation. Chemosphere 2002, 46, 905–912. [Google Scholar] [CrossRef]
- Sheydaei, M.; Aber, S.; Khataee, A. Degradation of amoxicillin in aqueous solution using nanolepidocrocite chips/H2O2/UV: Optimization and kinetics studies. J. Ind. Eng. Chem. 2014, 20, 1772–1778. [Google Scholar] [CrossRef]
- Jalil, A.A.; Triwahyono, S.; Adam, S.H.; Rahim, N.D.; Aziz, M.A.A.; Hairom, N.H.H.; Razali, N.A.M.; Abidin, M.A.Z.; Mohamadiah, M.K.A. Adsorption of methyl orange from aqueous solution onto calcined Lapindo volcanic mud. J. Hazard. Mater. 2010, 181, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, M.V.; Kim, D.S. Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: Kinetics, isotherms, and thermodynamic studies. Ecotoxicol. Environ. Saf. 2016, 128, 109–117. [Google Scholar] [CrossRef]
- Sun, J.-H.; Sun, S.-P.; Sun, J.-Y.; Sun, R.-X.; Qiao, L.-P.; Guo, H.-Q.; Fan, M.-H. Degradation of azo dye Acid black 1 using low concentration iron of Fenton process facilitated by ultrasonic irradiation. Ultrason. Sonochem. 2007, 14, 761–766. [Google Scholar] [CrossRef]
- Sun, S.-P.; Li, C.-J.; Sun, J.-H.; Shi, S.-H.; Fan, M.-H.; Zhou, Q. Decolorization of an azo dye Orange G in aqueous solution by Fenton oxidation process: Effect of system parameters and kinetic study. J. Hazard. Mater. 2009, 161, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Gharbani, P.; Mehrizad, A.; Mosavi, S.A. Optimization, kinetics and thermodynamics studies for photocatalytic degradation of Methylene Blue using cadmium selenide nanoparticles. NPJ Clean Water 2022, 5, 34. [Google Scholar] [CrossRef]
- Chekir, N.; Benhabiles, O.; Tassalit, D.; Laoufi, N.A.; Bentahar, F. Photocatalytic degradation of methylene blue in aqueous suspensions using TiO2 and ZnO. Desalin. Water Treat. 2016, 57, 6141–6147. [Google Scholar] [CrossRef]
- Kavitha, G.; Vinoth Kumar, J.; Pavithra, S.; Komal, M.; Sherlin Nivetha, M.; Kayalvizhi, R.; Abirami, N. Biogenic synthesis of argentum nanocomposites for visible light photocatalyst of dye degradation. Chem. Phys. Lett. 2022, 809, 140159. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High Photocatalytic Activity of ZnO−Carbon Nanofiber Heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef]
- Farghaly, A.; Maher, E.; Gad, A.; El-Bery, H. Synergistic photocatalytic degradation of methylene blue using TiO2 composites with activated carbon and reduced graphene oxide: A kinetic and mechanistic study. Appl. Water Sci. 2024, 14, 228. [Google Scholar] [CrossRef]
- Wei, G.-T.; Fan, C.-Y.; Zhang, L.-Y.; Ye, R.-C.; Wei, T.-Y.; Tong, Z.-F. Photo-Fenton degradation of methyl orange using H3PW12O40 supported Fe-bentonite catalyst. Catal. Commun. 2012, 17, 184–188. [Google Scholar] [CrossRef]
- Shah, R.K. Efficient photocatalytic degradation of methyl orange dye using facilely synthesized α-Fe2O3 nanoparticles. Arab. J. Chem. 2023, 16, 104444. [Google Scholar] [CrossRef]
- Tien, T.-M.; Chen, C.-H.; Huang, C.-T.; Chen, E.L. Photocatalytic Degradation of Methyl Orange Dyes Using Green Synthesized MoS2/Co3O4 Nanohybrids. Catalysts 2022, 12, 1474. [Google Scholar] [CrossRef]
- Hariani, P.L.; Said, M.; Rachmat, A.; Salni, S.; Aprianti, N.; Amatullah, A.F. Synthesis of NiFe2O4/SiO2/NiO Magnetic and Application for the Photocatalytic Degradation of Methyl Orange Dye under UV Irradiation. Bull. Chem. React. Eng. Catal. 2022, 17, 699–711. [Google Scholar] [CrossRef]
- Pang, Y.L.; Law, Z.X.; Lim, S.; Chan, Y.Y.; Shuit, S.H.; Chong, W.C.; Lai, C.W. Enhanced photocatalytic degradation of methyl orange by coconut shell–derived biochar composites under visible LED light irradiation. Environ. Sci. Pollut. Res. 2021, 28, 27457–27473. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhou, Z.; Yu, Y.; Zhang, H.; Qian, Y.; Yang, Y.; Duo, S.L. Fabrication of nanometer-sized high-silica SAPO-5 and its enhanced photocatalytic performance for methyl orange degradation. Res. Chem. Intermed. 2019, 45, 1457–1473. [Google Scholar] [CrossRef]
- Znad, H.; Abbas, K.; Hena, S.; Awual, M.R. Synthesis a novel multilamellar mesoporous TiO2/ZSM-5 for photo-catalytic degradation of methyl orange dye in aqueous media. J. Environ. Chem. Eng. 2018, 6, 218–227. [Google Scholar] [CrossRef]
- Hamieh, M.; Tabaja, N.; Tlais, S.; Koubaissy, B.; Hammoud, M.; Chawraba, K.; Hamieh, T.; Toufaily, J. Development of a Novel Adsorbent Derived from Olive Mill Solid Wastes for Enhanced Removal of Methylene Blue. Materials 2024, 17, 4326. [Google Scholar] [CrossRef]
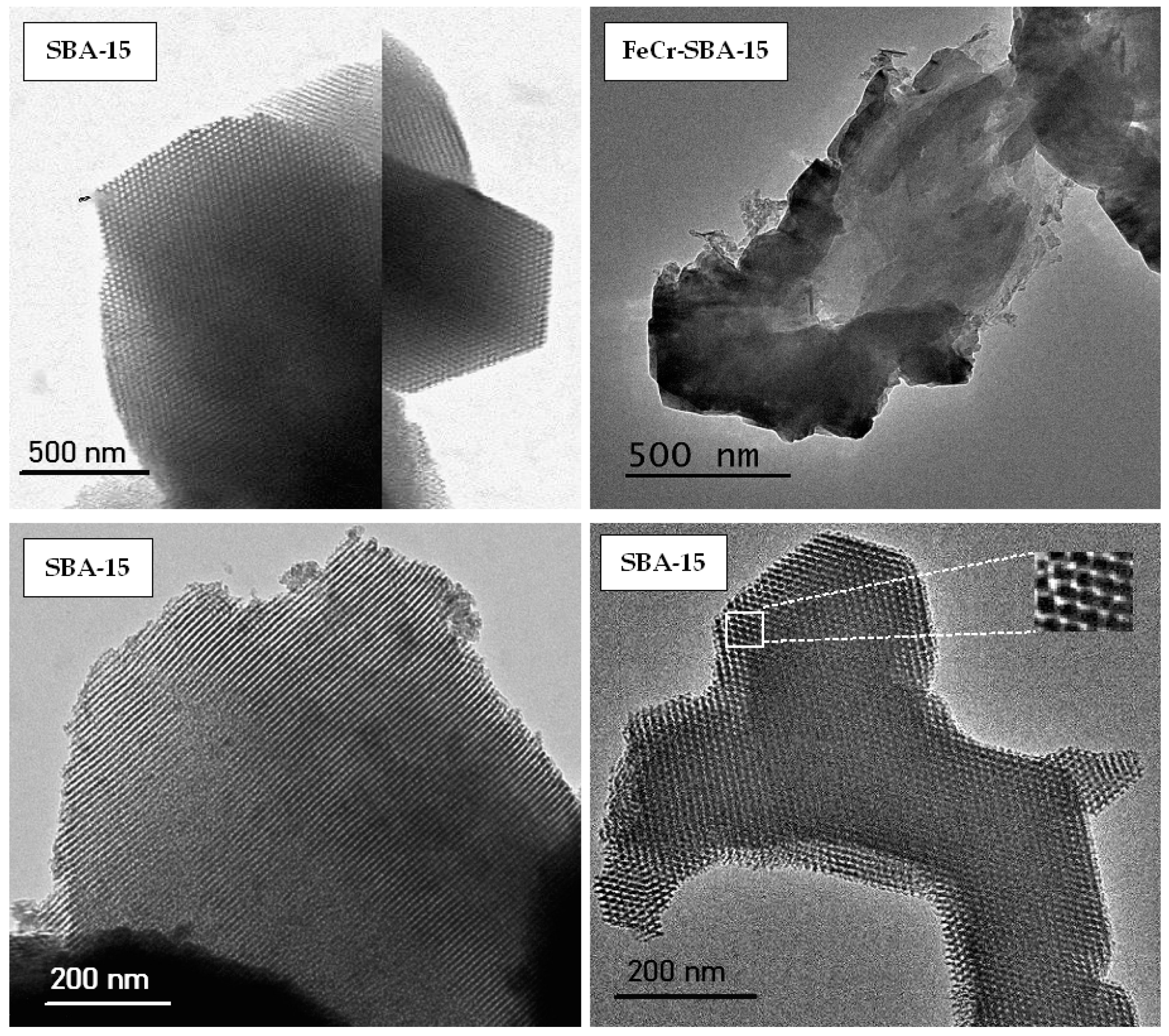


| Sample | SBET (m2 g−1) | Vp a (cm3 g−1) | Dpore b (nm) | Vµp c (cm3 g−1) |
|---|---|---|---|---|
| SBA-15 | 522 | 1.48 | 8.7 | 0.013 |
| FeCr-SBA-15 | 372 | 0.65 | 6.8 | 0.006 |
| AC | 1148 | 0.6 | 3.2 | 0.055 |
| Isotherm Models | Parameters | Values |
|---|---|---|
| Langmuir | qm | 458.71 |
| KL | 0.059 | |
| R2 | 0.856 | |
| Freundlich | n | 3.15 |
| KF | 89.73 | |
| R2 | 0.946 |
| Kinetic Models | Parameters | FeCr-SBA/AC | FeCr-SBA |
|---|---|---|---|
| Zero order | K₀ | 0.0439 | 0.061 |
| R2 | 0.8632 | 0.9356 | |
| First order | K₁ | 0.0173 | 0.0085 |
| R2 | 0.9912 | 0.9982 | |
| Second order | K2 | 0.0206 | 0.0016 |
| R2 | 0.8295 | 0.8896 |
| Photocatalyst | Catalyst Concentration (g L−1) | MO Concentration (ppm) | pH | Light Source | MO Degradation (%) | Reaction Time (min) | Reference |
|---|---|---|---|---|---|---|---|
| HPW-Fe-Bent | 0.75 | 10 | - | UV light (40 W) | 78.1 | 60 | [61] |
| α-Fe2O3 | 1 | 20 | 3 | UV lamp (15 W) | 82.17 | 100 | [62] |
| MoS2/Co3O4 | 0.2 | 20 | - | Xe lamp (350 W) | 95.6 | 170 | [63] |
| NiFe2O4/SiO2/NiO | 0.5 | 10 | 4 | UV light (40 W) | 95.76 | 120 | [64] |
| g-C3N4/biochar | 0.75 | 10 | 3.48 | LED (20 W) | 96.63 | 30 | [65] |
| High-silica SAPO-5 | 1.4 | 10 | - | Hg lamp (36 W) | 82.8 | 240 | [66] |
| TiO2/ZSM-5 | 2 | 20 | 7.5 | solar simulator (100 mW cm−2) | 99 | 180 | [67] |
| FeCr-SBA-15/activated carbon | 0.75 | 20 | 6.47 | Halogen Lamp (100 W) | 97 | 180 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamieh, M.; Tabaja, N.; Chawraba, K.; Hamie, Z.; Hammoud, M.; Tlais, S.; Hamieh, T.; Toufaily, J. Visible Light Photo-Fenton with Hybrid Activated Carbon and Metal Ferrites for Efficient Treatment of Methyl Orange (Azo Dye). Molecules 2025, 30, 1770. https://doi.org/10.3390/molecules30081770
Hamieh M, Tabaja N, Chawraba K, Hamie Z, Hammoud M, Tlais S, Hamieh T, Toufaily J. Visible Light Photo-Fenton with Hybrid Activated Carbon and Metal Ferrites for Efficient Treatment of Methyl Orange (Azo Dye). Molecules. 2025; 30(8):1770. https://doi.org/10.3390/molecules30081770
Chicago/Turabian StyleHamieh, Malak, Nabil Tabaja, Khaled Chawraba, Zeinab Hamie, Mohammad Hammoud, Sami Tlais, Tayssir Hamieh, and Joumana Toufaily. 2025. "Visible Light Photo-Fenton with Hybrid Activated Carbon and Metal Ferrites for Efficient Treatment of Methyl Orange (Azo Dye)" Molecules 30, no. 8: 1770. https://doi.org/10.3390/molecules30081770
APA StyleHamieh, M., Tabaja, N., Chawraba, K., Hamie, Z., Hammoud, M., Tlais, S., Hamieh, T., & Toufaily, J. (2025). Visible Light Photo-Fenton with Hybrid Activated Carbon and Metal Ferrites for Efficient Treatment of Methyl Orange (Azo Dye). Molecules, 30(8), 1770. https://doi.org/10.3390/molecules30081770









