Electrodeposition of Alloy Nanostructures (Co-Ni) in the Presence of Sodium Benzene Sulfonate (SBS) and Their Application in Alkaline Hydrogen Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Coating Structures and Compositions
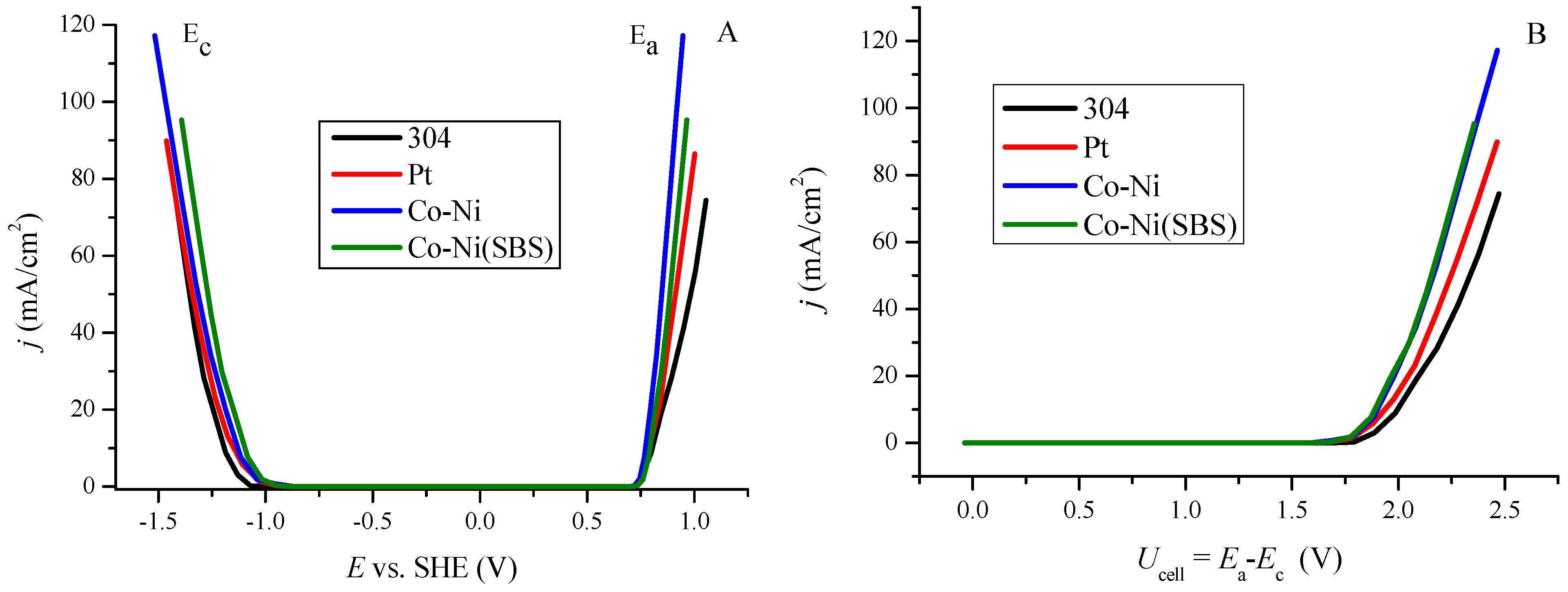
2.2. Determination and Comparison of Hydrogen Evolution Overpotential for Various Materials

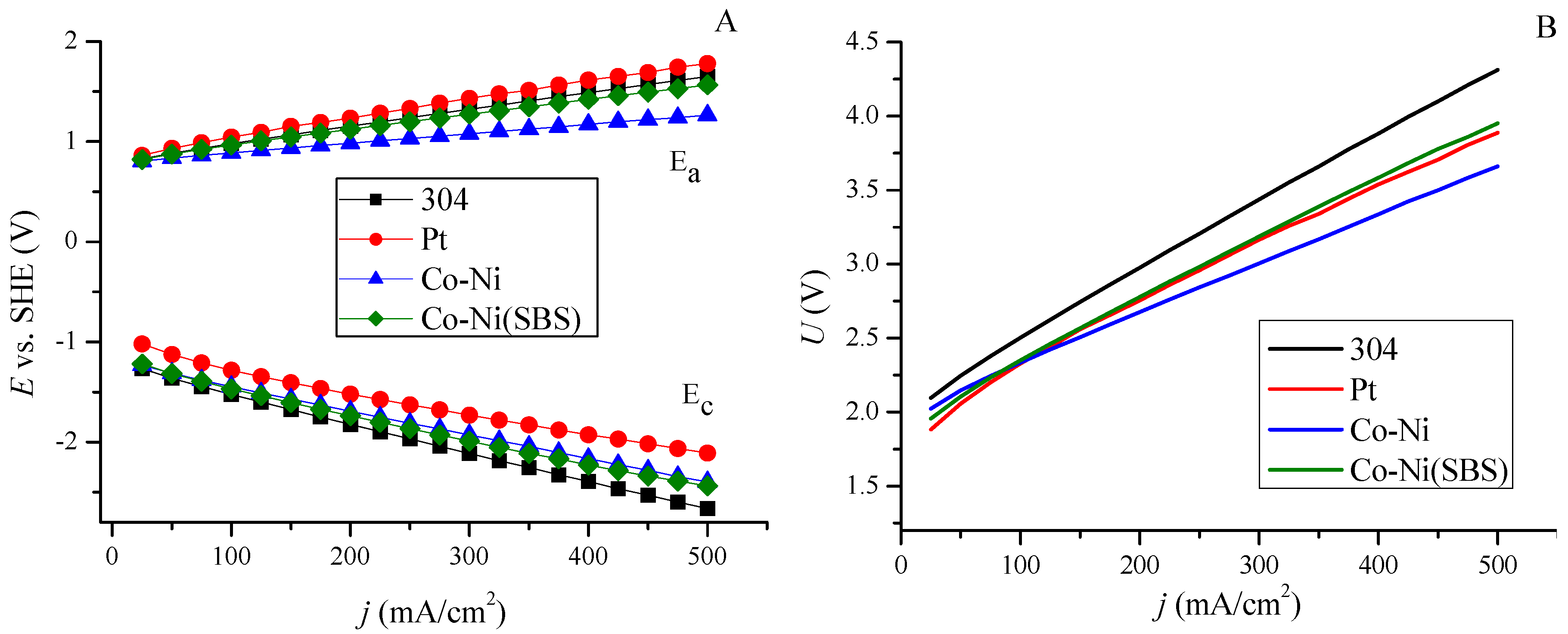
2.3. Hydrogen Evolution at Constant Voltage
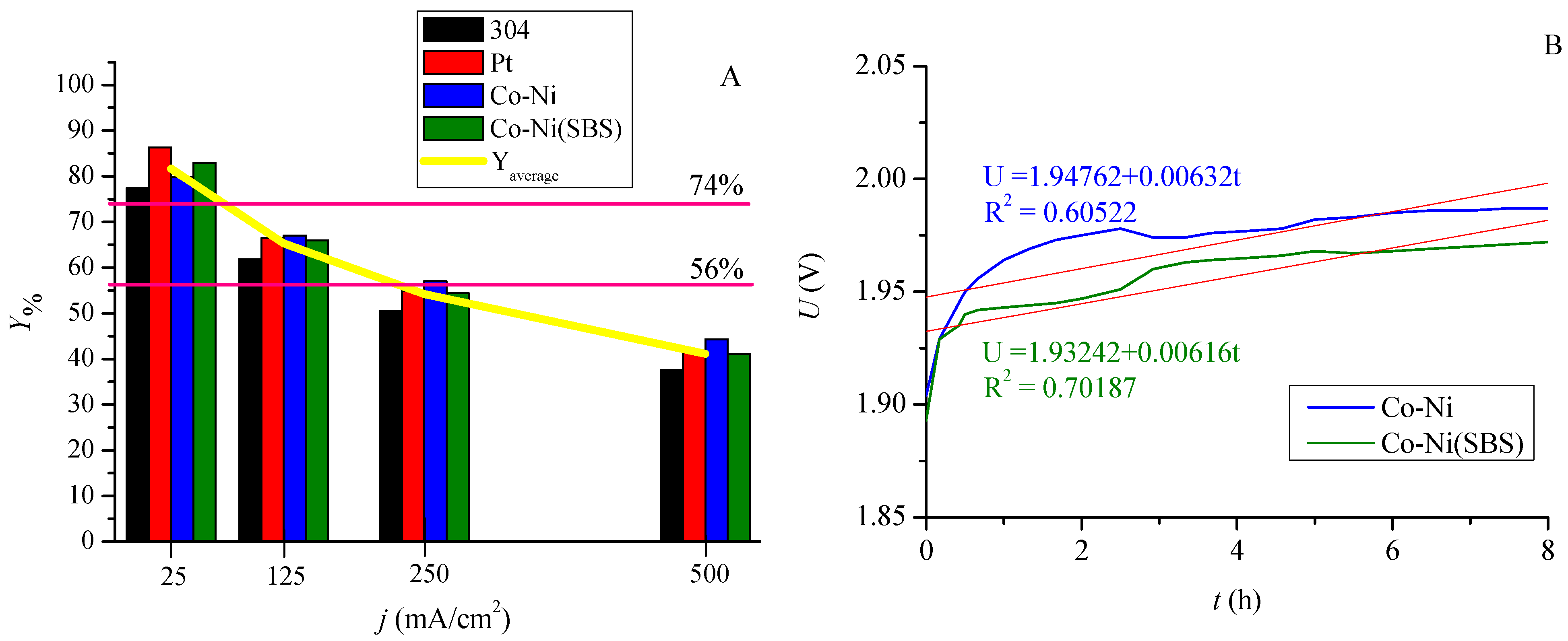
2.4. Hydrogen Evolution at Constant Current
2.5. Efficiency of Structures in the Hydrogen Evolution Process
3. Materials and Methods
3.1. Preparation of the Substrate for Deposition
3.2. Electrodeposition
3.3. Electrolytic Hydrogen Evolution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| CP | Reversible hydrogen electrode |
| HER | Hydrogen evolution reaction |
| OER | Oxygen evolution reaction |
| RES | Renewable energy sources |
| RHE | Reversible hydrogen electrode |
| SBS | Sodium benzene sulfonate |
| SCE | Saturated calomel electrode |
| SEM | Scanning electron microscope |
| SHE | Standard hydrogen electrode |
References
- Feidenhans, A.; Regmi, Y.N.; Wei, C.; Xia, D.; Kibsgaard, J.; King, L. Precious Metal Free Hydrogen Evolution Catalyst Design and Application. Chem. Rev. 2024, 124, 5617–5667. [Google Scholar] [CrossRef]
- Scarlat, N.; Prussi, M.; Padella, M. Quantification of the carbon intensity of electricity produced and used in Europe. Appl. Energy 2022, 305, 117901. [Google Scholar] [CrossRef]
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jäger-Waldau, A. Green hydrogen in Europe—A regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 228, 113649. [Google Scholar] [CrossRef]
- Faramarzi, S.; Khavari, A. An innovative mixed refrigerant hydrogen liquefaction cycle to store geothermal energy as liquid hydrogen. J. Energy Storage 2023, 72, 108008. [Google Scholar] [CrossRef]
- Srivastava, P.; García-Quismondo, E.; Palma, J.; González-Fernández, C. Coupling dark fermentation and microbial electrolysis cells for higher hydrogen yield: Technological competitiveness and challenges. Int. J. Hydrogen Energy 2024, 52, 223–239. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Large-scale green hydrogen production using alkaline water electrolysis based on seasonal solar radiation. Energy Harvest. Syst. 2023, 11, 20230011. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Zhang, Y.; Zhang, H.; Xiong, K.; Ye, X.; Liu, Q.; Zhu, J. Transition-metal atoms embedded MoTe2 single-atom catalyst for efficient electrocatalytic hydrogen evolution reaction. Appl. Surf. Sci. 2025, 680, 161335. [Google Scholar] [CrossRef]
- Yang, J.; Chai, C.; Jiang, C.; Liu, L.; Xi, J. MoS2–CoS2 heteronanosheet arrays coated on porous carbon microtube textile for overall water splitting. J. Power Sources 2021, 514, 230580. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, X.; Wei, W.; Chen, H.; Dong, T.; Wang, R.; Liu, M.; Ostrikov, K.; Peng, P.; Zang, S.-Q. Neighboring Platinum Atomic Sites Activate Platinum–Cobalt Nanoclusters as High-Performance ORR/OER/HER Electrocatalysts. Small 2023, 19, 2304294. [Google Scholar] [CrossRef]
- Lončar, A.; Jovanovič, P.; Hodnik, N.; Gaberšček, M. Determination of the Electroactive Surface Area of Supported Ir-Based Oxygen Evolution Catalysts by Impedance Spectroscopy: Observed Anomalies with Respect to Catalyst Loading. J. Electrochem. Soc. 2023, 170, 044504. [Google Scholar] [CrossRef]
- Hoisang, W.; Sakaushi, K. Key criteria for next-generation dimensionally stable electrodes towards large-scale green hydrogen production by water electrolysis. Curr. Opin. Electrochem. 2022, 36, 101136. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Khandelwal, M.; Dayong, F.; Sui, L.; Chung, J.S.; Misra, R.D.K.; Yin, P.; Kim, E.J.; Kim, W.; Vanchiappan, A.; et al. Developments and Perspectives on Robust Nano- and Microstructured Binder-Free Electrodes for Bifunctional Water Electrolysis and Beyond. Adv. Energy Mater. 2022, 12, 2200409. [Google Scholar] [CrossRef]
- Lyu, X.; Bai, Y.; Li, J.; Tao, R.; Yang, J.; Serov, A. Investigation of oxygen evolution reaction with 316 and 304 stainless-steel mesh electrodes in natural seawater electrolysis. J. Environ. Chem. Eng. 2023, 11, 109667. [Google Scholar] [CrossRef]
- Buttlera, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Lamy, C.; Millet, P. A critical review on the definitions used to calculate the energy efficiency coefficients of water electrolysis cells working under near ambient temperature conditions. J. Power Sources 2020, 447, 227350. [Google Scholar] [CrossRef]
- Bespalko, S.; Mizeraczyk, J. Overview of the Hydrogen Production by Plasma-Driven Solution Electrolysis. Energies 2022, 15, 7508. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, J.; Tang, J.; Liu, Z.; Deng, X. CNTs-supported noble metal-free high-entropy alloys heterostructure NiMoCoMnLa/CNTs@Ni for electrochemical hydrogen evolution reaction. Appl. Surf. Sci. 2024, 667, 160417. [Google Scholar] [CrossRef]
- Skitał, P.; Domańska, A. Modeling of the Simultaneous Hydrogen Evolution and Cobalt Electrodeposition. ChemPhysChem 2022, 23, e202200148. [Google Scholar] [CrossRef]
- Irandoos, E.; Barekati, N.S.; Farsi, H.; Farrokhi, A.; Horvath, G.; Li, Z. Cobalt–organic framework as a Bi–functional electrocatalyst for renewable hydrogen production by electrochemical water splitting. Appl. Energy Combust. Sci. 2024, 17, 100240. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. Progress in nickel chalcogenide electrocatalyzed hydrogen evolution reaction. J. Mater. Chem. A 2020, 8, 4174–4192. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Z.; Yao, Z.; Ma, Y.; Guo, F.; Chai, L. Facile fabrication of an enhanced electrodeposited nickel electrode for alkaline hydrogen evolution reaction. Electrochim. Acta. 2024, 477, 143792. [Google Scholar] [CrossRef]
- Hu, X.; Tian, X.; Lin, Y.-W.; Wang, Z. Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media. RSC Adv. 2019, 9, 31563–31571. [Google Scholar] [CrossRef] [PubMed]
- Sam, D.K.; Wang, W.; Gong, S.; Sam, E.K.; Lv, X.; Wang, J.; Liu, J. CO2 assisted synthesis of silk fibroin driven robust N-doped carbon aerogels coupled with nickel–cobalt particles as highly active electrocatalysts for HER. Int. J. Hydrogen Energy 2021, 46, 21525–21533. [Google Scholar] [CrossRef]
- Ahmadian, F.; Salarvand, V.; Saghafi, M.; Noghani, M.T.; Yousefifar, A. Production and characterization of high-performance cobalt–nickel selenide catalyst with excellent activity in HER. J. Mater. Res. Technol. 2021, 15, 3942–3950. [Google Scholar] [CrossRef]
- Wang, J.; Shao, H.; Ren, S.; Hu, A.; Li, M. Fabrication of porous Ni-Co catalytic electrode with high performance in hydrogen evolution reaction. Appl. Surf. Sci. 2021, 539, 148045. [Google Scholar] [CrossRef]
- Domańska, A.J.; Skitał, P.M. Mathematical modeling of the hydrogen evolution process on nickel, cobalt, and Co-Ni alloy coatings in acidic and alkaline environments. Chem. Process Eng. New Front. 2025, 46, e82. [Google Scholar] [CrossRef]
- Li, Z.; Wanga, J.; Zhang, J.; Huang, C.; Zhou, J.-J.; Xu, L.; Wang, L.; Lu, S.; Chen, L. MOF-on-MOF in situ-derived hybrid CoNi@C/N-doped C micro-rods arrays as an efficient catalyst for hydrogen evolution reaction in alkaline solution. Appl. Surf. Sci. 2024, 674, 160920. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Han, C.; Gao, J.; Pan, L.; Wu, J.; Zhu, X.D.; Zou, J.-J. NiCo-Based Electrocatalysts for the Alkaline Oxygen Evolution Reaction: A Review. ACS Catal. 2021, 11, 12485–12509. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, R.; Zou, J.-J.; Yang, H. Surface Design Strategy of Catalysts for Water Electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiong, T.; Huang, P.; Zhou, S.; Tan, Q.; Yang, H.; Huang, Y.; Balogun, M.-S. Nanostructured transition metal compounds coated 3D porous core-shell carbon fiber as monolith water splitting electrocatalysts: A general strategy. Chem. Eng. J. 2021, 423, 130279. [Google Scholar] [CrossRef]
- Zhanga, Y.; Zou, J.; Hea, Z.; Zhaoa, Y.; Kanga, X.; Zhaoa, Y.; Miao, Z. Facile synthesis of SnO2 nanostructures for enhanced electrochemical hydrogen evolution reaction. J. Alloys Compd. 2021, 865, 158597. [Google Scholar] [CrossRef]
- Cuppen, H.M.; Morata, O.; Herbs, E. Monte Carlo simulations of H2 formation on stochastically heated grains. Mon. Not. R. Astron. Soc. 2006, 367, 1757–1765. [Google Scholar] [CrossRef]
- Warrier, M.; Rai, A.; Schneider, R. A time dependent model to study the effect of surface roughness on reactive-diffusive transport in porous media. J. Nucl. Mater. 2009, 390-391, 203–206. [Google Scholar] [CrossRef]
- Xie, Y.; Miche, A.; Vivier, V.; Turmine, M. Electrodeposition of Ni-Co alloys from neat protic ionic liquid: Application to the hydrogen evolution reaction. Appl. Surf. Sci. 2023, 635, 157693. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, H.; Xue, S.; Song, H.; Zhang, G.; Xia, J.; Miao, X. Effect of graphene oxide on CoCrFeNi high-entropy alloy coatings prepared by the electrodeposition method. Surf. Coat. Technol. 2024, 485, 130919. [Google Scholar] [CrossRef]
- Guerrero, J.D.M.; Satpathy, B.; Prasad, P.S.; Das, S.; Das, K. Electrodeposition process optimization by response surface methodologies to obtain high-corrosion resistant Zn–Ni coatings. Surf. Coat. Technol. 2024, 479, 130564. [Google Scholar] [CrossRef]
- Falcón, J.M.; Aoki, I.V. Electrodeposition of Zn–Co alloy coatings on carbon steel and their corrosion resistance. Surf. Coat. Technol. 2024, 481, 130603. [Google Scholar] [CrossRef]
- Rudnik, E.; Wojnicki, M.; Włoch, G. Effect of gluconate addition on the electrodeposition of nickel from acidic baths. Surf. Coat. Technol. 2012, 207, 375–388. [Google Scholar] [CrossRef]
- Lv, B.; Hu, Z.; Wang, X.; Xu, B. Electrodeposition of nanocrystalline nickel assisted by flexible friction from an additive-free Watts bath. Surf. Coat. Technol. 2015, 270, 123–131. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; He, Z.; Chen, J.; Gao, W.; Cao, P. Superhydrophobic Ni nanocone surface prepared by electrodeposition and its overall performance. Surf. Coat. Technol. 2023, 464, 129548. [Google Scholar] [CrossRef]
- Ballesteros, J.C.; Díaz-Arista, P.; Meas, Y.; Ortega, R.; Trejo, G. Zinc electrodeposition in the presence of polyethylene glycol 20,000. Electrochim. Acta. 2007, 52, 3686–3696. [Google Scholar] [CrossRef]
- Zhongfei, W.; Yu, L.; Chengzhi, D.; Haimin, Z.; Ruirui, Z.; Hongyu, C. The critical role of boric acid as electrolyte additive on the electrochemical performance of lead-acid battery. J. Energy Storage 2020, 27, 101076. [Google Scholar] [CrossRef]
- Vivegnis, S.; Krid, M.; Delhalle, J.; Mekhalif, Z.; Renner, F.U. Use of pyrophosphate and boric acid additives in the copper-zinc alloy electrodeposition and chemical dealloying. J. Electroanal. Chem. 2019, 848, 113310. [Google Scholar] [CrossRef]
- Agarwal, S.; Khatri, M.S. Effect of pH and Boric Acid on Magnetic Properties of Electrodeposited Co Nanowires. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2022, 92, 111–116. [Google Scholar] [CrossRef]
- Skitał, P.M.; Sanecki, P.T.; Saletnik, D.; Kalembkiewicz, J. Electrodeposition of nickel from alkaline NH4OH/NH4Cl buffer solutions. Trans. Nonferrous Met. Soc. China 2019, 29, 222–232. [Google Scholar] [CrossRef]
- Domańska, A.J.; Skitał, P.M. Electrolytic deposition of zinc-nickel alloy coatings with organic addition. Ochrona Przed Korozją 2021, 64, 358–362. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, P.; Chen, P.; Hang, T.; Li, M.; Wu, Y. Super-flat and uni-oriented cobalt film electrodeposited by modulating the crystal nucleation and growth behavior. Appl. Surf. Sci. 2024, 645, 158795. [Google Scholar] [CrossRef]
- Safavi, M.S.; Tanhaei, M.; Ahmadipour, M.F.; Adli, R.G.; Mahdavi, S.; Walsh, F.C. Electrodeposited Ni-Co alloy-particle composite coatings: A comprehensive review. Surf. Coat. Technol. 2020, 382, 125153. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Nickel–cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int. J. Hydrogen Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Pariona, M.M.; Telegiński, V.; dos Santos, K.; dos Santos, E.L.R.; Riva, R. AFM study of the effects of laser surface remelting on the morphology of Al–Fe aerospace alloys. Mater. Charact. 2012, 74, 64–76. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Elektrochemical Methods. In Fundamental and Applications, 3rd ed.; Wiley: New York, NY, USA, 2022. [Google Scholar]
- Zhai, W.; Ma, Y.; Chen, D.; Ho, J.C.; Dai, Z.; Qu, Y. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. InfoMa 2022, 4, e12357. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Han, N.; Tian, Y.; Kallio, T.; Yu, C.; Jiang, L. 2023, Superaerophilic/superaerophobic cooperative electrode for efficient hydrogen evolution reaction via enhanced mass transfer. Sci. Adv. 2023, 9, eadd6978. [Google Scholar] [CrossRef]


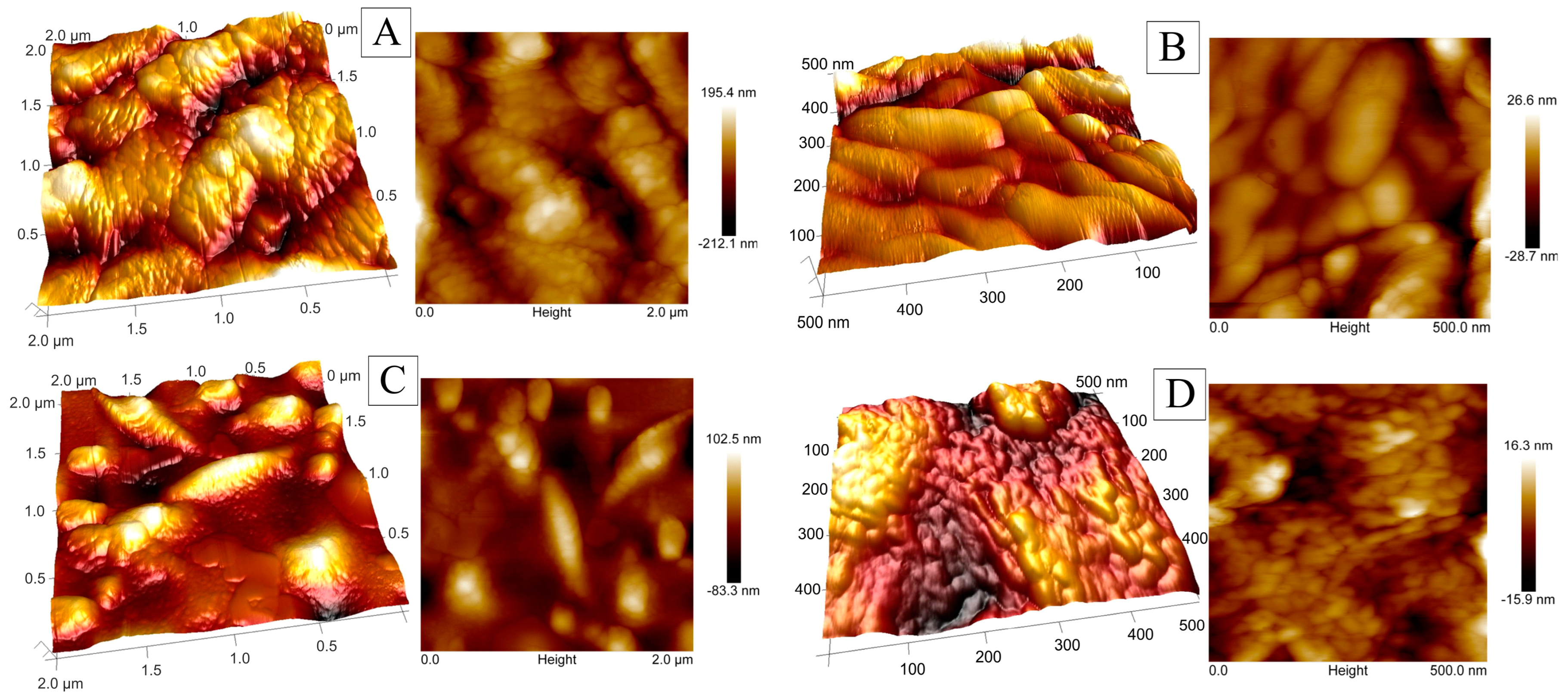
| Coating Designation | Elemental Content [% wt] ± SD | Co/Ni | |||||
|---|---|---|---|---|---|---|---|
| C | O | Cr | Fe | Co | Ni | ||
| Co-Ni | 2.06 ± 0.93 | 1.30 ± 0.74 | 0.50 ± 0.04 | 0.61 ± 0.08 | 70.23 ± 0.91 | 25.30 ± 0.44 | 2.78 |
| Co-Ni(SBS) | 1.51 ± 0.49 | 0.80 ± 0.38 | 0.22 ± 0.03 | 0.25 ± 0.03 | 72.65 ± 0.52 | 24.58 ± 0.57 | 2.95 |
| Roughness Parameter | Coating Designation | Scanning Surface [µm2] | ||
|---|---|---|---|---|
| 0.25 | 1 | 4 | ||
| Ra [nm] | Co-Ni | 5.61 | 16.0 | 47.5 |
| Co-Ni(SBS) | 2.90 | 8.17 | 18.3 | |
| Rq [nm] | Co-Ni | 7.17 | 20.7 | 58.4 |
| Co-Ni(SBS) | 3.71 | 10.8 | 24.7 | |
| Rz [nm] | Co-Ni | 8.07 | 52.9 | 122 |
| Co-Ni(SBS) | 7.18 | 28.2 | 67.6 | |
| Rp [nm] | Co-Ni | 25.1 | 62.4 | 179 |
| Co-Ni(SBS) | 14.4 | 42.8 | 121 | |
| Rv [nm] | Co-Ni | −34.9 | −87.9 | −198 |
| Co-Ni(SBS) | −16.0 | −41.7 | −83.0 | |
| Sample Determination | Low Current Density Values | High Current Density Values | ||||
|---|---|---|---|---|---|---|
| Tafel Slope [mV] | −logj0 [A/cm2] | R2 | Tafel Slope [mV] | −logj0 [A/cm2] | R2 | |
| 304 | 127.3 | 5.5 | 0.9985 | 378.2 | 8.4 | 0.9947 |
| Pt | 114.2 | 2.6 | 0.9793 | 654.4 | 9.0 | 0.9979 |
| Co-Ni | 122.4 | 4.0 | 0.9992 | 323.4 | 6.1 | 0.9965 |
| Co-Ni(SBS) | 287.1 | 6.4 | 0.9955 | 348.8 | 6.8 | 0.9987 |
| Determination of the Cathode Area | Current Density [mA/cm2] | Hydrogen Evolution Yield Parameter | ||||
|---|---|---|---|---|---|---|
| gH2/kWh | kWh/kgH2 | kJ/molH2 | kWh/molH2 | Y% | ||
| 304 | 25 | 19.68 | 50.8 | 369.2 | 0.103 | 77.50 |
| 125 | 15.70 | 63.7 | 462.8 | 0.129 | 61.83 | |
| 250 | 12.85 | 77.8 | 565.6 | 0.157 | 50.59 | |
| 500 | 9.55 | 104.7 | 760.9 | 0.212 | 37.60 | |
| Pt | 25 | 21.91 | 45.6 | 331.7 | 0.092 | 86.27 |
| 125 | 16.89 | 59.2 | 430.3 | 0.120 | 66.49 | |
| 250 | 13.92 | 71.8 | 521.9 | 0.145 | 54.83 | |
| 500 | 10.59 | 94.4 | 686.0 | 0.191 | 41.71 | |
| Co-Ni | 25 | 20.26 | 49.4 | 358.6 | 0.100 | 79.79 |
| 125 | 17.01 | 58.8 | 427.2 | 0.119 | 66.99 | |
| 250 | 14.49 | 69.0 | 501.6 | 0.139 | 57.05 | |
| 500 | 11.25 | 88.9 | 645.9 | 0.180 | 44.30 | |
| Co-Ni(SBS) | 25 | 21.08 | 47.4 | 344.7 | 0.096 | 83.01 |
| 125 | 16.74 | 59.7 | 434.0 | 0.121 | 65.93 | |
| 250 | 13.81 | 72.4 | 526.1 | 0.146 | 54.39 | |
| 500 | 10.42 | 96.0 | 697.2 | 0.194 | 41.04 | |
| Coating Designation | [mol/dm3] | [mol/dm3] | [%] | [mol/dm3] |
|---|---|---|---|---|
| Co-Ni | 0.06 | 0.04 | - | 0.5 |
| Co-Ni(SBS) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domańska, A.J.; Skitał, P.M. Electrodeposition of Alloy Nanostructures (Co-Ni) in the Presence of Sodium Benzene Sulfonate (SBS) and Their Application in Alkaline Hydrogen Evolution. Molecules 2025, 30, 1771. https://doi.org/10.3390/molecules30081771
Domańska AJ, Skitał PM. Electrodeposition of Alloy Nanostructures (Co-Ni) in the Presence of Sodium Benzene Sulfonate (SBS) and Their Application in Alkaline Hydrogen Evolution. Molecules. 2025; 30(8):1771. https://doi.org/10.3390/molecules30081771
Chicago/Turabian StyleDomańska, Aleksandra J., and Piotr M. Skitał. 2025. "Electrodeposition of Alloy Nanostructures (Co-Ni) in the Presence of Sodium Benzene Sulfonate (SBS) and Their Application in Alkaline Hydrogen Evolution" Molecules 30, no. 8: 1771. https://doi.org/10.3390/molecules30081771
APA StyleDomańska, A. J., & Skitał, P. M. (2025). Electrodeposition of Alloy Nanostructures (Co-Ni) in the Presence of Sodium Benzene Sulfonate (SBS) and Their Application in Alkaline Hydrogen Evolution. Molecules, 30(8), 1771. https://doi.org/10.3390/molecules30081771






