Phytochemical Characterization and Antioxidant Activity of Cajanus cajan Leaf Extracts for Nutraceutical Applications
Abstract
:1. Introduction
2. Results
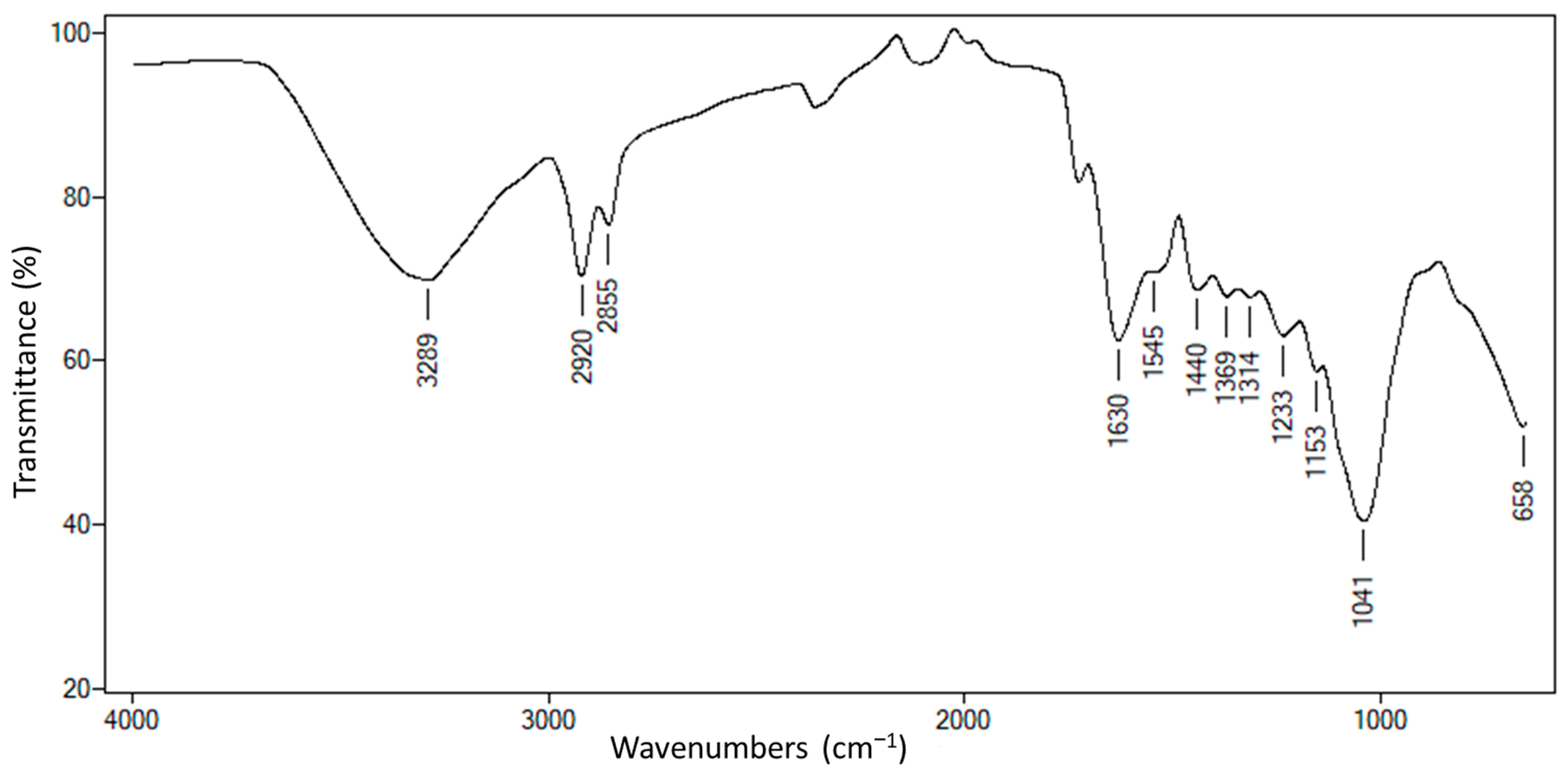
2.1. Chemical Composition of C. cajan Leaves
2.2. Phenolic Compounds, Flavonoids, and Anthocyanin Content
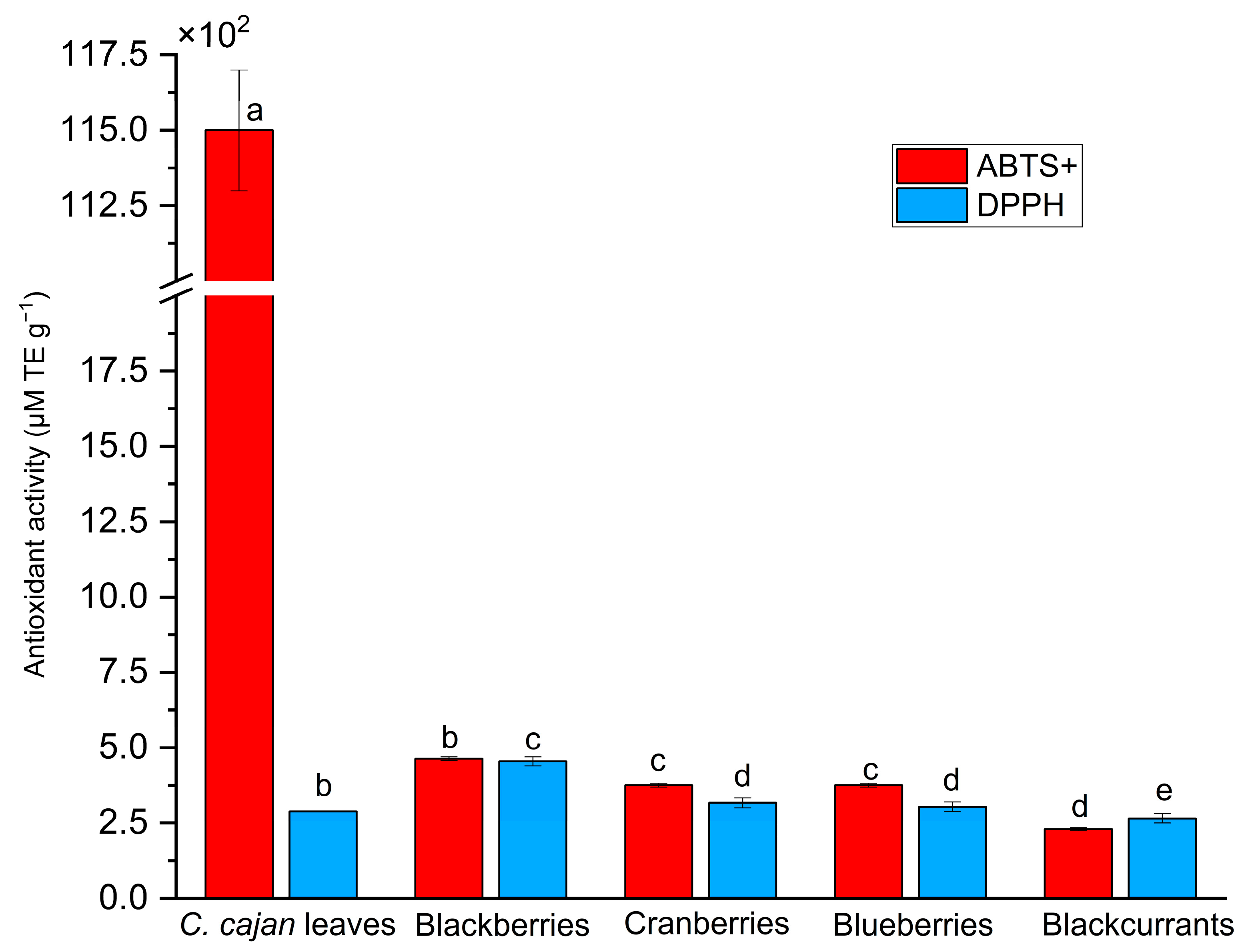
2.3. C. cajan Leaves Exhibit High Antioxidant Activity According to ABTS and DPPH Assays
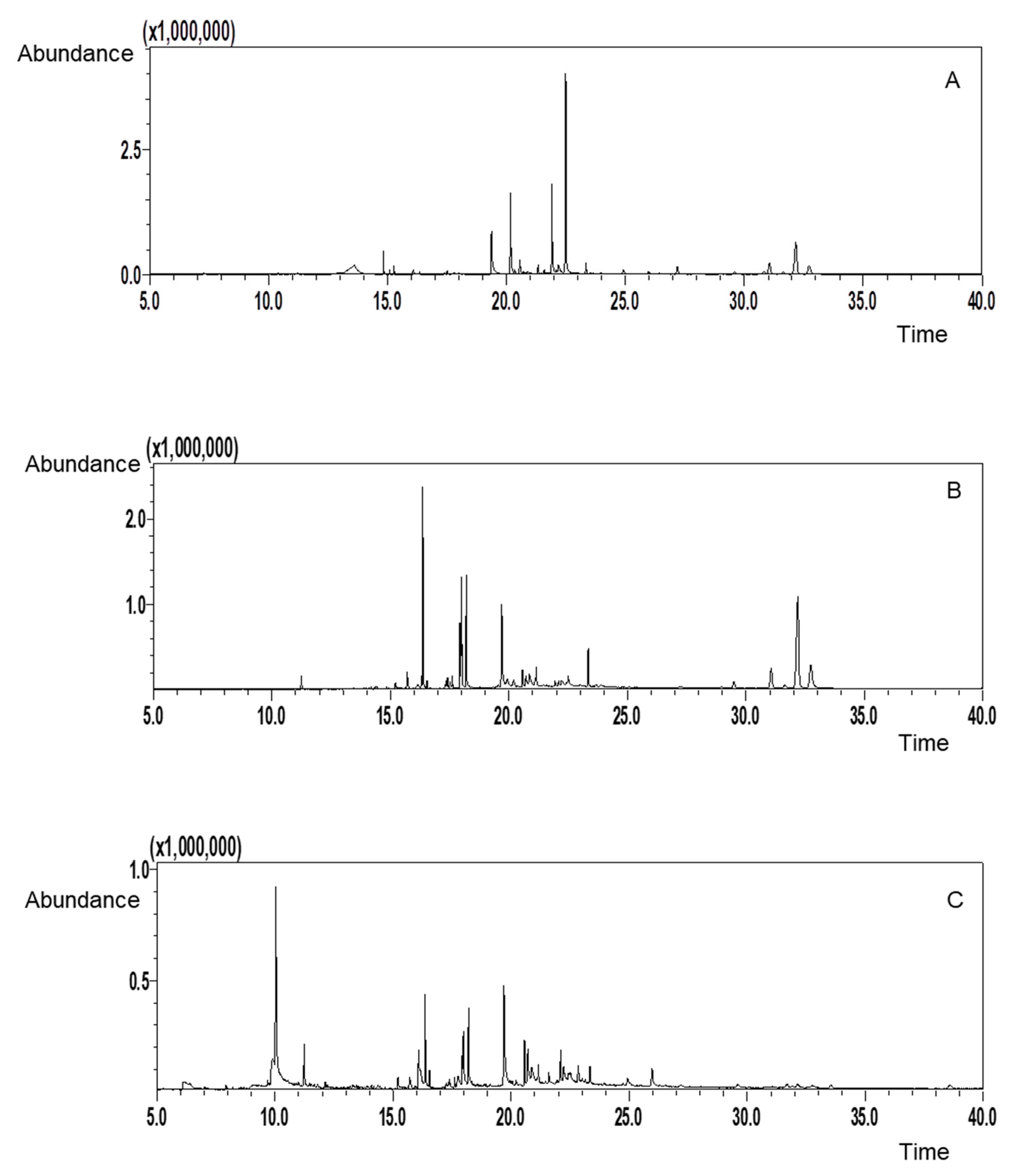
2.4. Identification of Active Compounds by GC-MS
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Raw Materials
4.3. Determination of Carbohydrate Composition and Chemical Characterization
4.4. Extraction of Bioactive Compounds Using Ultrasound-Assisted Extraction
4.5. Phenolic Compounds, Flavonoids, and Anthocyanin Content Evaluation
4.6. Determination of Antioxidant Activity Using the ABTS and DPPH Assays
4.7. Isolation and Purification of Bioactive Compounds via Liquid–Liquid Extraction (LLE)
4.8. Identification of Bioactive Compounds Using GC-MS
4.9. Statistical Analysis of Antioxidant Activity and Literature Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ABTS+ | ABTS radical cation |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GC-MS | Gas chromatography–mass spectrometry |
| FT-IR | Fourier transform infrared |
| TPC | Total phenolic content |
| GAE | Gallic acid equivalents |
| TFC | Total flavonoid content |
| QE | Quercetin equivalents |
| TAC | Total anthocyanin content |
| TEAC | Trolox equivalent antioxidant capacity |
| TE | Trolox equivalents |
| LLE | Liquid–liquid extraction |
| EtOAc | Ethyl acetate |
| SET | Single-electron transfer |
| HPLC | High-performance liquid chromatography |
| ATR | Attenuated total reflectance |
References
- Gargi, B.; Semwal, P.; Jameel Pasha, S.B.; Singh, P.; Painuli, S.; Thapliyal, A.; Cruz-Martins, N. Revisiting the Nutritional, Chemical and Biological Potential of Cajanus cajan (L.) Millsp. Molecules 2022, 27, 6877. [Google Scholar] [CrossRef]
- Gerrano, A.S.; Moalafi, A.; Seepe, H.A.; Amoo, S.; Shimelis, H. Nutritional and phytochemical compositions and their interrelationship in succulent pods of pigeonpea (Cajanus cajan [L.] Millsp.). Heliyon 2022, 8, e09078. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Crops and Livestock Products. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 27 October 2023).
- Locali-Pereira, A.R.; Boire, A.; Berton-Carabin, C.; Taboga, S.R.; Solé-Jamault, V.; Nicoletti, V.R. Pigeon Pea, An Emerging Source of Plant-Based Proteins. ACS Food Sci. Technol. 2023, 3, 1777–1799. [Google Scholar] [CrossRef]
- Olubodun-Obadun, T.G.; Ishola, I.O.; Folarin, O.R.; Oladoja, F.A.; Gilbert, T.T.; Aniekwensi, I.M.; Bisiriyu, A.; Joseph-Iwebi, N.A.; Adebanjo, F.O.; Olopade, J.O.; et al. Cajanus cajan (L) Millsp seeds extract prevents rotenone-induced motor- and non-motor features of Parkinson disease in mice: Insight into mechanisms of neuroprotection. J. Ethnopharmacol. 2024, 322, 117623. [Google Scholar] [CrossRef]
- Haji, A.; Teka, T.A.; Yirga Bereka, T.; Negasa Andersa, K.; Desalegn Nekera, K.; Geleta Abdi, G.; Lema Abelti, A.; Makiso Urugo, M. Nutritional Composition, Bioactive Compounds, Food Applications, and Health Benefits of Pigeon Pea (Cajanus cajan L. Millsp.): A Review. Legume Sci. 2024, 6, e233. [Google Scholar] [CrossRef]
- Oluwole, O.B.; Nicholas-Okpara, V.A.N.; Elemo, G.; Adeyoju, O.; Ibekwe, D.; Adegboyega, M.O. Medicinal uses, nutraceutical potentials and traditional farm production of Bambara beans and Pigeon pea. Glob. J. Epidemiol. Public. Health 2021, 6, 41–50. [Google Scholar] [CrossRef]
- Saxena, K.B.; Kumar, R.V.; Sultana, R. Quality nutrition through pigeonpea—A review. Health 2010, 2, 1335–1344. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Mukti, I.J.; Haque, A.K.M.F.; Mollik, M.A.H.; Parvin, K.; Jahan, R.; Chowdhury, M.H.; Rahman, T. An Ethnobotanical Survey and Pharmacological Evaluation of Medicinal Plants used by the Garo Tribal Community living in Netrakona district, Bangladesh. Adv. Nat. Appl. Sci. 2009, 3, 402–418. [Google Scholar]
- Upadhyay, B.; Parveen; Dhaker, A.K.; Kumar, A. Ethnomedicinal and ethnopharmaco-statistical studies of Eastern Rajasthan, India. J. Ethnopharmacol. 2010, 129, 64–86. [Google Scholar] [CrossRef]
- Pal, D.; Mishra, P.; Sachan, N.; Ghosh, A.K. Biological activities and medicinal properties of Cajanus cajan (L) Millsp. J. Adv. Pharm. Technol. Res. 2011, 2, 207–214. [Google Scholar] [CrossRef]
- Raveena-Devi, R.; Premalatha, R.; Saranya, A. Comparative analysis of phytochemical constituents and antibacterial activity of leaf, seed and root extract of Cajanus cajan (L.) Mill sp. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 485–494. [Google Scholar] [CrossRef]
- Agus, S.; Achmadi, S.S.; Mubarik, N.R. Antibacterial activity of naringenin-rich fraction of pigeon pea leaves toward Salmonella thypi. Asian Pac. J. Trop. Biomed. 2017, 7, 725–728. [Google Scholar] [CrossRef]
- Wang, X.; Yang, W.; Zhang, R.; He, Q.; Feng, L.; Yang, J.; Zhang, H.; Chen, B.; Chen, P.; Wang, Z. Cajanus cajan (L.) Millsp. Leaves: A Comprehensive Review of Phytochemistry, Pharmacological Properties, Safety, and Clinical Applications. Chem. Biodivers. 2025, e202500137. [Google Scholar] [CrossRef]
- Zahra, M.; Abrahamse, H.; George, B.P. Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants 2024, 13, 922. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A Comprehensive Review of Free Radicals, Oxidative Stress, and Antioxidants: Overview, Clinical Applications, Global Perspectives, Future Directions, and Mechanisms of Antioxidant Activity of Flavonoid Compounds. J. Chem. 2024, 2024, 5594386. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Yilwa, V.; Dikwa, K.; Emere, M.; Airoboman, P. Comparative assessment of the phytochemicals of the leaves and seeds of pigeon pea (Cajanus cajan (L.) Huth) plant. J. Adv. Sci. Eng. 2023, 8, 1–17. [Google Scholar]
- Pop, R.M.; Bocsan, I.C.; Buzoianu, A.D.; Chedea, V.S.; Socaci, S.A.; Pecoraro, M.; Popolo, A. Evaluation of the antioxidant activity of Nigella sativa L. and Allium ursinum extracts in a cellular model of doxorubicin-Induced cardiotoxicity. Molecules 2020, 25, 5259. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Kim, J.S. Antioxidant Activities of Selected Berries and Their Free, Esterified, and Insoluble-Bound Phenolic Acid Contents. Prev. Nutr. Food Sci. 2018, 23, 35–45. [Google Scholar] [CrossRef]
- Patel, N.K.; Bhutani, K.K. Pinostrobin and Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits TNF-α and IL-1β production: In vitro and in vivo experimentation. Phytomedicine 2014, 21, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Ogunbinu, A.O.; Flamini, G.; Cioni, P.L.; Adebayo, M.A.; Ogunwande, I.A. Constituents of Cajanus cajan (L.) Millsp., Moringa oleifera Lam., Heliotropium indicum L. and Bidens pilosa L. from Nigeria. Nat. Prod. Commun. 2009, 4, 573–578. [Google Scholar] [CrossRef]
- Anadebe, V.; Okafor, N.; Ezeugo, J.; Amanjide, I.; Ogide, B. GC-MS Analysis of phytochemical compounds in Cajanus cajan Leaf. J. Chem. Pharm. Res. 2017, 9, 360–363. [Google Scholar]
- Qi, X.-L.; Li, T.-T.; Wei, Z.-F.; Guo, N.; Luo, M.; Wang, W.; Zu, Y.-G.; Fu, Y.-J.; Peng, X. Solvent-free microwave extraction of essential oil from pigeon pea leaves [Cajanus cajan (L.) Millsp.] and evaluation of its antimicrobial activity. Ind. Crops Prod. 2014, 58, 322–328. [Google Scholar] [CrossRef]
- Huang, A.C.; Burrett, S.; Sefton, M.A.; Taylor, D.K. Production of the pepper aroma compound, (-)-rotundone, by aerial oxidation of α-guaiene. J. Agric. Food Chem. 2014, 62, 10809–10815. [Google Scholar] [CrossRef] [PubMed]
- Anggraeni, N.D.; Nurjanah, S.; Lembong, E. Uji Aktivitas Antibakteri α-guaiene Minyak Nilam terhadap Bakteri Staphylococcus aureus DAN Staphylococcus epidermidis. Gontor Agrotech. Sci. J. 2020, 6, 413–423. [Google Scholar] [CrossRef]
- Chaudhary, A.; Sood, S.; Das, P.; Kaur, P.; Mahajan, I.; Gulati, A.; Singh, B. Synthesis of novel antimicrobial aryl himachalene derivatives from naturally occurring himachalenes. Excli J. 2014, 13, 1216–1225. [Google Scholar]
- Duke, J.A. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economic Plants; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Maiolini, T.C.S.; Nicácio, K.J.; Rosa, W.; Miranda, D.O.; Santos, M.F.C.; Bueno, P.C.P.; Lago, J.H.G.; Sartorelli, P.; Dias, D.F.; Chagas de Paula, D.A.; et al. Potential anti-inflammatory biomarkers from Myrtaceae essential oils revealed by untargeted metabolomics. Nat. Prod. Res. 2023, 39, 985–992. [Google Scholar] [CrossRef]
- PubChem. PubChem Compound Summary for CID 5366244, 3,7,11,15-Tetramethyl-2-Hexadecen-1-OL. National Library of Medicine (US), National Center for Biotechnology Information, 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3_7_11_15-Tetramethyl-2-hexadecen-1-OL (accessed on 13 May 2024).
- Islam, M.T.; de Alencar, M.V.; da Conceição Machado, K.; da Conceição Machado, K.; de Carvalho Melo-Cavalcante, A.A.; de Sousa, D.P.; de Freitas, R.M. Phytol in a pharma-medico-stance. Chem. Biol. Interact. 2015, 240, 60–73. [Google Scholar] [CrossRef]
- Bobe, G.; Zhang, Z.; Kopp, R.; Garzotto, M.; Shannon, J.; Takata, Y. Phytol and its metabolites phytanic and pristanic acids for risk of cancer: Current evidence and future directions. Eur. J. Cancer Prev. 2020, 29, 191–200. [Google Scholar] [CrossRef]
- Kim, E.N.; Trang, N.M.; Kang, H.; Kim, K.H.; Jeong, G.S. Phytol Suppresses Osteoclast Differentiation and Oxidative Stress through Nrf2/HO-1 Regulation in RANKL-Induced RAW264.7 Cells. Cells 2022, 11, 3596. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.R.; Abdullah, N.; Ahmad, S.; Ismail, I.S.; Zakaria, M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. BMC Complement. Altern. Med. 2015, 15, 11. [Google Scholar] [CrossRef]
- Sagna, A.; Nair, R.V.R.; Hulyalkar, N.; Rajasekharan, S.; Nair, V.T.G.; Sivakumar, K.C.; Suja, S.R.; Baby, S.; Sreekumar, E. Ethyl palmitate, an anti-chikungunya virus principle from Sauropus androgynus, a medicinal plant used to alleviate fever in ethnomedicine. J. Ethnopharmacol. 2023, 309, 116366. [Google Scholar] [CrossRef]
- Ismail, N.Z.; Md Toha, Z.; Muhamad, M.; Nik Mohamed Kamal, N.N.S.; Mohamad Zain, N.N.; Arsad, H. Antioxidant Effects, Antiproliferative Effects, and Molecular Docking of Clinacanthus nutans Leaf Extracts. Molecules 2020, 25, 2067. [Google Scholar] [CrossRef] [PubMed]
- PubChem. PubChem Compound Summary for CID 6436081, Linolenyl alcohol. National Library of Medicine (US), National Center for Biotechnology Information, 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Linolenyl-alcohol (accessed on 13 May 2024).
- Cruz-Salomón, K.D.C.; Cruz-Rodríguez, R.I.; Espinosa-Juárez, J.V.; Cruz-Salomón, A.; Briones-Aranda, A.; Ruiz-Lau, N.; Ruíz-Valdiviezo, V.M. In Vivo and In Silico Study of the Antinociceptive and Toxicological Effect of the Extracts of Petiveria alliacea L. Leaves. Pharmaceuticals 2022, 15, 943. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, P.D.O.; Boleti, A.P.d.A.; Rüdiger, A.L.; Lourenço, G.A.; da Veiga Junior, V.F.; Lima, E.S. Anti-Inflammatory Activity of Triterpenes Isolated from Protium paniculatum Oil-Resins. Evid.-Based Complement. Altern. Med. 2015, 2015, 293768. [Google Scholar] [CrossRef]
- Oh, K.K.; Adnan, M.; Cho, D.H. Elucidating Drug-Like Compounds and Potential Mechanisms of Corn Silk (Stigma Maydis) against Obesity: A Network Pharmacology Study. Curr. Issues Mol. Biol. 2021, 43, 1906–1936. [Google Scholar] [CrossRef]
- Huang, J.; Yi, L.; Yang, X.; Zheng, Q.; Zhong, J.; Ye, S.; Li, X.; Li, H.; Chen, D.; Li, C. Neural stem cells transplantation combined with ethyl stearate improve PD rats motor behavior by promoting NSCs migration and differentiation. CNS Neurosci. Ther. 2023, 29, 1571–1584. [Google Scholar] [CrossRef]
- Cheng, L.; Ji, T.; Zhang, M.; Fang, B. Recent advances in squalene: Biological activities, sources, extraction, and delivery systems. Trends Food Sci. Technol. 2024, 146, 104392. [Google Scholar] [CrossRef]
- Combs, J.G.F.; McClung, J.P. Chapter 7—Vitamin E. In The Vitamins, 6th ed.; Combs, J.G.F., McClung, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 193–238. [Google Scholar]
- Usoro, O.B.; Mousa, S.A. Vitamin E Forms in Alzheimer’s Disease: A Review of Controversial and Clinical Experiences. Crit. Rev. Food Sci. Nutr. 2010, 50, 414–419. [Google Scholar] [CrossRef]
- Hensley, K.; Benaksas, E.J.; Bolli, R.; Comp, P.; Grammas, P.; Hamdheydari, L.; Mou, S.; Pye, Q.N.; Stoddard, M.F.; Wallis, G.; et al. New perspectives on vitamin E: γ-tocopherol and carboxyethylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004, 36, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shobana, R.; Ranjitha, J.; Anand, M.; Mahboob, S.; Vijayalakshmi, S. Chapter 8—Microbial production of hydrocarbon and its derivatives using different kinds of microorganisms. In Valorization of Biomass to Bioproducts; Gupta, V.K., Tuohy, M., Ramteke, P., Nguyen, Q., Bhat, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 137–149. [Google Scholar]
- Shakeel, T.; Fatma, Z.; Fatma, T.; Yazdani, S.S. Heterogeneity of Alkane Chain Length in Freshwater and Marine Cyanobacteria. Front. Bioeng. Biotechnol. 2015, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Mesa, T.; Munné-Bosch, S. α-Tocopherol in chloroplasts: Nothing more than an antioxidant? Curr. Opin. Plant Biol. 2023, 74, 102400. [Google Scholar] [CrossRef]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Kwun, M.S.; Lee, H.J.; Lee, D.G. β-amyrin-induced apoptosis in Candida albicans triggered by calcium. Fungal Biol. 2021, 125, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kwon, H.; Lee, J.H.; Cho, E.; Lee, Y.C.; Moon, M.; Jun, M.; Kim, D.H.; Jung, J.W. β-Amyrin Ameliorates Alzheimer’s Disease-Like Aberrant Synaptic Plasticity in the Mouse Hippocampus. Biomol. Ther. 2020, 28, 74–82. [Google Scholar] [CrossRef]
- Wu, H.; Xu, F.; Huang, X.; Li, X.; Yu, P.; Zhang, L.; Yang, X.; Kong, J.; Zhen, C.; Wang, X. Lupenone improves type 2 diabetic nephropathy by regulating NF-κB pathway-mediated inflammation and TGF-β1/Smad/CTGF-associated fibrosis. Phytomedicine 2023, 118, 154959. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.; Sun, K.; Kong, F.; Jiang, X.; Kong, Q. Lupenone improves motor dysfunction in spinal cord injury mice through inhibiting the inflammasome activation and pyroptosis in microglia via the nuclear factor kappa B pathway. Neural Regen. Res. 2024, 19, 1802–1811. [Google Scholar] [CrossRef]
- Li, D.; Guo, Y.Y.; Cen, X.F.; Qiu, H.L.; Chen, S.; Zeng, X.F.; Zeng, Q.; Xu, M.; Tang, Q.Z. Lupeol protects against cardiac hypertrophy via TLR4-PI3K-Akt-NF-κB pathways. Acta Pharmacol. Sin. 2022, 43, 1989–2002. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xie, L.; Deng, M.; Chen, H.; Song, J.; Long, J.; Li, X.; Luo, J. Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res. 2021, 164, 105373. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Kaari, M.; Joseph, J.; Manikkam, R.; Kalyanasundaram, R.; Sivaraj, A.; Anbalmani, S.; Murthy, S.; Sahu, A.K.; Said, M.; Dastager, S.G. A novel finding: 2, 4-Di-tert-butylphenol from Streptomyces bacillaris ANS2 effective against Mycobacterium tuberculosis and cancer cell lines. Appl. Biochem. Biotechnol. 2023, 195, 6572–6585. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhao, H.; Liu, S.; Tian, C.; Gao, M.; Wang, Y.; Dong, J.; Zhang, L. 2,4-Di-tert-butylphenol and 7-hydroxy-3-(2-methylpropyl)-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione: Two natural products from Serratia marcescens Ha1 and their herbicidal activities. Pest. Manag. Sci. 2024, 80, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Skanda, S.; Vijayakumar, B.S. Antioxidant and Anti-inflammatory Metabolites of a Soil-Derived Fungus Aspergillus arcoverdensis SSSIHL-01. Curr. Microbiol. 2021, 78, 1317–1323. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Cho, H.-Y.; Lee, Y.-B. Human risk assessment of di-isobutyl phthalate through the application of a developed physiologically based pharmacokinetic model of di-isobutyl phthalate and its major metabolite mono-isobutyl phthalate. Arch. Toxicol. 2021, 95, 2385–2402. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Jang, J.-H.; Cho, H.-Y.; Lee, Y.-B. Toxicokinetic studies of di-isobutyl phthalate focusing on the exploration of gender differences in rats. Chemosphere 2022, 286, 131706. [Google Scholar] [CrossRef]
- Wu, C.Y.-C.; e Silva, A.C.; Citadin, C.T.; Clemons, G.A.; Acosta, C.H.; Knox, B.A.; Grames, M.S.; Rodgers, K.M.; Lee, R.H.-C.; Lin, H.W. Palmitic acid methyl ester inhibits cardiac arrest-induced neuroinflammation and mitochondrial dysfunction. Prostaglandins Leukot. Essent. Fat. Acids 2021, 165, 102227. [Google Scholar] [CrossRef]
- Hamed, A.; Mantawy, E.; El-Bakly, W.; Abdel-Mottaleb, Y.; Azab, S. Methyl palmitate: The naturally occurring cardioprotective agent. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 47–62. [Google Scholar] [CrossRef]
- Kamariah Bakar, K.B.; Habsah Mohamad, H.M.; Jalifah Latip, J.L.; Tan HockSeng, T.H.; Herng GanMing, H.G. Fatty acids compositions of Sargassum granuliferum and Dictyota dichotoma and their anti-fouling activities. J. Sustain. Sci. Manag. 2017, 12, 8–16. [Google Scholar]
- von der Ohe, P.; Aalizadeh, R. S13|EUCOSMETICS|Combined Inventory of Ingredients Employed in Cosmetic Products (2000) and Revised Inventory (2006) (NORMAN-SLE-S13.0.1.3). Zenodo 2020. [Google Scholar] [CrossRef]
- Peng, J.; Chen, Z.; Chen, X.; Zheng, R.; Lu, S.; Seyab, M.; Yang, F.; Li, Q.; Tang, Q. Insecticidal potential of a Consolida ajacis extract and its major compound (ethyl linoleate) against the diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2023, 195, 105557. [Google Scholar] [CrossRef]
- Ko, G.A.; Kim Cho, S. Ethyl linoleate inhibits α-MSH-induced melanogenesis through Akt/GSK3β/β-catenin signal pathway. Korean J. Physiol. Pharmacol. 2018, 22, 53–61. [Google Scholar] [CrossRef]
- Maya-López, M.; Rubio-López, L.C.; Rodríguez-Alvarez, I.V.; Orduño-Piceno, J.; Flores-Valdivia, Y.; Colonnello, A.; Rangel-López, E.; Túnez, I.; Prospéro-García, O.; Santamaría, A. A Cannabinoid Receptor-Mediated Mechanism Participates in the Neuroprotective Effects of Oleamide Against Excitotoxic Damage in Rat Brain Synaptosomes and Cortical Slices. Neurotox. Res. 2020, 37, 126–135. [Google Scholar] [CrossRef]
- Camp, J.E.; Greatrex, B.W. Levoglucosenone: Bio-Based Platform for Drug Discovery. Front. Chem. 2022, 10, 902239. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.; Patel, R.; Hazarika, H.; Dey, P.; Goswami, D.; Chattopadhyay, P. Chemical composition and bioefficacy for larvicidal and pupicidal activity of essential oils against two mosquito species. Int. J. Mosq. Res. 2017, 4, 112–118. [Google Scholar]
- Đukić, N.; Andrić, G.; Glinwood, R.; Ninkovic, V.; Andjelković, B.; Radonjić, A. The effect of 1-pentadecene on Tribolium castaneum behaviour: Repellent or attractant? Pest. Manag. Sci. 2021, 77, 4034–4039. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Wu, G.; Libby, M.; Wu, K.; Jeong, K.J.; Kim, Y.J. Synthesis of biologically derived poly(pyrogallol) nanofibers for antibacterial applications. J. Mater. Chem. B 2023, 11, 3356–3363. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, G.; Chen, W.; Chen, J.; Li, Z.; Zheng, X.; Yin, H.; Hu, X. Pyrogallol and Fluconazole Interact Synergistically In Vitro against Candida glabrata through an Efflux-Associated Mechanism. Antimicrob. Agents Chemother. 2021, 65, e0010021. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, Y.H.; Han, G.U.; Kim, S.G.; Bhang, D.H.; Kim, B.G.; Moon, S.H.; Shin, S.H.; Ryu, B.Y. Diisobutyl phthalate (DiBP)-induced male germ cell toxicity and its alleviation approach. Food Chem. Toxicol. 2024, 184, 114387. [Google Scholar] [CrossRef]
- Moharana, M.; Pattanayak, S.K. Chapter 3—Biosensors: A better biomarker for diseases diagnosis. In Smart Biosensors in Medical Care; Chaki, J., Dey, N., De, D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 49–64. [Google Scholar]
- Czubacka, E.; Czerczak, S.; Kupczewska-Dobecka, M. The overview of current evidence on the reproductive toxicity of dibutyl phthalate. Int. J. Occup. Med. Environ. Health 2021, 34, 15–37. [Google Scholar] [CrossRef]
- Yan, Y.; Zhu, F.; Zhu, C.; Chen, Z.; Liu, S.; Wang, C.; Gu, C. Dibutyl phthalate release from polyvinyl chloride microplastics: Influence of plastic properties and environmental factors. Water Res. 2021, 204, 117597. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gao, Y.; Lin, J.; Liu, F.; Yang, L.; Zhou, J.; Xue, Y.; Li, Y.; Chang, Z.; Li, J.; et al. Dietary elaidic acid boosts tumoral antigen presentation and cancer immunity via ACSL5. Cell Metab. 2024, 36, 822–838.e8. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, H.; Fujii, K.; Kadochi, Y.; Mori, S.; Nishiguchi, Y.; Fujiwara, R.; Kishi, S.; Sasaki, T.; Kuniyasu, H. Elaidic acid, a trans-fatty acid, enhances the metastasis of colorectal cancer cells. Pathobiology 2017, 84, 144–151. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Comparison of surface tension, density, viscosity and contact angle of ethyl oleate to those of ethanol and oleic acid. J. Mol. Liq. 2024, 400, 124525. [Google Scholar] [CrossRef]
- El-Sharif, H.F.; Patel, S.; Ndunda, E.N.; Reddy, S.M. Electrochemical detection of dioctyl phthalate using molecularly imprinted polymer modified screen-printed electrodes. Anal. Chim. Acta 2022, 1196, 339547. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Sun, Y.; Jiang, X.; Liu, T.; Kang, B.; Freguia, S.; Feng, L.; Chen, Y. Dioctyl phthalate enhances volatile fatty acids production from sludge anaerobic fermentation: Insights of electron transport and metabolic functions. Sci. Total Environ. 2023, 859, 160102. [Google Scholar] [CrossRef]
- Luo, Y.M.; Guo, H.Y.; Wang, Z.J.; Liu, Z.R. Effects of Dioctyl Phthalate on Performance of Asphalt Sealant. Adv. Mater. Sci. Eng. 2022, 2022, 5385586. [Google Scholar] [CrossRef]
- Sahu, M.; Verma, D.; Harris, H. Phytochemicalanalysis of the leaf, stem and seed extracts of Cajanus cajan L (dicotyledoneae: Fabaceae. World J. Pharm. Pharm. Sci. 2014, 3, 694–733. [Google Scholar]
- Yang, S.-E.; Vo, T.-L.T.; Chen, C.-L.; Yang, N.-C.; Chen, C.-I.; Song, T.-Y. Nutritional composition, bioactive compounds and functional evaluation of various parts of Cajanus cajan (L.) Millsp. Agriculture 2020, 10, 558. [Google Scholar] [CrossRef]
- Monrroy, M.; Araúz, O.; García, J.R. Active Compound Identification in Extracts of N. lappaceum Peel and Evaluation of Antioxidant Capacity. J. Chem. 2020, 2020, 4301891. [Google Scholar] [CrossRef]
- Devi, R.R.; Premalatha, R.; Kayathri, R. Evaluation of total phenols, total flavonoids and in vitro antioxidant activity in the ethanolic leaf, seed and root extract of Cajanus cajan (L.) Mill sp. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 688–697. [Google Scholar] [CrossRef]
- Aja, P.M.; Alum, E.U.; Ezeani, N.N.; Nwali, B.U.; Edwin, N. Comparative Phytochemical Composition of Cajanus cajan Leaf and Seed. Int. J. Microbiol. Res. 2015, 6, 42–46. [Google Scholar]
- Sayem, A.S.M.; Ahmed, T.; Mithun, M.U.K.; Rashid, M.; Rana, M.R. Optimising ultrasound-assisted extraction conditions for maximising phenolic, flavonoid content and antioxidant activity in hog plum peel and seed: A response surface methodology approach. J. Agric. Food Res. 2024, 18, 101312. [Google Scholar] [CrossRef]
- Alum, E.U. Climate change and its impact on the bioactive compound profile of medicinal plants: Implications for global health. Plant Signal Behav. 2024, 19, 2419683. [Google Scholar] [CrossRef] [PubMed]
- Klimienė, A.; Klimas, R.; Shutava, H.; Razmuvienė, L. Dependence of the Concentration of Bioactive Compounds in Origanum vulgare on Chemical Properties of the Soil. Plants 2021, 10, 750. [Google Scholar] [CrossRef]
- Salem, O.; Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Szablewski, T.; Cegielska-Radziejewska, R.; Świerk, D.; Stuper-Szablewska, K. New Insights into Bioactive Compounds of Wild-Growing Medicinal Plants. Appl. Sci. 2023, 13, 13196. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Ariviani, S.; Nastiti, G.P. Investigation of the sensory quality, nutritional value and antioxidant capacity of flakes prepared using various pigeon pea-based flours. Food Res. 2024, 8, 30–37. [Google Scholar] [CrossRef]
- Maneechai, S.; Rinthong, P.; Pumyen, K.; Koonkratok, P.; Sripirom, C. Free radical scavenging activity of extracts from Cajanus cajan (L.) Millsp. Planta Med. 2013, 79, PN59. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Monrroy, M.; Garcia, J.R.; Troncoso, E.; Freer, J. Fourier transformed near infrared (FT-NIR) spectroscopy for the estimation of parameters in pretreated lignocellulosic materials for bioethanol production. J. Chem. Technol. Biotechnol. 2015, 90, 1281–1289. [Google Scholar] [CrossRef]
- Ullah Khan, S.; Ullah, F.; Hussain, M.; Zahid Ihsan, M.; Mehmood, S.; Ali, L.; Saud, S.; Fahad, S.; Hassan, S.; Zeeshan, M.; et al. Phytochemical analysis and phytotoxic evaluation of Chenopodium glaucum L. J. King Saud. Univ. Sci. 2023, 35, 102571. [Google Scholar] [CrossRef]
- Xie, J.-H.; Dong, C.-j.; Nie, S.-P.; Li, F.; Wang, Z.-J.; Shen, M.-Y.; Xie, M.-Y. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]


| Compounds | Compounds Type | Mol. Formula | Tr (Min) | Peak Area (%) | Mol. Wt. (g mol−1) | Top Peak | Frag. Ions (m/z) 2nd and 3rd Highest | Extract |
|---|---|---|---|---|---|---|---|---|
| α-Guaiene | Sesquiterpenes | C15H24 | 10.41 | 0.2 | 204 | 105 | 147 and 107 | Crude |
| (−)-α-Himachalene | Sesquiterpenes | C15H24 | 10.61 | 0.2 | 204 | 189 | 119 and 105 | Crude |
| cis-(−)-2,4a,5,6,9a-Hexahydro-3,5,5,9-tetramethyl(1H) benzocycloheptene | Aromatic hydrocarbon | C15H24 | 10.96 | 0.1 | 204 | 93 | 133 and 105 | Crude |
| α -Selinene | Sesquiterpenes | C15H24 | 11.17 | 0.1 | 204 | 189 | 93 and 204 | Crude |
| Phytol | Acyclic diterpene alcohol | C20H40O | 15.27 | 0.9 | 296 | 82 | 81 and 95 | Crude |
| Hexadecanoic acid | Phenolic acid, saturated fatty acid | C16H32O2 | 16.09 | 1.4 and 4.1 | 256 | 73 | 60 and 55 | Crude and LLE with hexane |
| Ethyl palmitate | Fatty acid ethyl ester | C18H36O2 | 16.37 | 0.2, 13.3 and 5.9 | 285 | 88 | 101 and 55 | Crude and LLE with EtOAc and hexane |
| (E,7R,11R)-Phytol | Diterpenoid | C20H40O | 17.53 | 0.5 | 296 | 71 | 123 and 57 | Crude and LLE with EtOAc |
| Linolenyl alcohol | Fatty primary alcohol | C18H30O2 | 17.80 | 0.5 | 278 | 79 | 55 and 95 | Crude |
| Ethyl linolenate | Fatty acid ethyl ester | C20H34O2 | 18.02 | 0.2 and 8.1 | 306 | 79 | 67 and 95 | Crude and LLE with EtOAc |
| Ethyl stearate | Fatty acid ethyl ester | C20H40O2 | 18.22 | 0.1, 6.8 and 5.2 | 312 | 88 | 101 and 55 | Crude and LLE with EtOAc and hexane |
| 1-Piperidineacetonitrile,. alpha. -styryl | Heterocyclic | C15H18N2 | 19.39 | 9.2 | 226 | 226 | 225 and 165 | Crude |
| Pinostrobin chalcone | Chalcones | C16H14O4 | 20.19 | 10.9 | 270 | 270 | 193 and 166 | Crude |
| 4-(4-Methoxyphenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline | Tetrahydroisoquinolines | C29H50O2 | 20.90 | 0.8 | 299 | 98 | 239 and 57 | Crude |
| Squalene | Triterpenoid | C30H50 | 23.37 | 1.4, 2.8 and 1.04 | 410 | 69 | 81 and 95 | Crude and LLE with EtOAc and hexane |
| γ-Tocopherol/Vitamin E | Tocopherol | C28H48O2 | 26.00 | 0.8 and 2.0 | 416 | 416 | 151 and 417 | Crude and LLE with hexane |
| n-Heptadecane | Alkane | C17H36 | 26.44 | 0.2 | 240 | 57 | 71 and 85 | Crude |
| Vitamin E | Tocopherol | C29H50O2 | 27.22 | 1.8 | 431 | 430 | 165 and 432 | Crude |
| β-Amyrin | Pentacyclic triterpenoid | C30H50O | 31.00 | 3.5 and 0.7 | 426 | 218 | 203 and 219 | Crude and LLE with EtOAc |
| Lup-20(29)-en-3-one | Triterpenoid | C30H48O | 32.16 | 12.7 and 21.0 | 425 | 205 | 109 and 424 | Crude and LLE with EtOAc |
| Lupeol | Pentacyclic triterpenoid | C30H50O | 32.77 | 3.4 and 6.6 | 426 | 189 | 218 and 207 | Crude and LLE with EtOAc |
| 2,4-Di-tert-butylphenol | Phenol | C14H22O | 11.24 | 1.3 and 2.9 | 206 | 191 | 206 and 57 | LLE with EtOAc and hexane |
| Di-isobutyl phthalate | Phthalate ester | C16H22O4 | 15.20 | 0.4 | 278 | 149 | 223 and 57 | LLE with EtOAc |
| Methyl palmitate | Fatty acid methyl ester | C17H34O2 | 15.73 | 1.8 and 1.6 | 270 | 74 | 87 and 55 | LLE with EtOAc and hexane |
| Ethyl 9-hexadecenoate | Fatty acid ester | C18H34O2 | 16.30 | 1.0 | 282 | 55 | 88 and 96 | LLE with EtOAc |
| 8,11-Octadecadienoic acid, methyl ester | Fatty acid methyl ester | C19H34O2 | 17.33 | 0.8 | 294 | 67 | 81 and 55 | LLE with EtOAc |
| cis-11,14,17-Eicosatrienoic acid methyl ester | Fatty acid methyl ester | C21H36O2 | 17.40 | 1.0 | 320 | 79 | 67and 95 | LLE with EtOAc |
| Methyl isostearate | Ester | C19H38O2 | 17.60 | 0.7 and 0.5 | 298 | 74 | 87 and 298 | LLE with EtOAc and hexane |
| Ethyl linoleate | Fatty acid ethyl ester | C20H36O2 | 17.93 | 4.4 and 1.9 | 308 | 67 | 81 and 95 | LLE with EtOAc and hexane |
| Oleamide | Fatty amide | C18H35NO | 19.72 | 7.8 and 13 | 218 | 59 | 72 and 55 | LLE with EtOAc and hexane |
| 9,12-Octadecadienoic acid, ethyl ester | Fatty acid ethyl esters | C19H38O4 | 20.85 | 2.9 | 330 | 98 | 239 ad 57 | LLE with EtOAc |
| Monoethylhexyl phthalic acid | Mono(2-ethylhexyl) ester of benzene-1,2-dicarboxylic acid | C16H22O4 | 21.10 | 2.4 | 278 | 149 | 167 and 57 | LLE with EtOAc |
| β-amyrone | Pentacyclic triterpenes | C30H48O | 31.05 | 4.2 | 424 | 218 | 203 and 219 | LLE with EtOAc |
| Levoglucosenone | Anhydrohexose and a deoxyketohexose | C6H6O3 | 6.15 | 1.4 | 126 | 98 | 96 and 68 | LLE with hexane |
| Benzene, 1,3-bis(1,1-dimethylethyl)- | Phenylpropanes | C14H22 | 7.90 | 0.2 | 190 | 175 | 57 and 90 | LLE with hexane |
| 1-Pentadecene | Alkene | C15H30 | 9.68 | 0.4 | 210 | 55 | 83 and 69 | LLE with hexane |
| Pyrogallol | Phenolic | C6H6O3 | 9.91 | 6.4 | 126 | 126 | 52 and 80 | LLE with hexane |
| 1-Nonadecene | Alkene | C19H38 | 12.10 | 0.3 | 266 | 83 | 55 and 97 | LLE with hexane |
| Di-isobutyl phthalate | Phthalate ester | C16H22O4 | 15.20 | 1.1 | 278 | 149 | 57 and 150 | LLE with hexane |
| Dibutyl phthalate | Phthalate ester | C16H22O4 | 16.15 | 2.8 | 278 | 149 | 150 and 205 | LLE with hexane |
| 8-Octadecenoic acid, methyl ester | Oleic acid methyl ester | C19H36O2 | 17.38 | 0.6 | 296 | 55 | 74 and 69 | LLE with hexane |
| Elaidic acid | Trans-isomer of oleic acid | C18H34O2 | 17.77 | 2.4 | 282 | 55 | 69 and 83 | LLE with hexane |
| Ethyl oleate | Fatty acid ethyl ester | C20H38O2 | 18.00 | 4.9 | 310 | 55 | 69 and 83 | LLE with hexane |
| Dioctyl phthalate | Phthalate ester | C24H38O4 | 21.15 | 1.4 | 390 | 149 | 167 and 279 | LLE with hexane |
| Compound | Properties/Use | References |
|---|---|---|
| α-Guaiene | Precursor to rotundone (peppery aroma and flavor). Possesses antimicrobial activity. | [26,27] |
| (−)-α-Himachalene | Antimicrobial agent | [28] |
| α-selinene | Anti-plasmodial and anti-inflammatory biomarkers. | [29,30] |
| Phytol | Precursor of vitamin E and vitamin K1. Modulates transcription in cells via transcription factors PPAR-alpha and retinoid X receptor (RXR). Flavoring agent. Antimicrobial, cytotoxic, antitumor, antimutagenic, anti-teratogenic, antibiotic-chemotherapeutic, antidiabetic, lipid lowering, antispasmodic, anticonvulsant, antinociceptive, antioxidant, anti-inflammatory, anxiolytic, antidepressant, immunoadjuvancy, hair growth facilitator, hair fall defense and antidandruff activities. Inhibits osteoclast differentiation. | [31,32,33,34] |
| Hexadecanoic acid | Anti-inflammatory agent | [35] |
| Ethyl palmitate | Antiviral agent | [36] |
| Linolenyl alcohol | Antibacterial and anticancer agent | [37,38] |
| Ethyl linolenate | Antinociceptive activity | [39] |
| β-amyrone | Anti-inflammatory and anti-obesity agent | [40,41] |
| Ethyl stearate | Protective effect against the neurotoxin 6-hydroxydopamine | [42] |
| Squalene | Antitumor, immunity enhancement, antioxidant, detoxifier, skin senility resistance, hypolipidemic, and antibacterial activities. | [43] |
| γ-tocopherol | Antioxidant, anti-inflammatory, cancer prevention, and contributions to natriuresis. | [44,45,46] |
| Heptadecane | Potential biofuel precursor | [47,48] |
| D-α-Tocopherol | Antioxidant and modulates lipid peroxidation. | [44,49,50] |
| β-Amyrin | Antimicrobial activity and protects against Alzheimer’s disease. | [51,52] |
| Lupenone | Innovative drug for preventing and treating diabetic nephropathy. Anti-inflammatory agent | [53,54] |
| Lupeol | Anti-inflammatory, antiapoptotic, anticancer, antioxidant, and antimicrobial activities. Protective effects on cardiovascular diseases. | [55,56] |
| 2,4-Di-tert-butylphenol | Antitubercular, anticancer, antioxidant, anti-inflammatory, insecticidal, herbicidal, and antimicrobial activity. Exhibits broad toxicity in human and animal cells. | [57,58,59,60] |
| Di-isobutyl phthalate | Human health risks, including fetal toxicity. | [61,62] |
| Methyl palmitate | Neuroprotective and cardioprotective activity. | [63,64] |
| cis-11,14,17-Eicosatrienoic acid methyl ester | Antifouling activity. | [65] |
| Methyl isostearate | Skin conditioning agent Emulsifier | [66] |
| Ethyl Linoleate | Insecticidal, antibacterial, and anti-inflammatory activity. | [67,68] |
| Oleamide | Neuroprotective effects. | [69] |
| Levoglucosenone | Bio-based platform for drug discovery. | [70] |
| 1,3-ditert-butylbenzene | Mosquitocidal activity. | [71] |
| 1-Pentadecene | Insect repellent. | [72] |
| Pyrogallol | Antibacterial and antifungal agent. | [73,74] |
| 1-Nonadecene | Anti-inflammatory activity | [60] |
| Di-isobutyl phthalate | Plasticizer and anti-androgenic effects. | [75] |
| Dibutyl phthalate | Biosensors, plasticizers, and endocrine disruptor. | [76,77,78] |
| Elaidic acid | Boosts tumoral antigen presentation and cancer immunity. Treatment of colorectal cancer. | [79,80] |
| Ethyl oleate | Promotes the drying of fruits, vegetables, and grains Effective microemulsion ingredient to increase the bioavailability of a drug Gasoline additive | [81] |
| Dioctyl phthalate | Plasticizer | [82,83,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monrroy, M.; García, J.R. Phytochemical Characterization and Antioxidant Activity of Cajanus cajan Leaf Extracts for Nutraceutical Applications. Molecules 2025, 30, 1773. https://doi.org/10.3390/molecules30081773
Monrroy M, García JR. Phytochemical Characterization and Antioxidant Activity of Cajanus cajan Leaf Extracts for Nutraceutical Applications. Molecules. 2025; 30(8):1773. https://doi.org/10.3390/molecules30081773
Chicago/Turabian StyleMonrroy, Mariel, and José Renán García. 2025. "Phytochemical Characterization and Antioxidant Activity of Cajanus cajan Leaf Extracts for Nutraceutical Applications" Molecules 30, no. 8: 1773. https://doi.org/10.3390/molecules30081773
APA StyleMonrroy, M., & García, J. R. (2025). Phytochemical Characterization and Antioxidant Activity of Cajanus cajan Leaf Extracts for Nutraceutical Applications. Molecules, 30(8), 1773. https://doi.org/10.3390/molecules30081773









