Bacteriophages Improve the Effectiveness of Rhamnolipids in Combating the Biofilm of Candida albicans
Abstract
1. Introduction
2. Results and Discussion
2.1. Critical Micelle Concentration (CMC)
2.2. Influence of RLs on the Activity of Bacteriophages
2.3. Antiadhesive Properties of RLs, Bacteriophages and Their Combination
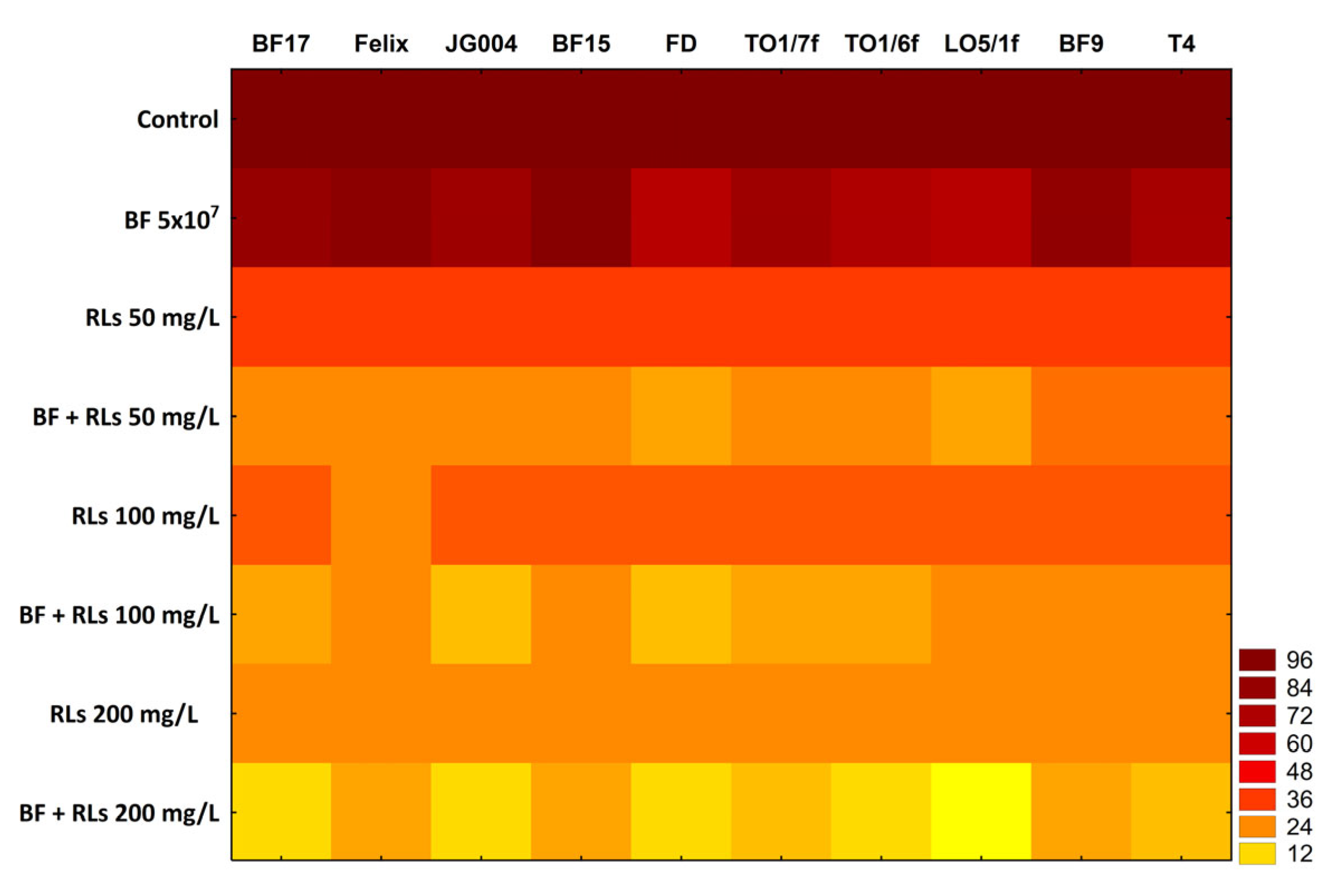
2.4. Anti-Biofilm Properties of RLs, Bacteriophages and Their Mixtures
2.5. Expression of Genes Responsible for Biofilm Formation by C. albicans in the Presence of RLs, Phages, and Their Combinations
2.6. Microscopic Observation of Hyphae Formation by Candida Cells
3. Materials and Methods
3.1. Strains, Media, and Compounds
3.2. Critical Micelle Concentration (CMC)
3.3. Bacteriophages
3.4. Amplification of Bacteriophages
3.5. Influence of RLs on the Activity of Bacteriophages
3.6. Bacterial Control Lysates
3.7. Effect of Rhamnolipids and Bacteriophages on the Growth of Candida albicans
3.8. Anti-Adhesion Assays
3.9. In Vitro Anti-Biofilm Assay
3.10. Quantification of Gene Expression by Quantitative Real-Time PCR (qRT-PCR)
3.11. Hypha Formation
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talapko, M.J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The virulence factors and clinical manifestations of infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Massey, J.; Zarnowski, R.; Andes, D. Role of the extracellular matrix in Candida biofilm antifungal resistance. FEMS Microbiol. Rev. 2023, 47, fuad059. [Google Scholar] [CrossRef]
- Ajetunmobi, O.H.; Badali, H.; Romo, J.A.; Ramage, G.; Lopez-Ribot, J.L. Antifungal therapy of Candida biofilms: Past, present and future. Biofilm 2023, 5, 100126. [Google Scholar] [CrossRef]
- Ohadi, M.; Forootanfar, H.; Dehghannoudeh, N.; Banat, I.M.; Dehghannoudeh, G. The role of surfactants and biosurfactants in the wound healing process: A review. J. Wound Care 2023, 32 (Suppl. S4a), xxxix–xlvi. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I. M Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Vieira, I.M.M.; Santos, B.L.P.; Ruzene, D.S.; Silva, D.P. An overview of current research and developments in biosurfactants. J. Ind. Eng. Chem. 2021, 100, 1–18. [Google Scholar] [CrossRef]
- Sanches, M.A.; Luzeiro, I.G.; Alves Cortez, A.C.; Simplício de Souza, É.; Albuquerque, P.M.; Chopra, H.K.; Braga de Souza, J.V. Production of biosurfactants by Ascomycetes. Int. J. Microbiol. 2021, 2021, 6669263. [Google Scholar] [CrossRef] [PubMed]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Giri, S.S.; Ryu, E.; Sukumaran, V.; Park, S.C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019, 132, 66–72. [Google Scholar] [CrossRef]
- Kumar, R.; Barbhuiya, R.I.; Bohra, V.; Wong, J.W.; Singh, A.; Kaur, G. Sustainable rhamnolipids production in the next decade–advancing with Burkholderia thailandensis as a potent biocatalytic strain. Microbiol. Res. 2023, 272, 127386. [Google Scholar] [CrossRef]
- Li, Q. Rhamnolipid synthesis and production with diverse resources. Front. Chem. Sci. Eng. 2017, 11, 27–36. [Google Scholar] [CrossRef]
- Esposito, R.; Speciale, I.; De Castro, C.; D’Errico, G.; Russo Krauss, I. Rhamnolipid Self-Aggregation in Aqueous Media: A Long Journey toward the Definition of Structure–Property Relationships. Int. J. Mol. Sci. 2023, 24, 5395. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tiwari, P.; Pandey, L.M. Surface, interfacial and thermodynamic aspects of the Rhamnolipid-salt systems. J. Mol. Liq. 2023, 384, 122245. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Jończyk-Matysiak, E.; Łodej, N.; Kula, D.; Owczarek, B.; Orwat, F.; Międzybrodzki, R.; Neuberg, J.; Bagińska, N.; Weber-Dąbrowska, B.; Górski, A. Factors determining phage stability/activity: Challenges in practical phage application. Expert Rev. Anti-Infect. Ther. 2019, 17, 583–606. [Google Scholar] [CrossRef]
- Górski, A.; Borysowski, J.; Miȩdzybrodzki, R. Bacteriophage interactions with epithelial cells: Therapeutic implications. Front. Microbiol. 2021, 11, 631161. [Google Scholar] [CrossRef] [PubMed]
- Bichet, M.C.; Adderley, J.; Avellaneda-Franco, L.; Magnin-Bougma, I.; Torriero-Smith, N.; Gearing, L.J.; Deffrasnes, C.; David, C.; Pepin, G.; Gantier, M.P.; et al. Mammalian cells internalize bacteriophages and use them as a resource to enhance cellular growth and survival. PLoS Biol. 2023, 21, e3002341. [Google Scholar] [CrossRef]
- Nazik, H.; Joubert, L.M.; Secor, P.R.; Sweere, J.M.; Bollyky, P.L.; Sass, G.; Cegelski, L.; Stevens, D.A. Pseudomonas phage inhibition of Candida albicans. Microbiology 2017, 163, 1568–1577. [Google Scholar] [CrossRef]
- Penner, J.C.; Ferreira, J.A.; Secor, P.R.; Sweere, J.M.; Birukova, M.K.; Joubert, L.M.; Haagensen, J.A.J.; Garcia, O.; Malkovskiy, A.V.; Kaber, G.; et al. Pf4 bacteriophage produced by Pseudomonas aeruginosa inhibits Aspergillus fumigatus metabolism via iron sequestration. Microbiology 2016, 162, 1583–1594. [Google Scholar] [CrossRef]
- Havenga, B.; Reyneke, B.; Waso-Reyneke, M.; Ndlovu, T.; Khan, S.; Khan, W. Biological Control of Acinetobacter baumannii: In Vitro and in vivo activity, limitations, and combination therapies. Microorganisms 2022, 10, 1052. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Lin, J.; Wang, W.; Li, S. High-yield di-rhamnolipid production by Pseudomonas aeruginosa YM4 and its potential application in MEOR. Molecules 2019, 24, 1433. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Datta, P.; Kumar, B.; Tiwari, P.; Pandey, L.M. Production of novel rhamnolipids via biodegradation of waste cooking oil using Pseudomonas aeruginosa MTCC7815. Biodegradation 2019, 30, 301–312. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, M.; Xu, X. Microbial conversion of agro-processing waste (peanut meal) to rhamnolipid by Pseudomonas aeruginosa: Solid-state fermentation, water extraction, medium optimization and potential applications. Bioresour. Technol. 2023, 369, 128426. [Google Scholar] [CrossRef]
- Vollenbroich, D.; Özel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, G.E.; Abu-Serie, M.M.; Abou-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Teleb, M.; Abdel-Fattah, Y.R. Bioprocess development for biosurfactant production by Natrialba sp. M6 with effective direct virucidal and anti-replicative potential against HCV and HSV. Sci. Rep. 2022, 12, 16577. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Rameshkumar, M.R.; Nivedha, A.; Sundar, K.; Arunagirinathan, N.; Valan Arasu, M. Biosurfactants: An Antiviral Perspective. In Multifunctional Microbial Biosurfactants; Springer Nature: Cham, Switzerland, 2023; pp. 431–454. [Google Scholar]
- Chattopadhyay, D.; Chattopadhyay, S.; Lyon, W.G.; Wilson, J.T. Effect of surfactants on the survival and sorption of viruses. Environ. Sci. Technol. 2002, 36, 4017–4024. [Google Scholar] [CrossRef]
- Vodolazkaya, N.; Laguta, A.; Farafonov, V.; Nikolskaya, M.; Balklava, Z.; Khayat, R.; Stich, M.; Mchedlov-Petrossyan, N.; Nerukh, D. Influence of various colloidal surfactants on the stability of MS2 bacteriophage suspension. The charge distribution on the PCV2 virus surface. J. Mol. Liq. 2023, 387, 122644. [Google Scholar] [CrossRef]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of environmental factors on phage–bacteria interaction and on the efficacy and infectivity of phage P100. Front. Microbiol. 2016, 7, 1152. [Google Scholar] [CrossRef]
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. J. Ind. Microbiol. Biotechnol. 2007, 34, 577–588. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef]
- Turbhekar, R.; Malik, N.; Dey, D.; Thakare, D. Disruption of Candida albicans biofilms by rhamnolipid obtained from Pseudomonas aeruginosa. Int. J. Res. Stud. Biosci. 2015, 3, 73–78. [Google Scholar]
- Anjos, I.; Bettencourt, A.F.; Ribeiro, I.A. Antimicrobial Biosurfactants Towards the Inhibition of Biofilm Formation. In Urinary Stents: Current State and Future Perspectives; Springer Nature: Cham, Switzerland, 2022; pp. 291–304. [Google Scholar] [CrossRef]
- Tambone, E.; Bonomi, E.; Ghensi, P.; Maniglio, D.; Ceresa, C.; Agostinacchio, F.; Caciagli, P.; Nollo, G.; Piccoli, F.; Caola, I.; et al. Rhamnolipid coating reduces microbial biofilm formation on titanium implants: An in vitro study. BMC Oral Health 2021, 21, 49. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-grade silicone coated with rhamnolipid R89 is effective against Staphylococcus spp. biofilms. Molecules 2019, 24, 3843. [Google Scholar] [CrossRef]
- Alara, J.A.; Alara, O.R. Antimicrobial and anti-biofilm potentials of biosurfactants. In Industrial Applications of Biosurfactants and Microorganisms; Academic Press: Cambridge, MA, USA, 2024; pp. 307–339. [Google Scholar] [CrossRef]
- Richter, Ł.; Księżarczyk, K.; Paszkowska, K.; Janczuk-Richter, M.; Niedziółka-Jönsson, J.; Gapiński, J.; Łoś, M.; Hołyst, R.; Paczesny, J. Adsorption of bacteriophages on polypropylene labware affects the reproducibility of phage research. Sci. Rep. 2021, 11, 7387. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Barros, J.; Costa, F. Antibiotic-Free Solutions for the Development of Biofilm Prevention Coatings. In Urinary Stents: Current State and Future Perspectives; Springer Nature: Cham, Switzerland, 2022; pp. 259–272. [Google Scholar] [CrossRef]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm formation in medically important Candida species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef]
- Staniszewska, M.; Bondaryk, M.; Malewski, T.; Schaller, M. The expression of the Candida albicans gene SAP4 during hyphal formation in human serum and in adhesion to monolayer cell culture of colorectal carcinoma Caco-2 (ATCC). Open Life Sci. 2014, 9, 796–810. [Google Scholar] [CrossRef]
- Haque, F.; Alfatah, M.; Ganesan, K.; Bhattacharyya, M.S. Inhibitory effect of sophorolipid on Candida albicans biofilm formation and hyphal growth. Sci. Rep. 2016, 6, 23575. [Google Scholar] [CrossRef] [PubMed]
- Saadati, F.; Shahryari, S.; Sani, N.M.; Farajzadeh, D.; Zahiri, H.S.; Vali, H.; Noghabi, K.A. Effect of MA01 rhamnolipid on cell viability and expression of quorum-sensing (QS) genes involved in biofilm formation by methicillin-resistant Staphylococcus aureus. Sci. Rep. 2022, 12, 14833. [Google Scholar] [CrossRef]
- Harper, D.R.; Parracho, H.M.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Skaradzińska, A.; Ochocka, M.; Śliwka, P.; Kuźmińska-Bajor, M.; Skaradziński, G.; Friese, A.; Roschanski, N.; Murugaiyan, J.; Roesler, U. Bacteriophage amplification–A comparison of selected methods. J. Virol. Methods 2020, 282, 113856. [Google Scholar] [CrossRef]
- Adams, M.H. Enumeration of bacteriophage particles. Bacteriophages 1959, 29, 27–34. [Google Scholar]
- Kurzepa-Skaradzinska, A.; Skaradzinski, G.; Weber-Dabrowska, B.; Zaczek, M.; Maj, T.; Slawek, A.; Switalska, M.; Maciejewska, M.; Wietrzyk, J.; Rymwoicz, W.; et al. Influence of bacteriophage preparations on migration of HL-60 leukemia cells in vitro. Anticancer Res. 2013, 33, 1569–1574. [Google Scholar] [PubMed]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial compounds produced by vaginal Lactobacillus crispatus are able to strongly inhibit Candida albicans growth, hyphal formation and regulate virulence-related gene expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Yap, M.L.; Rossmann, M.G. Structure and function of bacteriophage T4. Future Microbiol. 2014, 9, 1319–1327. [Google Scholar] [CrossRef]
- Mesyanzhinov, V.V. Bacteriophage T4: Structure, assembly, and initiation infection studied in three dimensions. Adv. Virus Res. 2004, 63, 287–352. [Google Scholar] [CrossRef] [PubMed]
- Garbe, J.; Bunk, B.; Rohde, M.; Schobert, M. Sequencing and characterization of Pseudomonas aeruginosa phage JG004. BMC Microbiol. 2011, 11, 102. [Google Scholar] [CrossRef]
- Whichard, J.M.; Weigt, L.A.; Borris, D.J.; Li, L.L.; Zhang, Q.; Kapur, V.; Pierson, F.W.; Linghor, E.J.; She, Y.; Kropinski, A.M.; et al. Complete genomic sequence of bacteriophage Felix O1. Viruses 2010, 2, 710–730. [Google Scholar] [CrossRef]
- Prisco, A.; De Berardinis, P. Filamentous bacteriophage FD as an antigen delivery system in vaccination. Int. J. Mol. Sci. 2012, 13, 5179–5194. [Google Scholar] [CrossRef]
- Śliwka, P.; Weber-Dąbrowska, B.; Żaczek, M.; Kuźmińska-Bajor, M.; Dusza, I.; Skaradzińska, A. Characterization and comparative genomic analysis of three virulent E. coli bacteriophages with the potential to reduce antibiotic-resistant bacteria in the environment. Int. J. Mol. Sci. 2023, 24, 5696. [Google Scholar] [CrossRef]




| Bacteriophage | Phage Titer [pfu/mL] | Phage Titer After Incubation with 125 mg/L RLs | Phage Titer After Incubation with 250 mg/L RLs | Phage Titer After Incubation with 500 mg/L RLs |
|---|---|---|---|---|
| BF9 | 7.51 ± 5.20 × 108 a | 1.55 ± 0.13 × 108 b | 1.38 ± 0.16 × 108 c | 1.23 ± 0.35 × 108 c |
| BF15 | 2.35 ± 0.62 × 109 a | 1.91 ± 0.14 × 109 ab | 1.68 ± 0.15 × 109 ab | 1.43 ± 0.23 × 109 c |
| BF17 | 1.70 ± 0.63 × 109 a | 9.51 ± 0.77 × 108 b | 1.60 ± 0.35 × 109 ab | 1.15 ± 0.19 × 109 a |
| FD | 2.50 ± 1.39 × 1010 a | 1.35 ± 0.32 × 1010 a | 9.50 ± 1.02 × 109 ab | 5.00 ± 4.44 × 109 b |
| Felix | 9.13 ± 1.00 × 109 a | 9.10 ± 0.44 × 109 a | 8.02 ± 0.38 × 109 a | 8.34 ± 0.23 × 109 a |
| JG004 | 1.97 ± 1.08 × 109 a | 1.02 ± 0.06 × 109 ab | 9.50 ± 1.39 × 108 ab | 8.25 ± 0.77 × 108 b |
| LO5/1f | 1.92 ± 1.71 × 109 a | 2.55 ± 1.36 × 108 ab | 2.75 ± 0.95 × 108 ab | 2.00 ± 1.74 × 108 b |
| T4 | 3.50 ± 1.30 × 108 a | 3.31 ± 1.00 × 108 a | 2.80 ± 0.42 × 108 a | 2.00 ± 0.35 × 108 a |
| TO1/6f | 5.10 ± 3.72 × 109 a | 2.05 ± 1.06 × 109 ab | 1.26 ± 0.32 × 109 ab | 8.35 ± 1.75 × 108 b |
| TO1/7f | 4.97 ± 2.30 × 109 a | 4.49 ± 1.25 × 109 a | 3.06 ± 0.48 × 109 ab | 2.02 ± 1.11 × 109 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dusza, I.; Jama, D.; Skaradziński, G.; Śliwka, P.; Janek, T.; Skaradzińska, A. Bacteriophages Improve the Effectiveness of Rhamnolipids in Combating the Biofilm of Candida albicans. Molecules 2025, 30, 1772. https://doi.org/10.3390/molecules30081772
Dusza I, Jama D, Skaradziński G, Śliwka P, Janek T, Skaradzińska A. Bacteriophages Improve the Effectiveness of Rhamnolipids in Combating the Biofilm of Candida albicans. Molecules. 2025; 30(8):1772. https://doi.org/10.3390/molecules30081772
Chicago/Turabian StyleDusza, Izabela, Dominika Jama, Grzegorz Skaradziński, Paulina Śliwka, Tomasz Janek, and Aneta Skaradzińska. 2025. "Bacteriophages Improve the Effectiveness of Rhamnolipids in Combating the Biofilm of Candida albicans" Molecules 30, no. 8: 1772. https://doi.org/10.3390/molecules30081772
APA StyleDusza, I., Jama, D., Skaradziński, G., Śliwka, P., Janek, T., & Skaradzińska, A. (2025). Bacteriophages Improve the Effectiveness of Rhamnolipids in Combating the Biofilm of Candida albicans. Molecules, 30(8), 1772. https://doi.org/10.3390/molecules30081772









