Edible Flowers in Modern Gastronomy: A Study of Their Volatilomic Fingerprint and Potential Health Benefits
Abstract
1. Introduction
2. Results
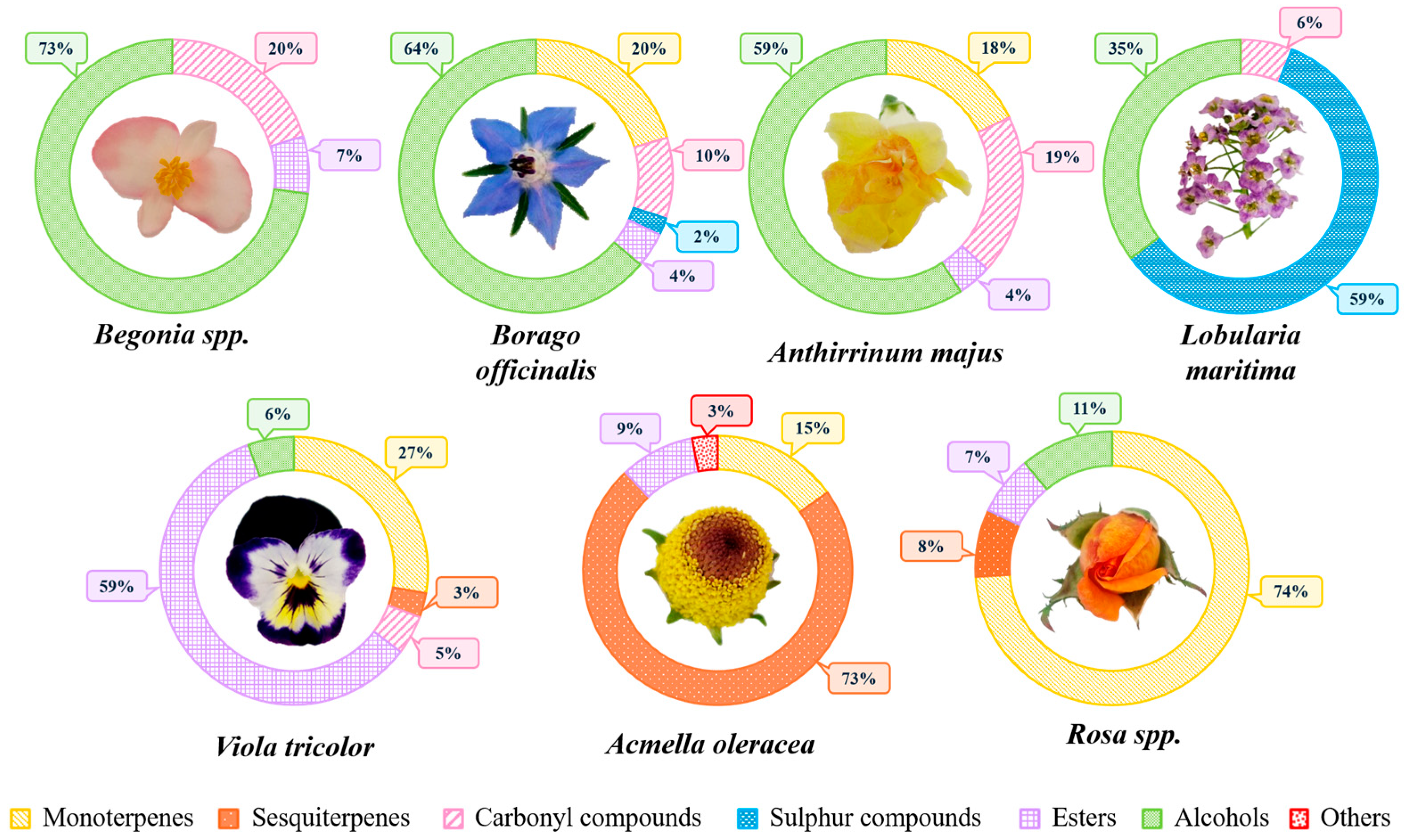
2.1. Volatilomic Fingerprinting from Edible Flowers
2.1.1. Begonia spp.
2.1.2. Borago officinalis
2.1.3. Anthirrinum majus
2.1.4. Lobularia maritima
2.1.5. Acmella oleracea
2.1.6. Viola tricolor
2.1.7. Rosa spp.
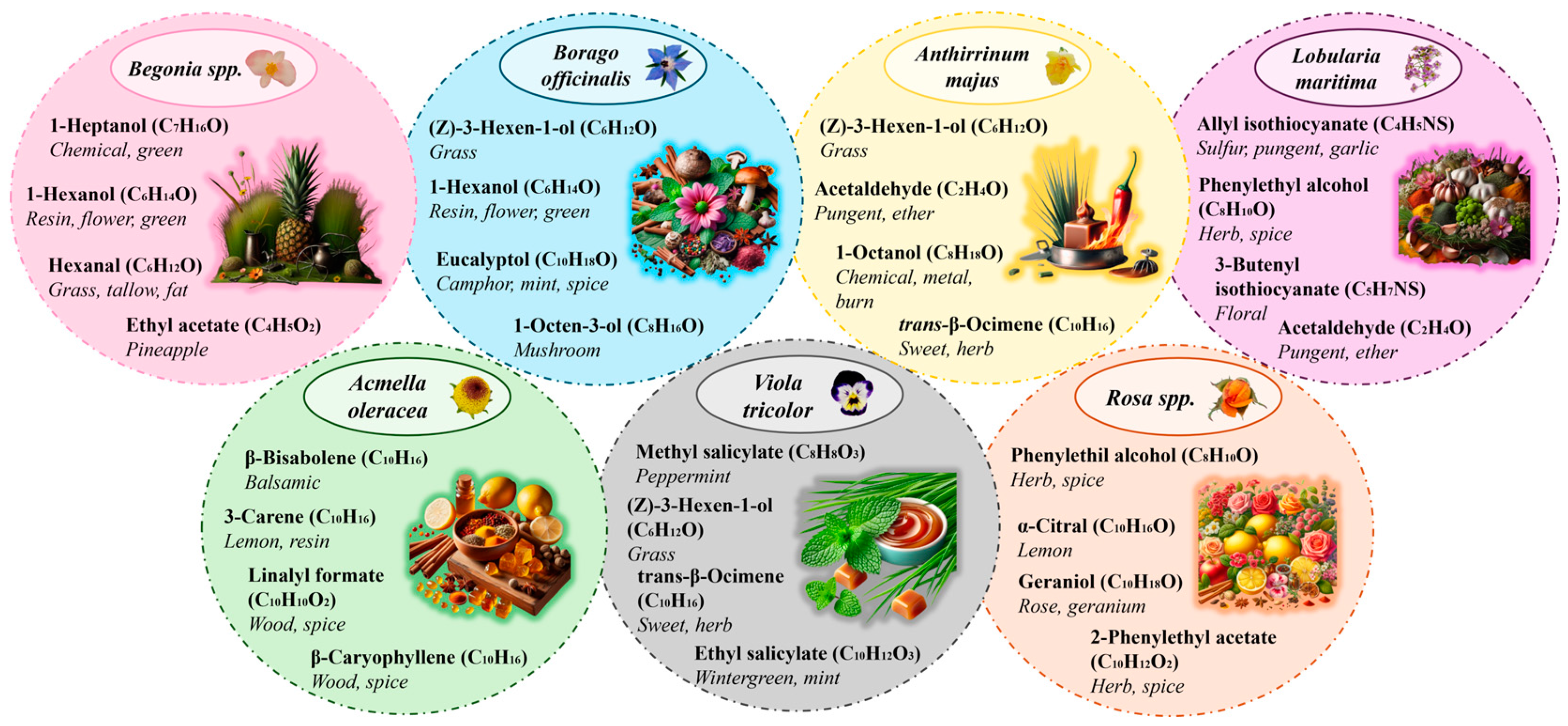
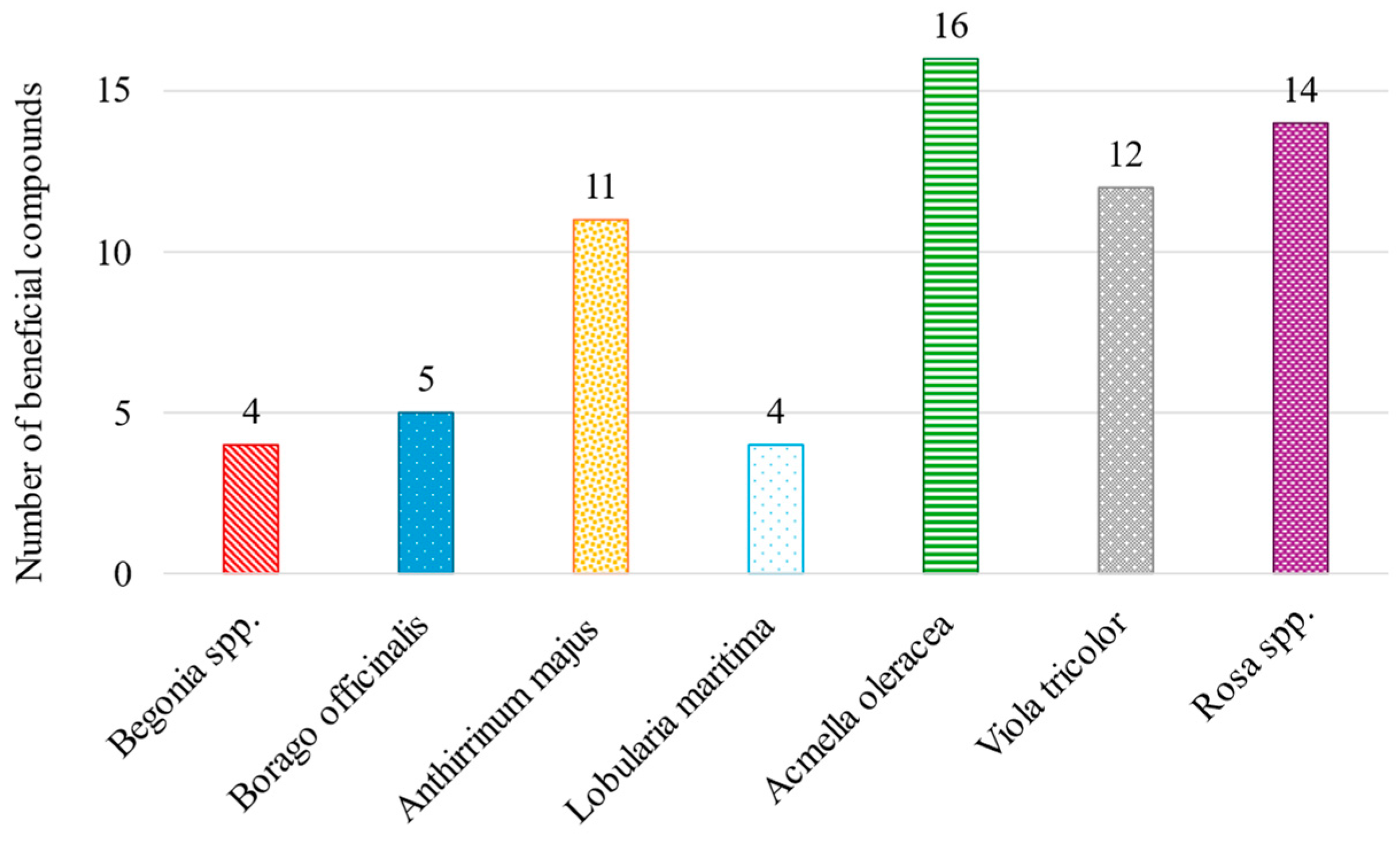
2.2. Odor of Some Identified VOMs and Their Potential Bioactive Effects
2.2.1. Begonia spp.
2.2.2. Borago officinalis
2.2.3. Anthirrinum majus
2.2.4. Lobularia maritima
2.2.5. Lobularia maritima
2.2.6. Viola tricolor
2.2.7. Rosa spp.
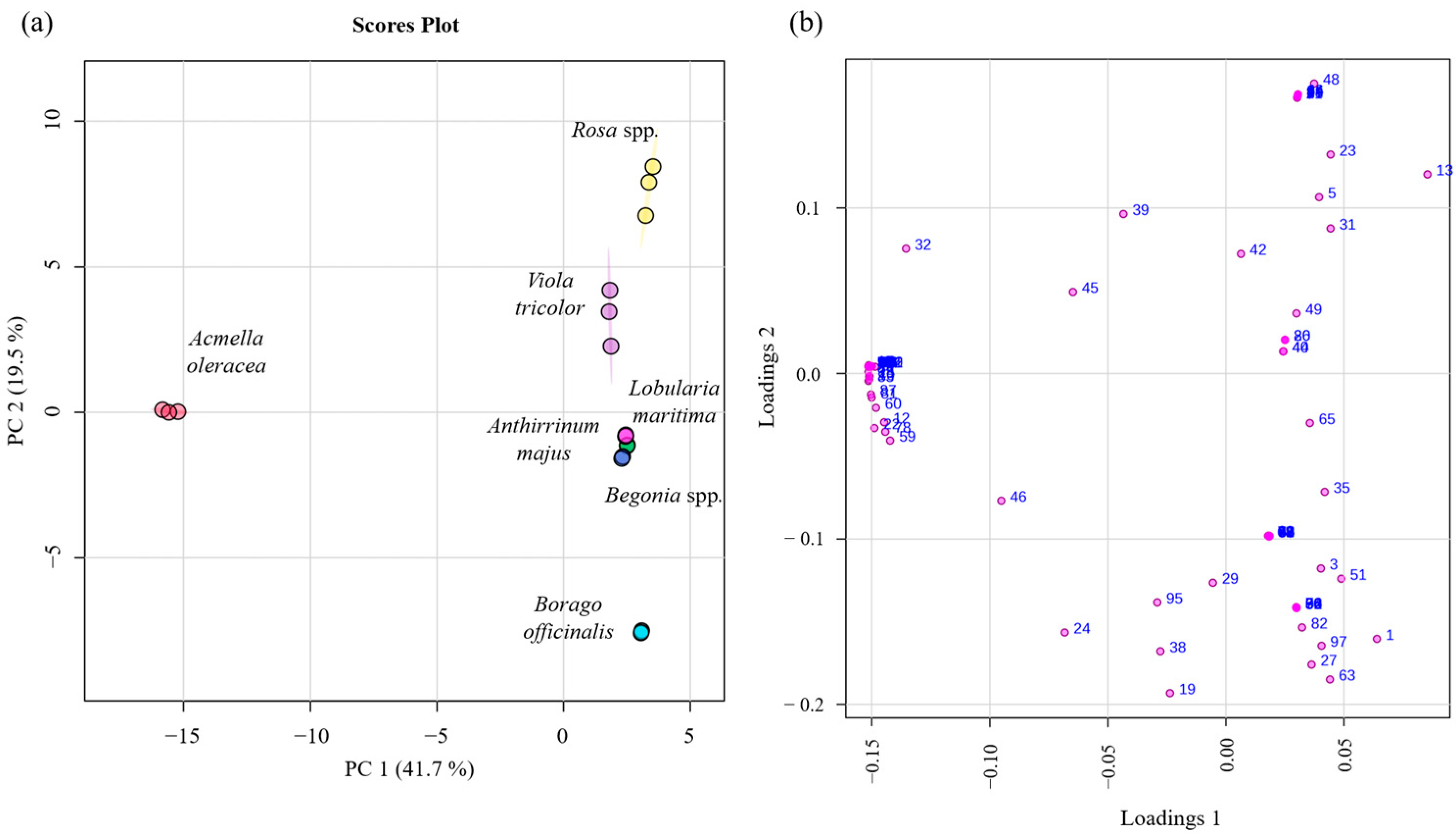
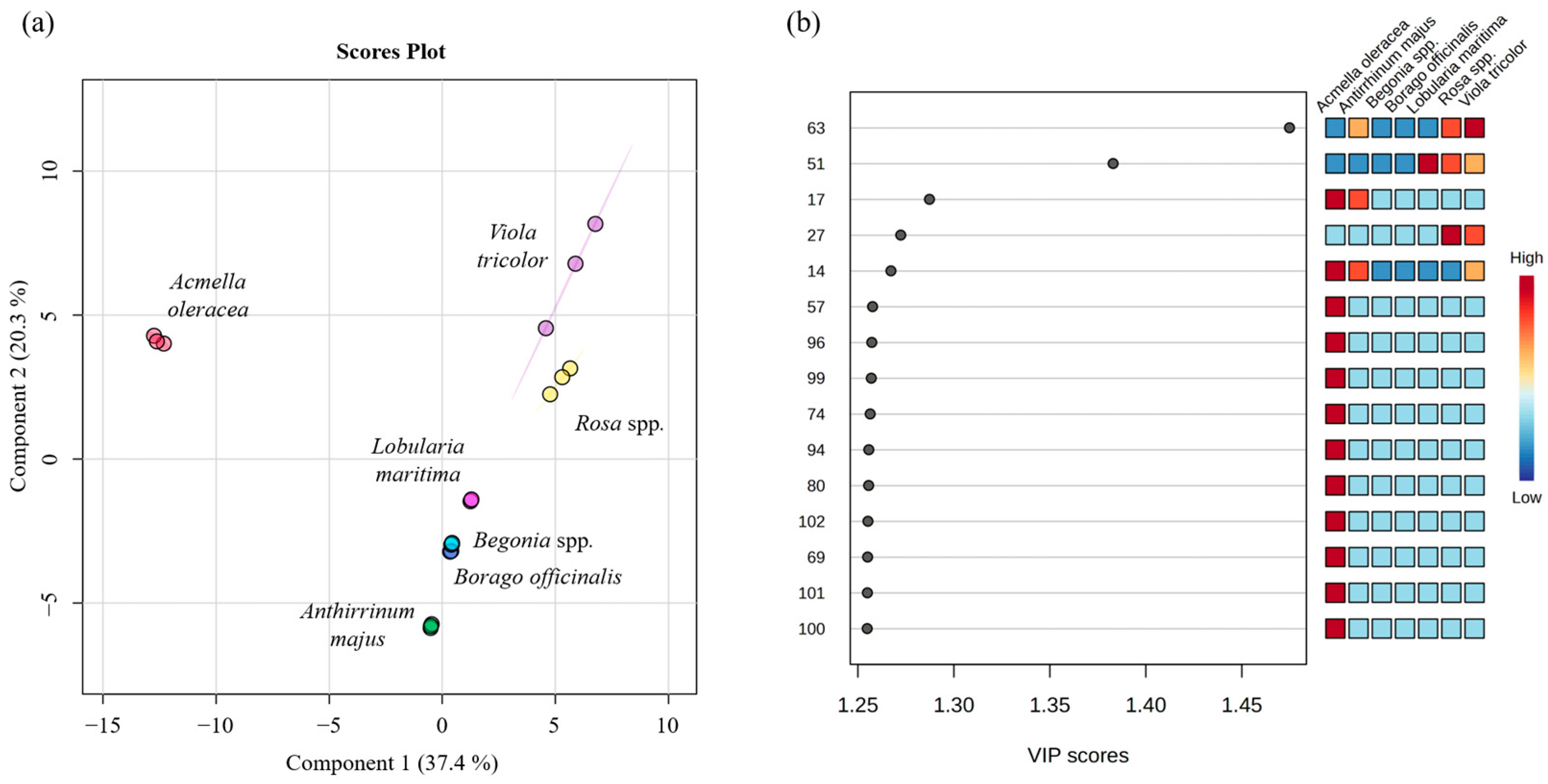
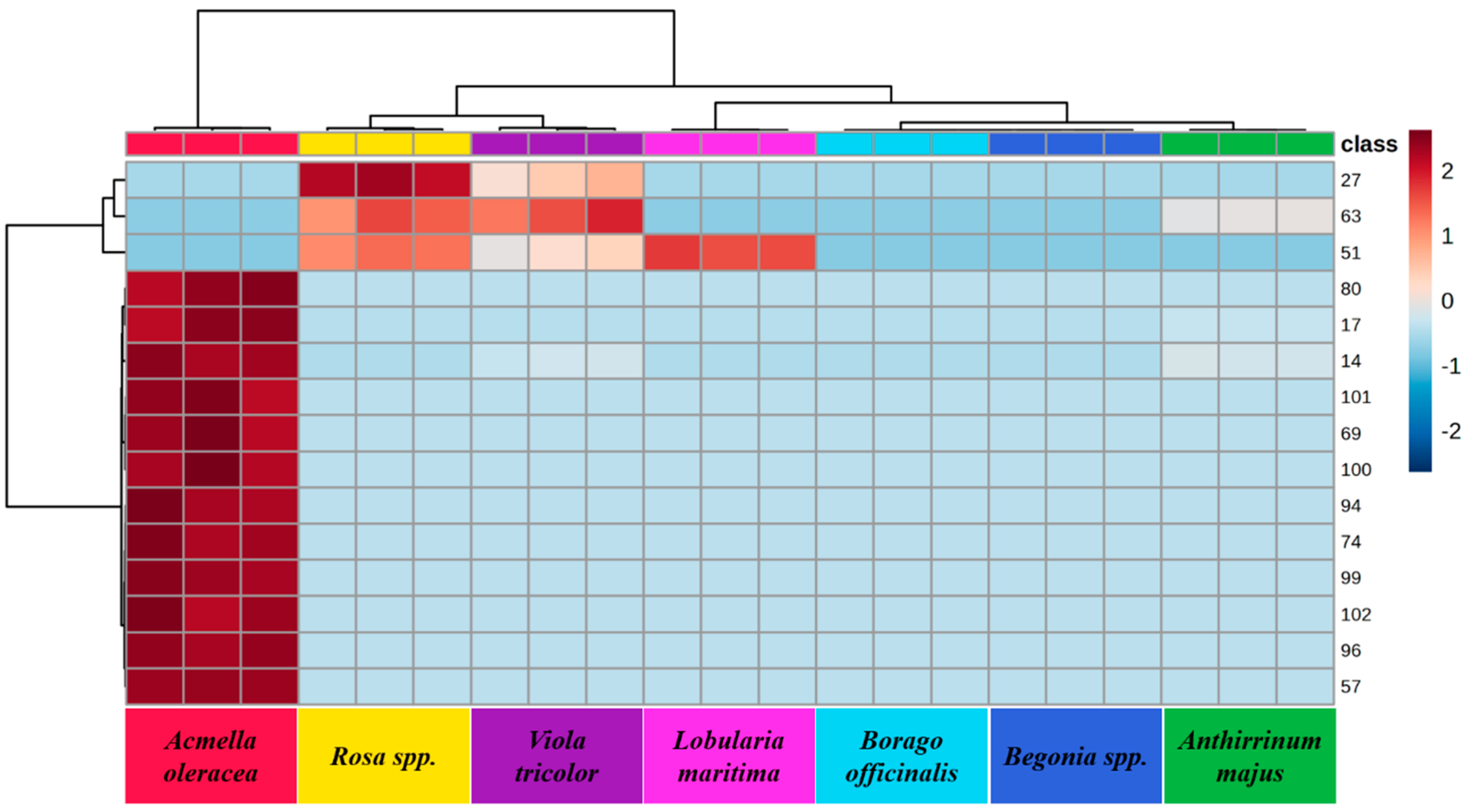
2.3. Multivariate Statistical Analysis
3. Materials and Methods
3.1. Chemical and Reagents
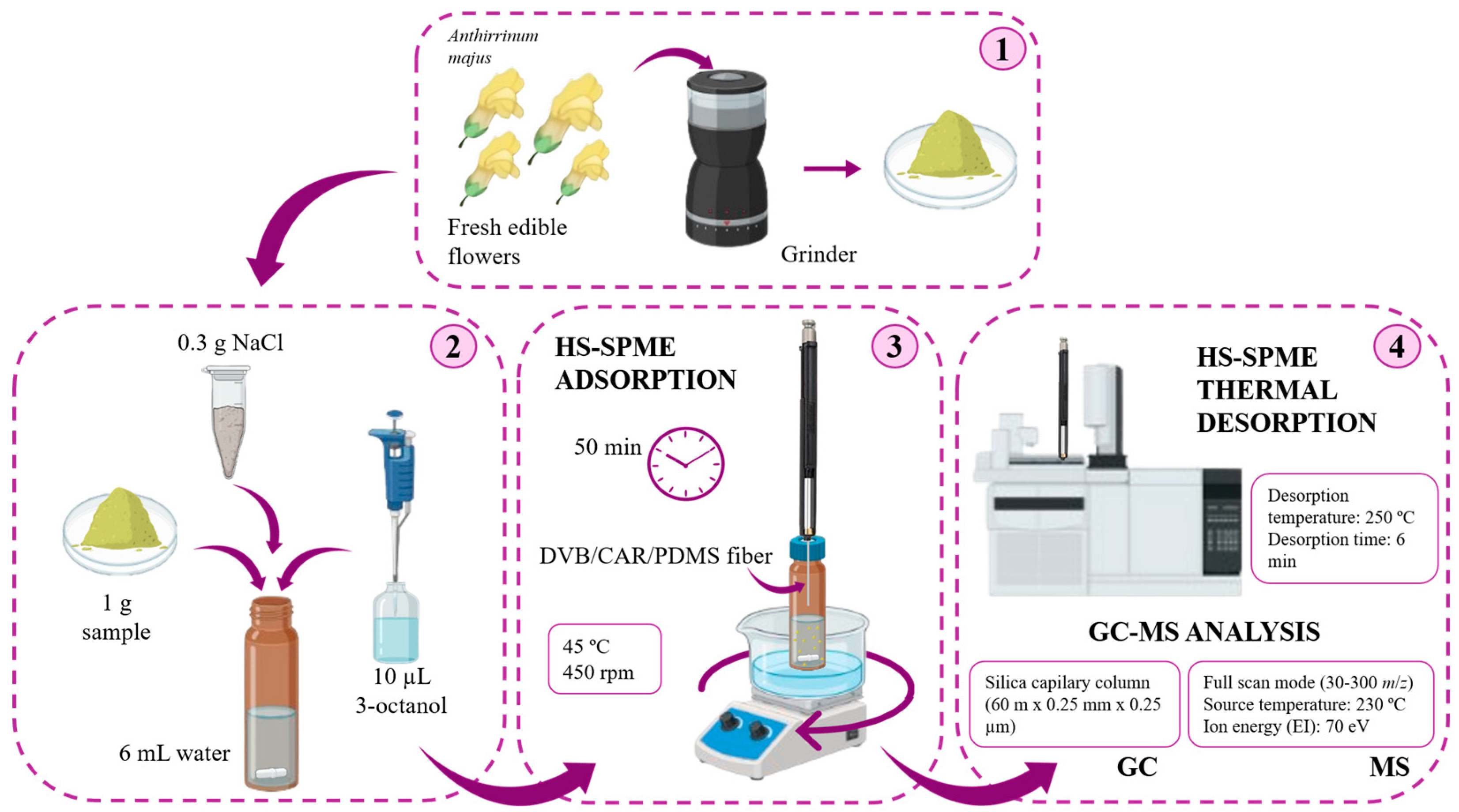
3.2. Fresh Edible Flowers
3.3. HS-SPME Procedure
3.4. GC-qMS Analysis
3.5. Statistical Analysis and Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOM | Volatile organic metabolite |
| HS-SPME | Headspace solid-phase microextraction |
| GC-MS | Gas chromatography coupled to mass spectrometry |
| RT | Retention time |
| KI | Kovats index |
| MF | Molecular formule |
| SD | Standard deviations |
| CC | Carbonyl compounds |
| SC | Sulphur compounds |
| E | Esters |
| FC | Furanic compounds |
| M | Monoterpenoids |
| A | Alcohols |
| S | Sesquiterpenoids |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares-discriminant analysis |
| PC | Principal component |
| VIP | Variable importance in the projection |
| HCA | Hierarchical cluster analysis |
| IS | Internal standard |
| DVB/CAR/PDMS | Divinylbenzene/carboxen/polydimethylsiloxane |
| FS | Full scan |
| q-MS | Quadrupole mass spectrometry detector |
| EI | Electron impact ionization |
| NIST | National Institute of Standards and Technology |
References
- Giannetti, V.; Biancolillo, A.; Marini, F.; Mariani, M.B.; Livi, G. Characterization of the aroma profile of edible flowers using HS-SPME/GC–MS and chemometrics. Food Res. Int. 2024, 178, 114001. [Google Scholar] [CrossRef] [PubMed]
- Lara-Cortés, E.; Osorio-Díaz, P.; Jiménez-Aparicio, A.; Bautista-Bañios, S. Nutritional content, functional properties and conservation of edible flowers. Arch. Latinoam. Nutr. 2013, 63, 197–208. [Google Scholar] [PubMed]
- Marchioni, I.; Gabriele, M.; Carmassi, G.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Najar, B. Phytochemical, nutritional and mineral content of four edible flowers. Foods 2024, 13, 939. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.S.; Pieracci, Y.; Carmassi, G.; Ruffoni, B.; Copetta, A.; Pistelli, L. Effect of drying post-harvest on the nutritional compounds of edible flowers. Horticulturae 2023, 9, 1248. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Pires, T.C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Singh, P.; Khan, M.; Hailemariam, H. Nutritional and health importance of Hibiscus sabdariffa: A review and indication for research needs. J. Nutr. Health Food Eng. 2017, 6, 125–128. [Google Scholar]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef]
- Walsh, M.J.; Baker, S.A. Clean eating and Instagram: Purity, defilement, and the idealization of food. Food Cult. Soc. 2020, 23, 570–588. [Google Scholar] [CrossRef]
- Misra, B. Plant volatilome resources. Curr. Metabolomics 2016, 4, 148–150. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Yan, H.; Bao, T.; Shan, X.; Caissard, J.C.; Zhang, L.; Fang, H.; Bai, X.; Zhang, J.; et al. The complexity of volatile terpene biosynthesis in roses: Particular insights into β-citronellol production. Plant Physiol. 2024, 196, 1908–1922. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Benfodda, Z.; Bénimélis, D.; Fontaine, J.X.; Molinié, R.; Meffre, P. Extraction and identification of volatile organic compounds in scentless flowers of 14 Tillandsia species using HS-SPME/GC-MS. Metabolites 2022, 12, 628. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. An overview on the market of edible flowers. Food Rev. Int. 2020, 36, 258–275. [Google Scholar] [CrossRef]
- Omar, J.; Alonso, I.; Garaikoetxea, A.; Etxebarria, N. Optimization of focused ultrasound extraction and supercritical fluid extraction of volatile compounds and antioxidants from aromatic plants. Food Anal. Methods 2013, 6, 1611–1620. [Google Scholar] [CrossRef]
- Vrca, I.; Čikeš Čulić, V.; Lozić, M.; Dunkić, N.; Kremer, D.; Ruščić, M.; Nazlić, M.; Dunkić, V. Isolation of volatile compounds by microwave-assisted extraction from six Veronica species and testing of their antiproliferative and apoptotic activities. Plants 2023, 12, 3244. [Google Scholar] [CrossRef]
- Longo, V.; Forleo, A.; Provenzano, S.P.; Coppola, L.; Zara, V.; Ferramosca, A.; Siciliano, P.; Capone, S. HS-SPME-GC-MS metabolomics approach for sperm quality evaluation by semen volatile organic compounds (VOCs) analysis. Biomed. Phys. Eng. Express 2018, 5, 015006. [Google Scholar] [CrossRef]
- Paul, I.; Goyal, P.; Bhadoria, P.S.; Mitra, A. Developing efficient methods for unravelling headspace floral volatilome in Murraya paniculata for understanding ecological interactions. In Applications of Biotechnology for Sustainable Development; Springer: Singapore, 2017; pp. 73–79. [Google Scholar] [CrossRef]
- Wong, Y.F.; Yan, D.; Shellie, R.A.; Sciarrone, D.; Marriott, P.J. Rapid plant volatiles screening using headspace SPME and person-portable gas chromatography–mass spectrometry. Chromatographia 2019, 82, 297–305. [Google Scholar] [CrossRef]
- Izcara, S.; Perestrelo, R.; Morante-Zarcero, S.; Sierra, I.; Câmara, J.S. Volatilomic fingerprinting from edible flowers. Unravelling some impact compounds behind its attractiveness. Food Biosci. 2022, 50, 102188. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—A review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive compounds and aroma profile of some Lamiaceae edible flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef]
- Marchioni, I.; Pistelli, L.; Ferri, B.; Copetta, A.; Ruffoni, B.; Pistelli, L.; Najar, B. Phytonutritional content and aroma profile changes during postharvest storage of edible flowers. Front. Plant Sci. 2020, 11, 590968. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Marchioni, I.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Volatilomic analysis of four edible flowers from Agastache genus. Molecules 2019, 24, 4480. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Malheiro, R.; Rodrigues, N.; Saraiva, J.A.; Ramalhosa, E. Borage, calendula, cosmos, Johnny Jump up, and pansy flowers: Volatiles, bioactive compounds, and sensory perception. Eur. Food Res. Technol. 2019, 245, 593–606. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Durán, E.; Castro, R.; Rodríguez-Dodero, M.C.; Natera, R.; García-Barroso, C. Study of the volatile composition and sensory characteristics of new Sherry vinegar-derived products by maceration with fruits. LWT—Food Sci. Technol. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 98, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Perestrelo, R.; Câmara, J.S.; Castilho, P.C. Establishment of Monstera deliciosa fruit volatile metabolomic profile at different ripening stages using solid-phase microextraction combined with gas chromatography–mass spectrometry. Food Res. Int. 2015, 67, 409–417. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Yang, G.; Huang, S.; Liao, S.; Chen, K.; Du, M.; Zalán, Z.; Hegyi, F.; Kan, J. Biocontrol potential of 1-pentanal emitted from lactic acid bacteria strains against Aspergillus flavus in red pepper (Capsicum annuum L.). Food Control 2022, 142, 109261. [Google Scholar] [CrossRef]
- Ayseli, M.T.; Ayseli, Y.İ. Flavors of the future: Health benefits of flavor precursors and volatile compounds in plant foods. Trends Food Sci. Technol. 2016, 48, 69–77. [Google Scholar] [CrossRef]
- Narayanan, M.; Chanthini, A.; Devarajan, N.; Saravanan, M.; Sabour, A.; Alshiekheid, M.; Chi, N.T.L.; Brindhadevi, K. Antibacterial and antioxidant efficacy of ethyl acetate extract of Cymodocea serrulata and assess the major bioactive components in the extract using GC-MS analysis. Process Biochem. 2023, 124, 24–32. [Google Scholar] [CrossRef]
- Kazak, F. A bioactive compound: Eucalyptol. In Functional Foods and Nutraceuticals: Bioactive Compounds; Livre de Lyon: Lyon, France, 2022; pp. 125–138. [Google Scholar]
- Zhang, Z.-M.; Wu, W.-W.; Li, G.-K. A GC–MS Study of the Volatile Organic Composition of Straw and Oyster Mushrooms During Maturity and its Relation to Antioxidant Activity. J. Chromatogr. Sci. 2008, 46, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zegin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.L.; Zhang, S.B.; Lv, Y.Y.; Zhai, H.C.; Hu, Y.S.; Cai, J.P. The antifungal mechanisms of plant volatile compound 1-octanol against Aspergillus flavus growth. Appl. Microbiol. Biotechnol. 2022, 106, 5179–5196. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α-and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from forests and human health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Mander, L.; Liu, H.W. Comprehensive Natural Products II: Chemistry and Biology. In Comprehensive Natural Products II: Chemistry and Biology, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2010; Volume 1. [Google Scholar]
- Rathore, S.S.; Saxena, S.N.; Singh, B. Potential health benefits of major seed spices. Int. J. Seed Spices 2013, 3, 1–12. [Google Scholar]
- Kang, G.Q.; Duan, W.G.; Lin, G.S.; Yu, Y.P.; Wang, X.Y.; Lu, S.Z. Synthesis of bioactive compounds from 3-carene (II): Synthesis, antifungal activity and 3D-QSAR study of (Z)- and (E)-3-caren-5-one oxime sulfonates. Molecules 2019, 24, 477. [Google Scholar] [CrossRef]
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1,8-cineole against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats liver. Toxicol. Ind. Health 2011, 27, 447–453. [Google Scholar] [CrossRef]
- Suhail, M.M.; Wu, W.; Cao, A.; Mondalek, F.G.; Fung, K.M.; Shih, P.T.; Fang, Y.T.; Woolley, C.; Young, G.; Lin, H.K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Med. Ther. 2011, 11, 1–14. [Google Scholar] [CrossRef]
- Miller, J.A.; Lang, J.E.; Ley, M.; Nagle, R.; Hsu, C.H.; Thompson, P.A.; Cordova, C.; Waer, A.; Chow, H.S. Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev. Res. 2013, 6, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Iglesias, M.J.; Novio, S.; García-Santiago, C.; Cartea, M.E.; Soengas, P.; Velasco, P.; Freire-Garabal, M. Effects of 3-butenyl isothiocyanate on phenotypically different prostate cancer cells. Int. J. Oncol. 2018, 53, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xue, P.; Sun, X.; Zhao, G. Determination of the volatile and polyphenol constituents and the antimicrobial, antioxidant, and tyrosinase inhibitory activities of the bioactive compounds from the by-product of Rosa rugosa Thunb. var. plena Regal tea. BMC Complement. Med. Ther. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Tarar, A.; Peng, S.; Cheema, S.; Peng, C.A. Anticancer activity, mechanism, and delivery of allyl isothiocyanate. Bioengineering 2022, 9, 470. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Lin, F.; Long, C. GC-TOF-MS-based metabolomics correlated with bioactivity assays unveiled seasonal variations in leaf essential oils of two species in Garcinia L. Ind. Crops Prod. 2023, 194, 116356. [Google Scholar] [CrossRef]
- Judžentienė, A.; Pečiulytė, D.; Nedveckytė, I. In Situ Antimicrobial Properties of Sabinene Hydrate, a Secondary Plant Metabolite. Molecules 2024, 29, 4252. [Google Scholar] [CrossRef]
- Singh, B.K.; Tripathi, M.; Chaudhari, B.P.; Pandey, P.K.; Kakkar, P. Natural terpenes prevent mitochondrial dysfunction, oxidative stress and release of apoptotic proteins during nimesulide-hepatotoxicity in rats. PLoS ONE 2012, 7, e34200. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.H.; Elmadfa, I. Biological relevance of terpenoids: Overview focusing on mono-, di-and tetraterpenes. Ann. Nut. Metab. 2003, 47, 95–106. [Google Scholar] [CrossRef]
- Graßmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Cardozo, M.T.; de Conti, A.; Ong, T.P.; Scolastici, C.; Purgatto, E.; Horst, M.A.; Bassoli, B.K.; Moreno, F.S. Chemopreventive effects of β-ionone and geraniol during rat hepatocarcinogenesis promotion: Distinct actions on cell proliferation, apoptosis, HMGCoA reductase, and RhoA. J. Nutr. Biochem. 2011, 22, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.M.; Lin, J.Y. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef]
- López, P.L.; Guerberoff, G.K.; Grosso, N.R.; Olmedo, R.H. Antioxidant-efficient indicator determinate by the relationship between β-myrcene/caryophyllene (α, β) on Hop (Humulus lupulus) essential oils under an accelerated oxidation test. Ind.Crops Prod. 2023, 205, 117399. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Regulation of β-phellandrene synthase gene expression, recombinant protein accumulation, and monoterpene hydrocarbons production in Synechocystis transformants. Planta 2014, 240, 309–324. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Liu, Z.; Xie, M.; Jiang, R.; Liu, W.; Wang, X.; Meng, S.; She, G. Naturally occurring methyl salicylate glycosides. Mini Revs. Med. Chem. 2014, 14, 56–63. [Google Scholar] [CrossRef]
- Sianipar, N.F.; Assidqi, K.; Hadisaputri, Y.E.; Salam, S.; Tarigan, R.; Purnamaningsih, R. Determination of bioactive compounds of superior mutant rodent tuber (Typhonium flagelliforme) in various fractions using GC-MS. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Banten, Indonesia, 2021; Volume 794, p. 012144. [Google Scholar] [CrossRef]
- Seo, J.; Lee, J.; Yang, H.Y.; Ju, J. Antirrhinum majus L. flower extract inhibits cell growth and metastatic properties in human colon and lung cancer cell lines. Food Sci. Nutr. 2020, 8, 6259–6268. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Dhibi, S.; Dhifi, W.; Ben Saad, R.; Brini, F.; Hfaidh, N.; Almeida, J.R.G.D.S.; Mnif, W. Lobularia maritima leave extract, a nutraceutical agent with antioxidant activity, protects against CCl₄-induced liver injury in mice. Drug Chem. Toxicol. 2022, 45, 604–616. [Google Scholar] [CrossRef]
- Teixeira, M.; Tao, W.; Fernandes, A.; Faria, A.; Ferreira, I.M.P.L.V.O.; He, J.; de Freitas, V.; Mateus, N.; Oliveira, H. Anthocyanin-rich edible flowers, current understanding of a potential new trend in dietary patterns. Trends Food Sci. Technol. 2023, 138, 708–725. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]
| Peak nº | RT a (min) | KIcalc b | KIlit c | VOMs | MF d | Chemical Family | Relative Area e ± SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Begonia spp. | Borago officinalis | Anthirrinum majus | Lobularia maritima | Acmella oleracea | Viola tricolor | Rosa spp. | |||||||
| 1 | 6.03 | 746 | 748 | Acetaldehyde | C2H4O | CC | - | - | 1.03 ± 0.03 | 2.16 ± 0.22 | - | 0.34 ± 0.02 | 1.12 ± 0.13 |
| 2 | 6.51 | 803 | - | Dimethyl sulfide | (CH3)2S | SC | - | 3.50 ± 0.27 | - | - | - | - | - |
| 3 | 8.66 | 917 | 915 | Ethyl acetate | C4H8O2 | E | 0.39 ± 0.06 | - | - | - | - | - | 1.41 ± 0.22 |
| 4 | 9.21 | 936 | 933 | 2-Methyl-butanal | C5H10O | CC | - | 0.48 ± 0.02 | - | - | - | - | - |
| 5 | 9.33 | 940 | 949 | 3-Methyl-butanal | C5H10O | CC | - | 0.46 ± 0.07 | 0.32 ± 0.05 | - | - | - | - |
| 6 | 10.44 | 973 | 975 | 2-Ethyl-furan | C6H8O | FC | - | 0.41 ± 0.02 | - | - | - | - | - |
| 7 | 11.23 | 995 | 997 | 3-Pentanone | C5H10O | CC | - | 0.82 ± 0.15 | - | - | - | - | - |
| 8 | 12.48 | 1024 | 1022 | Methyl 2-methylbutanoate | C6H12O2 | E | - | - | 0.17 ± 0.03 | - | - | - | - |
| 9 | 12.79 | 1031 | 1034 | 1-Penten-3-one | C5H8O | CC | - | 3.72 ± 0.51 | - | - | - | - | - |
| 10 | 13.36 | 1042 | 1046 | α-Pinene | C10H16 | M | - | - | 0.19 ± 0.01 | - | 27.00 ± 1.83 | 0.11 ± 0.01 | - |
| 11 | 14.69 | 1068 | 1076 | Sabinene | C10H16 | M | - | - | - | - | 0.20 ± 0.02 | - | - |
| 12 | 14.91 | 1072 | 1070 | Camphene | C10H16 | M | - | - | - | - | 0.78 ± 0.03 | 0.07 ± 0.01 | - |
| 13 | 15.83 | 1088 | 1090 | Hexanal | C6H12O | CC | 1.17 ± 0.05 | 5.84 ± 0.27 | 0.13 ± 0.02 | 1.93 ± 0.12 | - | 0.06 ± 0.01 | 0.26 ± 0.02 |
| 14 | 16.96 | 1107 | 1110 | β-Pinene | C10H16 | M | - | - | 0.25 ± 0.03 | - | 40.10 ± 3.49 | 0.08 ± 0.00 | - |
| 15 | 17.63 | 1120 | 1140 | α-Phellandrene | C10H16 | M | - | - | - | - | 34.35 ± 5.40 | - | - |
| 16 | 17.86 | 1124 | 1127 | 2-Pentenal | C5H8O | CC | - | 0.52 ± 0.02 | - | - | - | - | - |
| 17 | 19.06 | 1145 | 1147 | 3-Carene | C10H16 | M | - | - | 0.12 ± 0.01 | - | 259.59 ± 38.11 | - | - |
| 18 | 19.44 | 1151 | 1157 | 1-Penten-3-ol | C5H10O | A | - | 2.00 ± 0.31 | - | - | - | - | - |
| 19 | 19.91 | 1159 | 1159 | β-Myrcene | C10H16 | M | - | - | 0.21 ± 0.01 | - | 0.54 ± 0.04 | 0.98 ± 0.18 | 2.58 ± 0.15 |
| 20 | 20.81 | 1173 | 1176 | Heptanal | C7H14O | CC | - | - | 0.13 ± 0.02 | - | - | - | - |
| 21 | 21.68 | 1186 | 1180 | (E)-2-Hexenal | C6H10O | CC | - | 1.33 ± 0.25 | - | - | - | - | - |
| 22 | 21.94 | 1190 | 1187 | Limonene | C10H16 | M | - | - | 0.26 ± 0.04 | - | 34.42 ± 1.49 | 0.42 ± 0.04 | 0.47 ± 0.08 |
| 23 | 22.39 | 1196 | 1198 | Eucalyptol | C10H18O | M | - | 34.75 ± 3.87 | 0.10 ± 0.02 | - | - | 0.20 ± 0.04 | 0.11 ± 0.01 |
| 24 | 22.41 | 1196 | 1193 | (Z)-2-Hexenal | C6H10O | CC | - | - | - | - | 1.54 ± 0.23 | 1.42 ± 0.05 | 1.49 ± 0.24 |
| 25 | 22.51 | 1198 | 1198 | β-Phellandrene | C10H16 | M | - | - | - | - | 105.26 ± 3.03 | - | 0.14 ± 0.02 |
| 26 | 2281 | 1203 | 1209 | 3-Methyl-1-butanol | C5H12O | A | - | - | 0.37 ± 0.02 | - | - | - | - |
| 27 | 22.92 | 1205 | 1222 | Ethyl hexanoate | C8H16O2 | E | - | - | - | - | - | 0.07 ± 0.01 | 1.04 ± 0.17 |
| 28 | 24.67 | 1235 | 1242 | γ-Terpinene | C10H16 | M | - | - | - | - | 1.74 ± 0.25 | - | - |
| 29 | 24.88 | 1238 | 1243 | Trans-β-ocimene | C10H16 | M | - | - | 0.73 ± 0.10 | - | 1.68 ± 0.13 | 39.41 ± 1.76 | 1.56 ± 0.16 |
| 30 | 25.06 | 1241 | 1238 | 1-Pentanol | C5H12O | A | - | - | 0.15 ± 0.01 | - | - | - | - |
| 31 | 25.49 | 1248 | 1253 | 3-Octanone | C8H16O | CC | - | 3.28 ± 0.17 | - | - | - | - | 0.32 ± 0.04 |
| 32 | 25.83 | 1254 | 1257 | p-Cymene | C10H14 | M | - | 0.58 ± 0.03 | - | - | 0.42 ± 0.02 | - | - |
| 33 | 26.77 | 1268 | 1271 | α-Terpinolene | C10H16 | M | - | - | - | - | 0.57 ± 0.05 | - | - |
| 34 | 28.11 | 1288 | 1287 | 2-Penten-1-ol | C5H10O | A | - | 1.55 ± 0.04 | - | - | - | - | - |
| 35 | 28.25 | 1290 | 1291 | 3-Hexen-1-ol acetate | C8H14O2 | E | - | 7.14 ± 0.53 | - | - | - | - | 7.73 ± 0.78 |
| 36 | 28.75 | 1297 | 1305 | 2-Hexen-1-ol acetate | C8H14O2 | E | - | - | - | - | - | - | 4.04 ± 0.49 |
| 37 | 29.07 | 1303 | 1327 | Ethyl heptanoate | C9H18O2 | E | - | - | - | - | - | 0.07 ± 0.00 | - |
| 38 | 29.28 | 1306 | 1317 | 6-Methyl-5-hepten-2-one | C8H14O | CC | - | - | 0.35 ± 0.04 | - | 0.19 ± 0.01 | 0.11 ± 0.01 | 0.65 ± 0.08 |
| 39 | 30.41 | 1326 | 1321 | 1-Hexanol | C6H14O | A | 1.57 ± 0.23 | 26.93 ± 2.06 | 0.73 ± 0.10 | - | 2.70 ± 0.05 | 0.52 ± 0.02 | 1.85 ± 0.29 |
| 40 | 30.46 | 1327 | 1325 | Allyl isothiocyanate | C4H5NS | SC | - | - | - | 9.39 ± 1.63 | - | - | - |
| 41 | 30.57 | 1329 | 1331 | (E)-3-Hexen-1-ol | C6H12O | A | - | 0.74 ± 0.13 | - | - | - | - | - |
| 42 | 31.76 | 1349 | 1357 | (Z)-3-Hexen-1-ol | C6H12O | A | - | 59.38 ± 5.91 | 1.83 ± 0.17 | - | 1.47 ± 0.06 | 6.18 ± 0.02 | - |
| 43 | 31.82 | 1350 | 1351 | Trans-alloocimene | C10H16 | M | - | - | - | - | 1.28 ± 0.19 | - | - |
| 44 | 31.84 | 1351 | 1361 | (E)-2-Hexen-1-ol | C6H12O | A | - | - | - | 1.13 ± 0.11 | - | - | - |
| 45 | 32.92 | 1368 | 1373 | (E)-4-Hexen-1-ol | C6H12O | A | - | 8.01 ± 0.89 | 0.21 ± 0.01 | - | 1.13 ± 0.01 | 0.17 ± 0.03 | 0.73 ± 0.11 |
| 46 | 33.43 | 1377 | 1374 | Fenchone | C10H16O | M | - | - | - | - | 0.27 ± 0.03 | 0.20 ± 0.02 | - |
| 47 | 34.95 | 1400 | 1429 | Ethyl octanoate | C10H20O2 | E | - | - | - | - | - | 0.30 ± 0.02 | - |
| 48 | 35.42 | 1409 | 1420 | 1-Octen-3-ol | C8H16O | A | - | 11.68 ± 2.01 | 0.28 ± 0.02 | - | - | - | - |
| 49 | 35.75 | 1416 | 1430 | 1-Heptanol | C7H16O | A | 2.63 ± 0.21 | - | 0.36 ± 0.02 | - | - | - | - |
| 50 | 36.12 | 1422 | 1431 | 6-Methyl-5-hepten-2-ol | C8H14O | A | - | - | - | - | - | - | 0.41 ± 0.03 |
| 51 | 36.52 | 1430 | 1416 | 3-Butenyl isothiocyanate | C5H7NS | SC | - | - | - | 29.92 ± 1.74 | - | 0.34 ± 0.01 | 2.57 ± 0.15 |
| 52 | 37.11 | 1441 | 1442 | Citronellal | C10H18O | M | - | - | - | - | - | - | 0.24 ± 0.02 |
| 53 | 37.33 | 1445 | 1445 | α-Cubebene | C10H16 | S | - | - | - | - | 10.93 ± 1.78 | - | - |
| 54 | 37.52 | 1448 | 1444 | 2-Ethyl-1-hexanol | C8H18O | A | - | - | - | - | - | - | 0.36 ± 0.01 |
| 55 | 37.84 | 1454 | 1452 | δ-Elemene | C10H16 | S | - | - | - | - | 11.59 ± 1.57 | - | - |
| 56 | 37.90 | 1455 | 1440 | (Z)-3-Hexenyl 3-methylbutanoate | C10H18O2 | E | - | - | - | - | - | 0.37 ± 0.06 | - |
| 57 | 38.40 | 1464 | 1473 | Cyclosativene | C10H16 | S | - | - | - | - | 9.15 ± 0.05 | - | - |
| 58 | 38.59 | 1467 | 1466 | 2-Hexenyl butanoate | C10H18O2 | E | - | - | - | - | - | 0.30 ± 0.05 | - |
| 59 | 38.84 | 1472 | 1477 | Copaene | C10H16 | S | - | - | - | - | 10.63 ± 0.69 | - | 1.86 ± 0.31 |
| 60 | 39.12 | 1476 | 1495 | β-Bourbonene | C10H16 | S | - | - | - | - | 9.27 ± 0.52 | 0.34 ± 0.03 | - |
| 61 | 39.58 | 1484 | 1491 | Benzaldehyde | C7H6O | CC | - | - | - | - | - | 1.42 ± 0.22 | - |
| 62 | 40.33 | 1497 | 1509 | (E)-2-Nonenal | C9H18O | CC | - | - | - | - | - | 1.58 ± 0.20 | - |
| 63 | 40.62 | 1502 | 1503 | Ethyl nonanoate | C10H20O2 | E | - | - | 0.25 ± 0.04 | - | - | 1.02 ± 0.07 | 1.63 ± 0.29 |
| 64 | 40.89 | 1507 | 1512 | Theaspirane B | C10H16O | S | - | - | - | - | - | - | 1.71 ± 0.07 |
| 65 | 41.21 | 1514 | 1516 | 1-Octanol | C8H18O | A | - | - | 2.24 ± 0.30 | - | - | - | 0.34 ± 0.06 |
| 66 | 41.29 | 1515 | 1535 | Cyperene | C10H16 | S | - | - | - | - | 19.41 ± 3.14 | - | - |
| 67 | 41.61 | 1521 | 1519 | β-Cubebene | C10H16 | S | - | - | - | - | 12.66 ± 3.54 | - | - |
| 68 | 42.92 | 1546 | 1548 | (E,Z)-2,6-Nonadienal | C10H18O | CC | - | - | - | - | - | 0.80 ± 0.11 | - |
| 69 | 43.99 | 1566 | 1553 | Linalyl formate | C10H10O2 | E | - | - | - | - | 337.61 ± 26.25 | - | - |
| 70 | 45.00 | 1584 | 1581 | β-Caryophyllene | C10H16 | S | - | - | - | - | 1599.59 ± 213.65 | 1.88 ± 0.32 | 0.23 ± 0.01 |
| 71 | 45.72 | 1596 | 1589 | Aromandrene | C10H16 | S | - | - | - | - | 1.90 ± 0.20 | - | - |
| 72 | 45.82 | 1598 | 1605 | Benzeneacetaldehyde | C8H8O | CC | - | - | - | - | - | 1.39 ± 0.10 | - |
| 73 | 46.68 | 1616 | 1635 | δ-Cadinene | C10H16 | S | - | - | - | - | 5.89 ± 0.70 | - | - |
| 74 | 47.00 | 1622 | 1622 | Humelene | C10H16 | S | - | - | - | - | 10.32 ± 0.57 | - | - |
| 75 | 47.07 | 1624 | 1634 | Ethyl benzoate | C11H10O2 | E | - | - | - | - | - | 0.26 ± 0.02 | - |
| 76 | 47.79 | 1638 | 1638 | β-Ciclocitral | C10H10O2 | M | - | - | - | - | - | - | 3.79 ± 0.65 |
| 77 | 47.84 | 1639 | 1648 | β-Farnesene | C10H16 | S | - | - | - | - | 2.52 ± 0.34 | - | - |
| 78 | 48.47 | 1652 | 1644 | α-Caryophyllene | C10H16 | S | - | - | - | - | 144.7 ± 14.5 | - | 0.64 ± 0.04 |
| 79 | 49.17 | 1665 | 1685 | (E)-3-Nonen-1-ol | C9H18O | A | - | - | - | - | - | 0.12 ± 0.02 | - |
| 80 | 49.41 | 1670 | 1670 | α-Muurolene | C10H16 | S | - | - | - | - | 47.51 ± 3.15 | - | - |
| 81 | 50.13 | 1684 | 1685 | Germacrene D | C10H16 | S | - | - | - | - | 51.10 ± 0.90 | 1.21 ± 0.12 | - |
| 82 | 50.30 | 1687 | 1692 | α-Citral | C10H16O | S | - | - | - | - | - | 0.12 ± 0.02 | 31.10 ± 1.93 |
| 83 | 50.53 | 1691 | 1672 | Epizonarene | C10H16 | CC | - | - | - | - | 231.91 ± 27.06 | 0.53 ± 0.08 | - |
| 84 | 50.83 | 1697 | 1694 | β-Bisabolene | C10H16 | S | - | - | - | - | 411.64 ± 66.82 | 0.10 ± 0.01 | - |
| 85 | 51.39 | 1708 | 1711 | Geranyl acetate | C12H18O2 | S | - | - | - | - | - | - | 8.74 ± 0.54 |
| 86 | 51.47 | 1710 | 1716 | Bicyclogermacrene | C10H16 | S | - | - | - | - | 14.45 ± 2.34 | - | - |
| 87 | 51.82 | 1717 | 1706 | α-Farnesene | C10H16 | S | - | - | - | - | 25.49 ± 2.61 | 0.48 ± 0.07 | - |
| 88 | 52.67 | 1734 | 1710 | δ-Amorphene | C10H16 | S | - | - | - | - | 71.05 ± 10.91 | - | - |
| 89 | 52.80 | 1736 | 1730 | Methyl salicylate | C8H8O3 | E | - | - | - | - | - | 65.81 ± 5.92 | - |
| 90 | 53.26 | 1745 | 1725 | γ-Muurolene | C10H16 | M | - | - | - | - | - | - | 13.69 ± 1.77 |
| 91 | 53.81 | 1756 | 1738 | δ-Selinene | C10H16 | S | - | - | - | - | 6.61 ± 0.87 | - | - |
| 92 | 54.14 | 1762 | 1780 | 2-Phenylethyl acetate | C10H12O2 | E | - | - | - | - | - | - | 19.80 ± 1.32 |
| 93 | 54.19 | 1763 | 1780 | Ethyl salicylate | C10H12O3 | E | - | - | - | - | - | 23.19 ± 1.89 | - |
| 94 | 54.21 | 1764 | 1753 | β-Muurolene | C10H16 | S | - | - | - | - | 23.10 ± 1.60 | - | - |
| 95 | 55.11 | 1781 | 1797 | Geraniol | C10H18O | M | - | - | - | - | 65.26 ± 2.07 | - | 371.74 ± 55.28 |
| 96 | 55.93 | 1796 | 1812 | β-Amorphene | C10H16 | S | - | - | - | - | 6.67 ± 0.21 | - | - |
| 97 | 58.37 | 1847 | 1858 | Phenylethyl alcohol | C8H10O | A | - | - | - | 22.42 ± 0.72 | - | 1.35 ± 0.23 | 49.19 ± 8.47 |
| 98 | 61.37 | 1902 | 1914 | α-Calacorene | C10H16 | S | - | - | - | - | 1.25 ± 0.24 | - | - |
| 99 | 62.51 | 1908 | 1928 | Caryophyllene oxide | C10H16O | S | - | - | - | - | 5.36 ± 0.22 | - | - |
| 100 | 64.09 | 2016 | 2008 | Nerolidol | C10H18O | S | - | - | - | - | 1.85 ± 0.15 | - | - |
| 101 | 72.07 | 2154 | 2167 | T-Cadinol | C10H18O | S | - | - | - | - | 1.17 ± 0.09 | - | - |
| 102 | 74.62 | 2165 | - | Cariophylladienol I | C10H16O | S | - | - | - | - | 1.08 ± 0.08 | - | - |
| Peak nº | VOMs | Edible Flowers | Potential Bioactive Effects | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Begonia spp. | Borago officinalis | Anthirrinum majus | Lobularia maritima | Acmella oleracea | Viola tricolor | Rosa spp. | |||
| 3 | Ethyl acetate | x | x | Antimicrobial | |||||
| 10 | α-Pinene | x | x | x | Antidiabetic, antifungal, antioxidant, antiproliferative, antitumor, cytotoxic | ||||
| 11 | Sabinene | x | Antibacterial, antifungal | ||||||
| 12 | Camphene | x | x | Antimicrobial, antioxidant | |||||
| 13 | Hexanal | x | x | x | x | x | x | Antimicrobial | |
| 14 | β-Pinene | x | x | x | Antitumor, anti-inflammatory, antimicrobial, antioxidant, antineoplastic, chemoprotective | ||||
| 17 | 3-Carene | x | x | Antimicrobial, antioxidant, anticancer | |||||
| 19 | β-Myrcene | x | x | x | x | Analgesic, anti-inflammatory, antibiotic, anticancer, antioxidant | |||
| 22 | Limonene | x | x | x | x | Antimutagenic, antitumor, antioxidant, antimicrobial, antiproliferative, chemoprotective | |||
| 23 | Eucalyptol | x | x | x | x | Anti-inflammatory, antioxidative, antihyperglycemic, antimicrobial, antihypertensive, anti-tumoral, antinociceptive, antipyretic, analgesic | |||
| 25 | β-Phellandrene | x | x | Antibacterial, antifungal, anticancer, antidiabetic, antioxidant, analgesic, antiviral, anti-inflammatory | |||||
| 28 | γ-Terpinene | x | Anti-inflammatory, antioxidant | ||||||
| 32 | p-Cymene | x | x | Antibacterial, anti-inflammatory, antifungal, antioxidant, cytotoxic | |||||
| 33 | α-Terpinolene | x | Antioxidant | ||||||
| 35 | 1-Octanol | x | x | Antifungal | |||||
| 39 | 1-Hexanol | x | x | x | x | x | x | Antifungal | |
| 40 | Allyl isothiocyanate | x | Anticancer, antibacterial, antifungal, anti-inflammatory, antioxidant | ||||||
| 48 | 1-Octen-3-ol | x | x | Antioxidant | |||||
| 49 | 1-Heptanol | x | x | Antifungal | |||||
| 51 | 3-Butenyl isothiocyanate | x | x | x | Cytotoxic, anticancer | ||||
| 70 | β-Caryophyllene | x | x | x | Antibacterial, antidiabetic, antioxidant, antiproliferative, cytotoxic | ||||
| 84 | β-Bisabolene | x | Cytotoxic, anti-inflammatory, antibacterial | ||||||
| 89 | Methyl salicylate | x | Anti-inflammatory, analgesic, antipyretic, antifungal | ||||||
| 90 | γ-Muurolene | x | Anti-inflammatory, antimicrobial, antioxidant, cytotoxic | ||||||
| 95 | Geraniol | x | x | Chemopreventive activity, antimutagenic, anti-inflammatory | |||||
| 97 | Phenylethyl alcohol | x | x | x | Antimicrobial, antioxidant, antienzymatic | ||||
| 99 | Caryophyllene oxide | x | Antibacterial, antioxidant, antiproliferative, cytotoxic, analgesic | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Pintor, B.; Perestelo, R.; Morante-Zarcero, S.; Sierra, I.; Câmara, J.S. Edible Flowers in Modern Gastronomy: A Study of Their Volatilomic Fingerprint and Potential Health Benefits. Molecules 2025, 30, 1799. https://doi.org/10.3390/molecules30081799
Fernández-Pintor B, Perestelo R, Morante-Zarcero S, Sierra I, Câmara JS. Edible Flowers in Modern Gastronomy: A Study of Their Volatilomic Fingerprint and Potential Health Benefits. Molecules. 2025; 30(8):1799. https://doi.org/10.3390/molecules30081799
Chicago/Turabian StyleFernández-Pintor, Begoña, Rosa Perestelo, Sonia Morante-Zarcero, Isabel Sierra, and José S. Câmara. 2025. "Edible Flowers in Modern Gastronomy: A Study of Their Volatilomic Fingerprint and Potential Health Benefits" Molecules 30, no. 8: 1799. https://doi.org/10.3390/molecules30081799
APA StyleFernández-Pintor, B., Perestelo, R., Morante-Zarcero, S., Sierra, I., & Câmara, J. S. (2025). Edible Flowers in Modern Gastronomy: A Study of Their Volatilomic Fingerprint and Potential Health Benefits. Molecules, 30(8), 1799. https://doi.org/10.3390/molecules30081799










