Construction of a Covalent Crosslinked Membrane Exhibiting Superhydrophilicity and Underwater Superoleophobicity for the Efficient Separation of High-Viscosity Oil–Water Emulsion Under Gravity
Abstract
1. Introduction
2. Results and Discussion
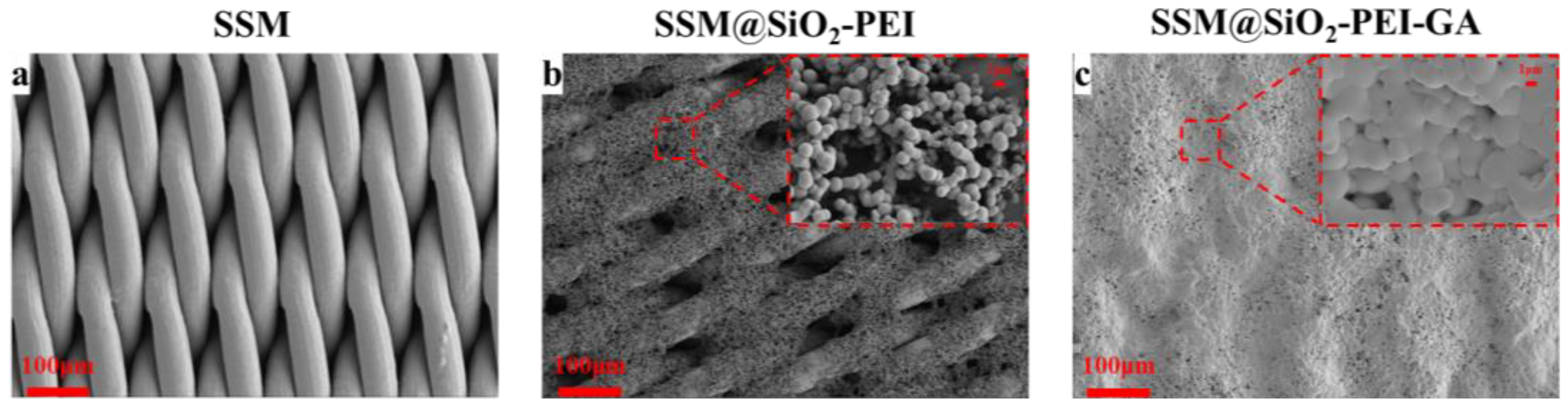
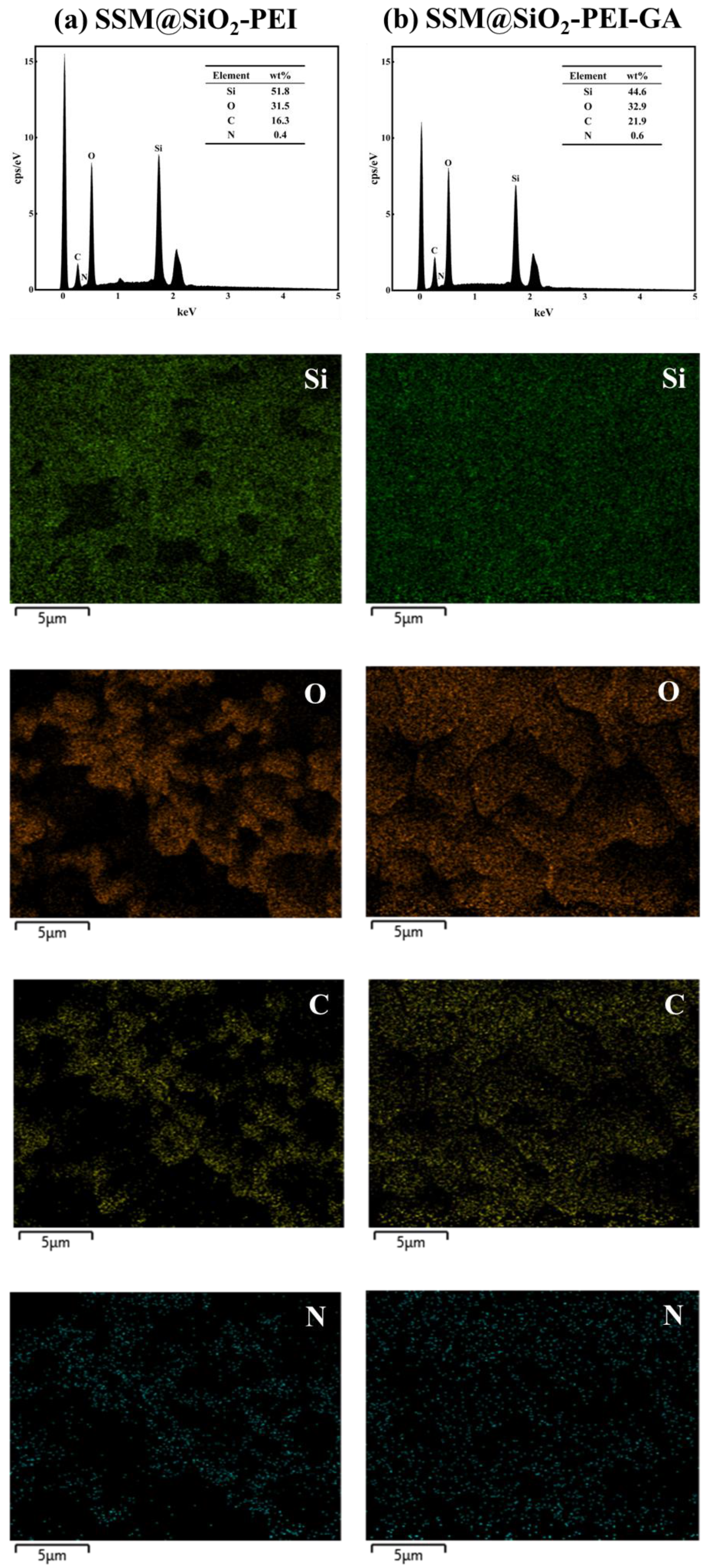
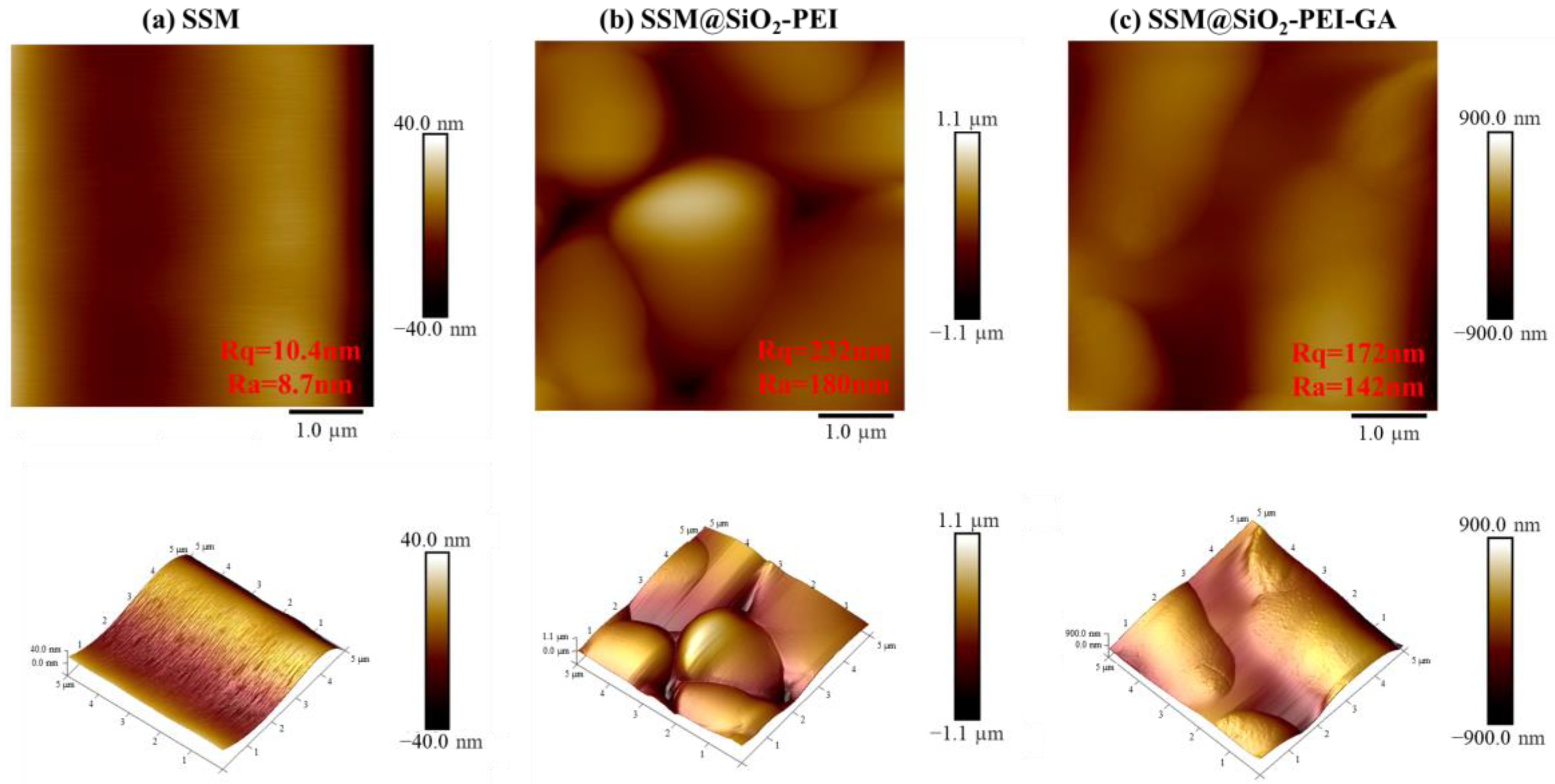
2.1. Characterization
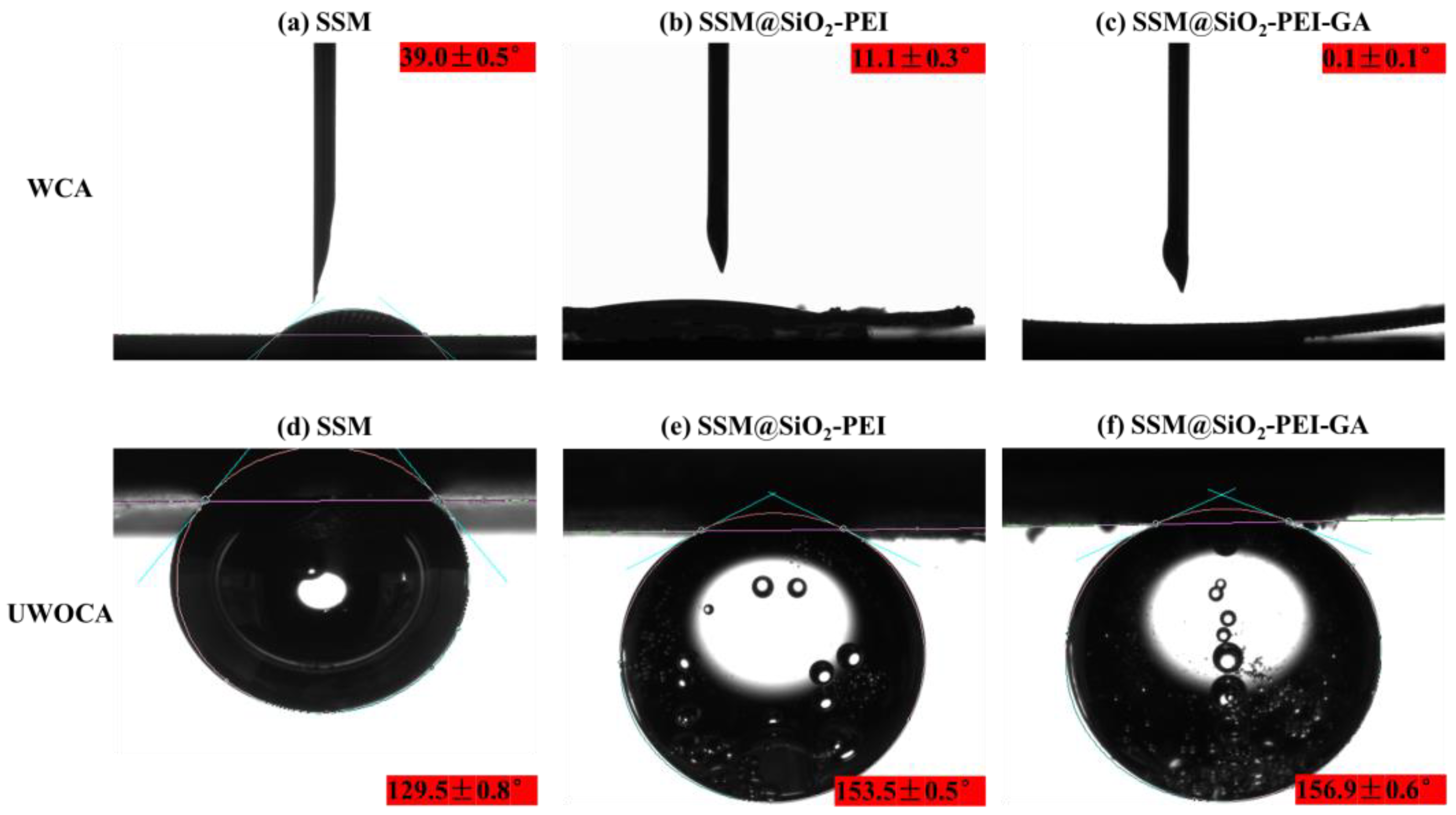
2.2. Surface Wettability and Oil Resistance Performance
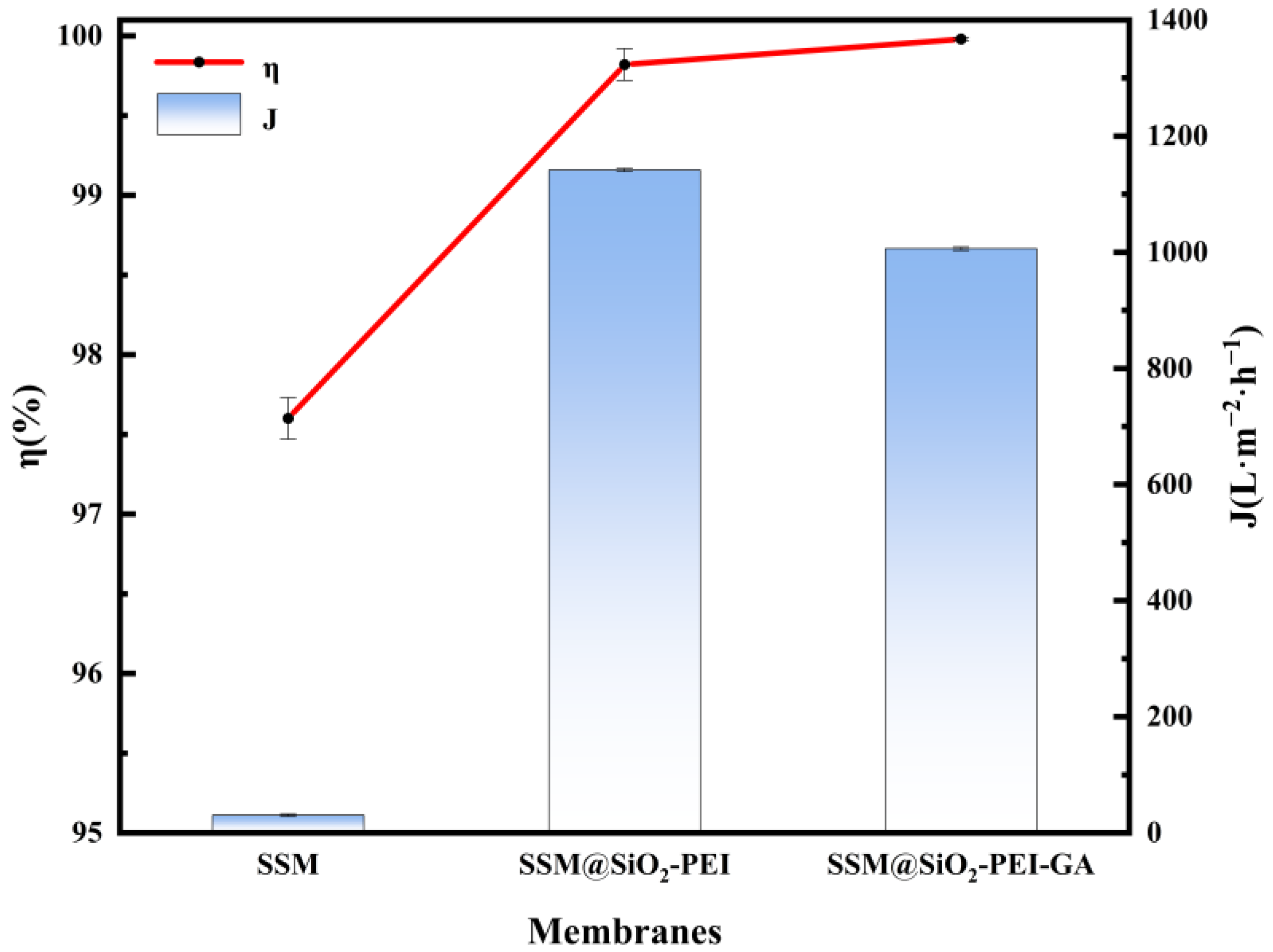
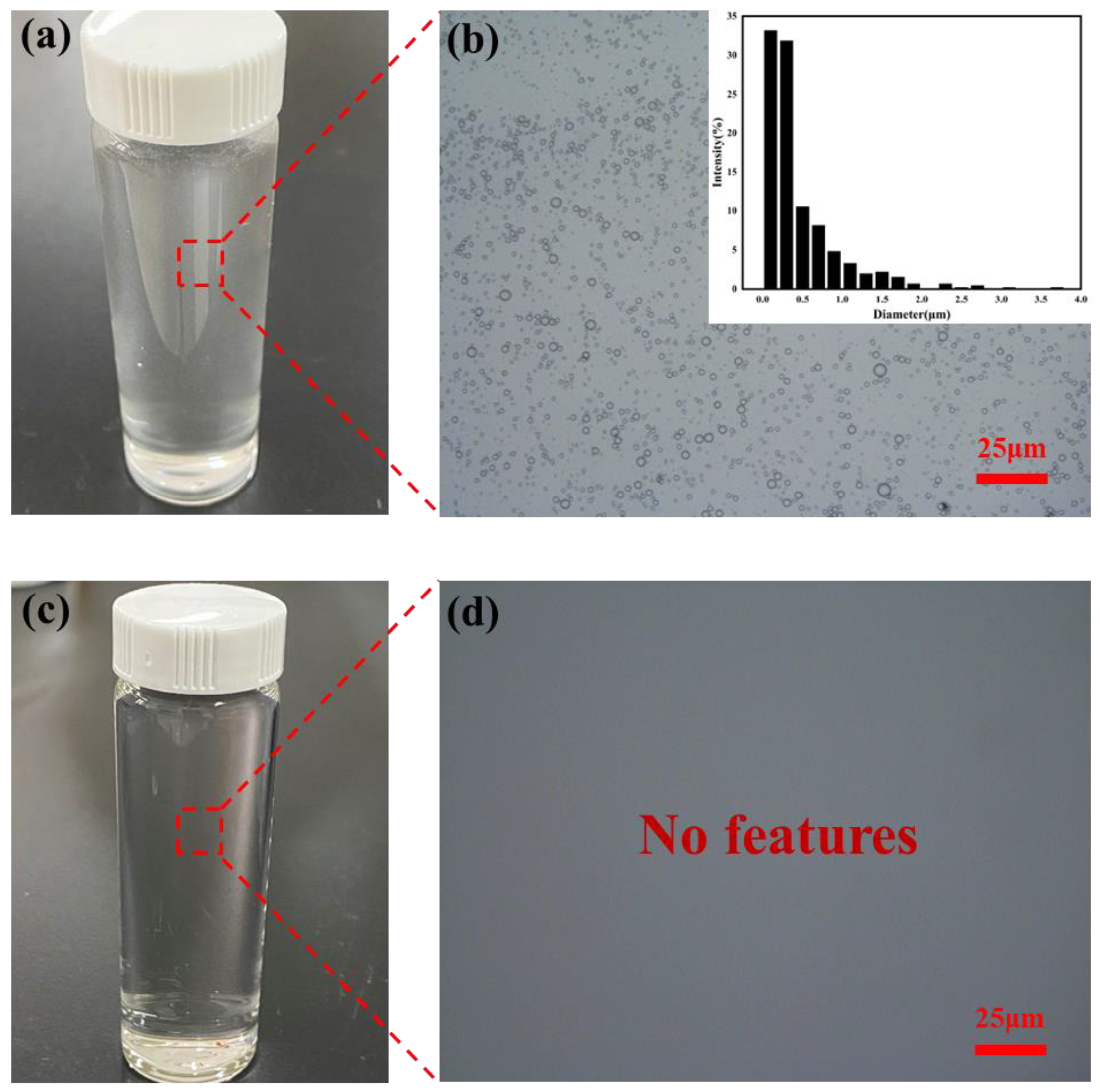
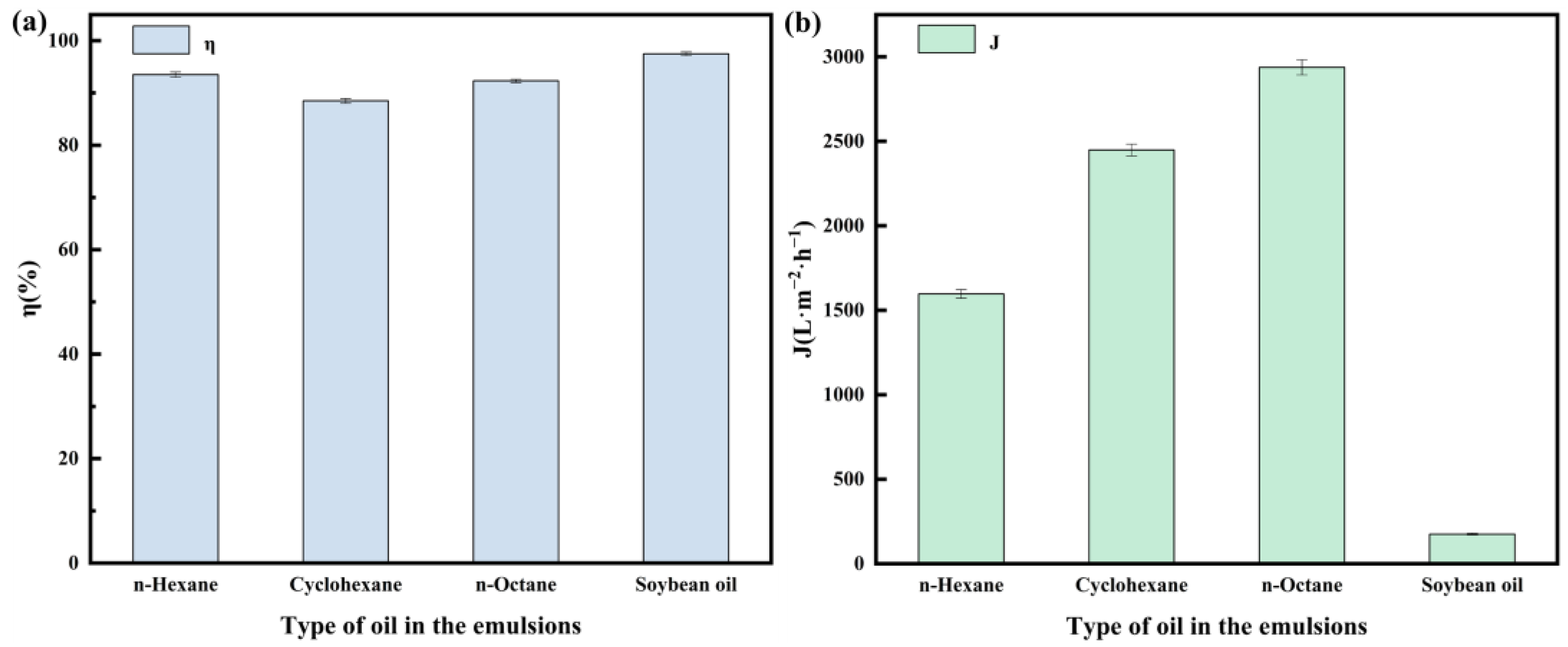
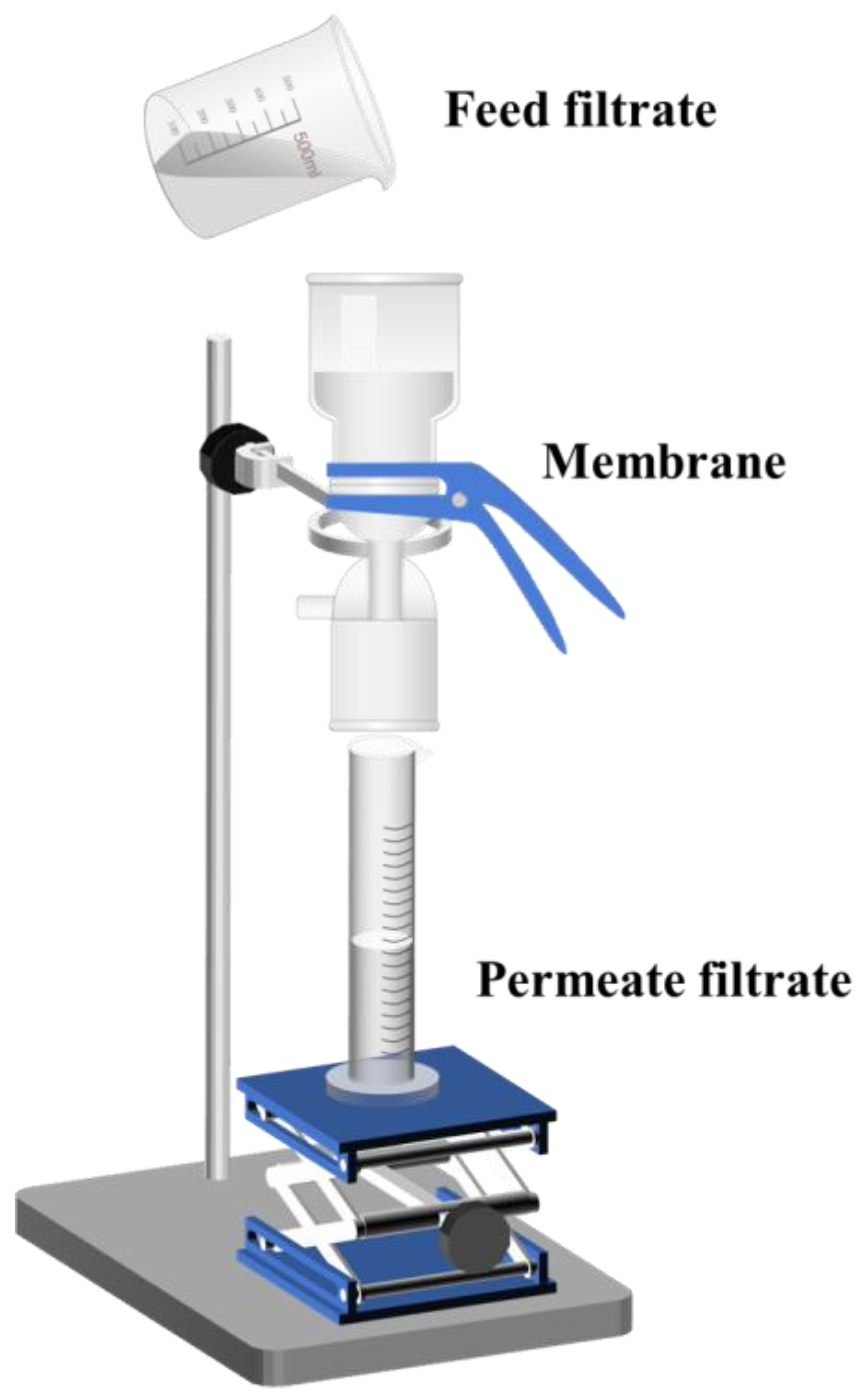
2.3. Oil–Water Separation Performance
2.4. Reusability and Antifouling Performance Evaluation
3. Simulation Results and Analysis
4. Materials and Methods
4.1. Materials
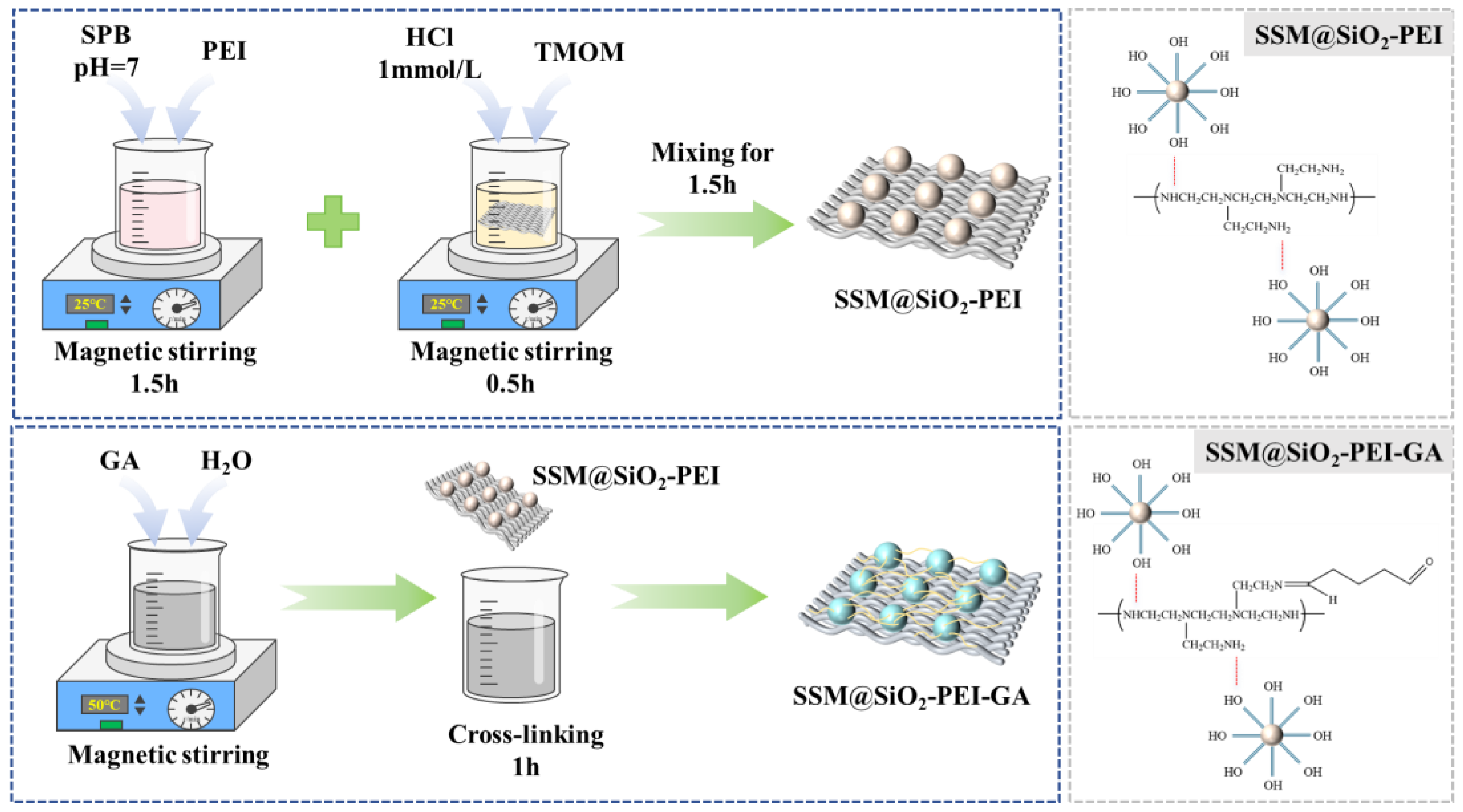
4.2. Preparation of SSM@SiO2-PEI-GA
4.3. Oil–Water Separation Experiments
4.4. Characterization of Membranes
4.5. Molecular Dynamics Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Zhang, L.; Lai, X.; Li, H.; Zeng, X. Highly hydrophobic F-rGO@wood sponge for efficient clean-up of viscous crude oil. Chem. Eng. J. 2020, 386, 123994. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Chen, C.; Wei, Y.; Xu, X.; Liu, H.; Kuang, C.; Yang, G.; Li, X.; Qing, Y.; et al. Multifunctional superhydrophobic coating constructed from rosin-based polymer and nano-boehmite particles for oil-water separation, flame retardancy and anti-icing. Prog. Org. Coat. 2025, 198, 108872. [Google Scholar] [CrossRef]
- Dan, H.; Ji, K.; Gao, Y.; Yin, W.; Gao, B.; Yue, Q. Fabrication of superhydrophobic Enteromorpha-derived carbon aerogels via NH4H2PO4 modification for multi-behavioral oil/water separation. Sci. Total Environ. 2022, 837, 155869. [Google Scholar] [CrossRef]
- Fan, Q.; Lu, T.; Deng, Y.; Zhang, Y.; Ma, W.; Xiong, R.; Huang, C. Bio-based materials with special wettability for oil-water separation. Sep. Purif. Technol. 2022, 297, 121445. [Google Scholar] [CrossRef]
- Xue, Z.; Cao, Y.; Liu, N.; Feng, L.; Jiang, L. Special wettable materials for oil/water separation. J. Mater. Chem. A 2014, 2, 2445–2460. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/Water Separation with Selective Superantiwetting/Superwetting Surface Materials. Angew. Chem. Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef]
- Liu, H.; Dai, K.; Deng, J.; Zhao, L.; Yu, H.; Zhang, H.; Tong, Y.; Wu, L.; Sun, L. Synthesis of antibacterial polyaluminium silicate sulfate/sepiolitenano composite coagulant for oilfield sewage treatment. J. Clean. Prod. 2022, 379, 134385. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, Z.; Li, M.; Cheng, C.; Wang, M.; Jiao, R.; Sun, H.; Li, A. Preparation of halloysite-based PVDF membrane for effective oil/water separation and dyes removal. Sep. Purif. Technol. 2025, 359, 130595. [Google Scholar] [CrossRef]
- Fu, Y.; Guo, Z. Natural polysaccharide-based aerogels and their applications in oil–water separations: A review. J. Mater. Chem. A 2022, 10, 8129–8158. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, X.; Chen, B.; Wang, N.; Chen, G. Electrospinning superhydrophobic–superoleophilic PVDF-SiO nanofibers membrane for oil–water separation. J. Appl. Polym. Sci. 2020, 137, 49546. [Google Scholar] [CrossRef]
- Tan, Q.; Chen, Z.; Zuo, J.; Wang, Y.; Jin, X.; Wen, X.; Xu, S.; Nong, Y.; Pi, P. A robust superwetting nickel foam with tuning pore features for stable and efficient separation of oil-in-water emulsions. Sep. Purif. Technol. 2024, 339, 126602. [Google Scholar] [CrossRef]
- Li, B.; Qian, X.; Ran, L.; Han, J.; Yang, C.; Jiao, T. Applications and challenges of superwetted oil-water separation membranes in air under liquids and in specific environments. Prog. Org. Coat. 2024, 195, 108673. [Google Scholar] [CrossRef]
- Baig, U.; Faizan, M.; Sajid, M. Multifunctional membranes with super-wetting characteristics for oil-water separation and removal of hazardous environmental pollutants from water: A review. Adv. Colloid Interface Sci. 2020, 285, 102276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, T.; Zhang, D.; Sun, S.; Liu, J.; Li, B.; Shi, Z. The preparation of superhydrophobic polylactic acid membrane with adjustable pore size by freeze solidification phase separation method for oil–water separation. Molecules 2023, 28, 5590. [Google Scholar] [CrossRef]
- Kong, W.; Li, F.; Pan, Y.; Zhao, X. Hygro-responsive, Photo-decomposed Superoleophobic/Superhydrophilic Coating for On-Demand Oil–Water Separation. ACS Appl. Mater. Interfaces 2021, 13, 35142–35152. [Google Scholar] [CrossRef]
- Yang, J.; Yin, L.; Tang, H.; Song, H.; Gao, X.; Liang, K.; Li, C. Polyelectrolyte-fluorosurfactant complex-based meshes with superhydrophilicity and superoleophobicity for oil/water separation. Chem. Eng. J. 2015, 268, 245–250. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Alolaimi, R.F.; Alzaid, M.; Al-Muaikel, N.S.; Alhumaimess, M.S.; Alsohaimi, I.H.; Rafea, M.A.; Zaki, M.E.A.; El-Sayed, M.Y.; Hassan, H.M.A. Oil/Water separation using thiol-functionalized mesoporous silica-impregnated polyacrylonitrile/polyvinylidene fluoride electrospun nanofiber hybrid nanostructures. Sep. Purif. Technol. 2025, 362, 131651. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Superhydrophilic and underwater superoleophobic membranes - A review of synthesis methods. Prog. Polym. Sci. 2019, 98, 101166. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, F.; Guo, Z. The antifouling mechanism and application of bio-inspired superwetting surfaces with effective antifouling performance. Adv. Colloid Interface Sci. 2024, 325, 103097. [Google Scholar] [CrossRef]
- Sun, W.; Ding, L.; Xu, P.; Zhu, B.; Ma, K.C.; Chen, Q. Facile preparation of marine carrageenan hydrogel-coated steel mesh with superhydrophilic and underwater superoleophobic performance for highly efficient oil–water separation. Water Environ. Res. 2025, 97, e70006. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Wang, T.; Cai, Y.; Feng, F.; He, F.; Yi, L. Demulsification and separation of crude oil-in-water emulsion using superhydrophilic silica-based nanocomposite membranes. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133863. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Chen, J.; Dong, L.; Tian, Y.; Cui, Z.; Li, J.; He, B.; Yan, F. Demulsifier-inspired superhydrophilic/underwater superoleophobic membrane modified with polyoxypropylene polyoxyethylene block polymer for enhanced oil/water separation properties. Molecules 2023, 28, 1282. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zheng, X.; Xu, T.; Ji, B.; Mei, J.; Li, Z. A self-cleaning TiO2 bacterial cellulose super-hydrophilic underwater super-oleophobic composite membrane for efficient oil–water separation. Molecules 2023, 28, 3396. [Google Scholar] [CrossRef]
- Xu, X.; Kao, H.; Yu, X.; Zhou, J.; Hou, P.; Xu, G.; Chen, J. Green Fabrication of Superhydrophilic/Underwater Superoleophobic Composite Membrane for High-Efficiency Oil/Water Separation in Harsh Environments. Langmuir 2024, 40, 11661–11669. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, F.; Ji, W.; Xia, L.; Li, L.; Chen, M.; Wang, H. Bioinspired Janus membrane of polyacrylonitrile/poly (vinylidene fluoride)@poly (vinylidene fluoride)-methyltriethoxysilane for oil-water separation. J. Membr. Sci. 2023, 687, 122090. [Google Scholar] [CrossRef]
- Zhang, D.; Lyu, Z.; Peng, D.; Peng, W.; Xue, W. Fabrication of Polytetrafluoroethylene Composite Membrane via Laser Perforating and Nanosilica Coating for Efficient Oil–Water Separation. J. Appl. Polym. Sci. 2025, 142, e56789. [Google Scholar] [CrossRef]
- Zeng, B.; Liang, J.; Fu, C.; Zhang, Z.; Xu, H.; Yu, D.; Luo, X.; Liu, W.; Deng, X.; Yang, G.; et al. Synergistic effect of nano-silica and eco-friendly hydrogel for the cost-effective and highly efficient oil-water separation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128136. [Google Scholar] [CrossRef]
- Hu, X.; Xie, H.; Wang, H.; Shen, L.; Li, R.; Lin, H.; Zhao, L.; Yu, G. Enhancing oil/water emulsion separation with inkjet printed beetle-inspired zeolitic imidazolate framework-67 modified membranes. Desalination 2025, 604, 118729. [Google Scholar] [CrossRef]
- Dou, Y.-L.; Lv, C.-J.; Yue, X.; Su, Y.; Yasin, A.; Ma, P.-C. Temperature and pH-responsive electrospun membrane with high flux recovery for emulsion separation. Sep. Purif. Technol. 2025, 358, 130247. [Google Scholar] [CrossRef]
- Xia, D.; Pan, H.; Yang, C.; Chen, Z.; Liao, M.; Lin, Q. One-step separation of oil-water emulsion with heavy metal ions by PVDF membrane modified with zwitterionic coating. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134855. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Zhang, G.; Wang, Q.; Shen, S.; Liu, D.; Hong, Y.; Wyman, I. Dialdehyde cellulose (DAC) and polyethyleneimine (PEI) coated polyvinylidene fluoride (PVDF) membrane for simultaneously removing emulsified oils and anionic dyes. J. Hazard. Mater. 2024, 471, 134341. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Wang, L.; Zhang, Y.; Liang, F.; Fan, Y. Modified ceramic membrane with pH/ethanol induced switchable superwettability for antifouling separation of oil-in-acidic water emulsions. Sep. Purif. Technol. 2022, 293, 121022. [Google Scholar] [CrossRef]
- Khan, I.A.; Falath, W.; Baig, N.; Aljundi, I. Modification of PVDF membranes using polyvinyl alcohol-crosslinked functionalized nano-silica sheets: High flux and antifouling properties for efficient oil-water separation. J. Taiwan Inst. Chem. Eng. 2024, 163, 105636. [Google Scholar] [CrossRef]
- Qing, W.; Li, X.; Wu, Y.; Shao, S.; Guo, H.; Yao, Z.; Chen, Y.; Zhang, W.; Tang, C.Y. In situ silica growth for superhydrophilic-underwater superoleophobic Silica/PVA nanofibrous membrane for gravity-driven oil-in-water emulsion separation. J. Membr. Sci. 2020, 612, 118476. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, J.; Yan, L.; Wang, K.; Zhang, Y.; Drioli, E.; Ma, J. Biodegradable nanofiber membranes based on interpenetrating network for highly efficient oil/water separation. Adv. Compos. Hybrid Mater. 2024, 7, 197. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Bangi, U.K.H.; Latthe, S.S.; Mahadik, S.A.; Rao, A.V. Self-cleaning silica coatings on glass by single step sol–gel route. Surf. Coat. Technol. 2011, 205, 5338–5344. [Google Scholar] [CrossRef]
- Hoijang, S.; Kunakham, T.; Manokruang, K.; Saiai, A.; Ananta, S.; Leem, G.; Srisombat, L. Silica-Coated Magnesium Ferrite Nanoparticles/Glutaraldehyde-Cross-Linked Polyethylenimine Composite Adsorbent for Ultrahigh Remediation of 2,4-Dichlorophenoxyacetic Acid. ACS Appl. Nano Mater. 2023, 6, 21967–21979. [Google Scholar] [CrossRef]
- Li, Z.; Du, G.; Yang, H.; Liu, T.; Yuan, J.; Liu, C.; Li, J.; Ran, X.; Gao, W.; Yang, L. Construction of a cellulose-based high-performance adhesive with a crosslinking structure bridged by Schiff base and ureido groups. Int. J. Biol. Macromol. 2022, 223, 971–979. [Google Scholar] [CrossRef]
- Lin, W.-T.; Fu, P.; Li, W.-L.; Yu, Y.-H.; Zhang, Z.-L.; Fan, H.-Y.; Huang, X.-J.; Xu, Z.-K.; Wan, L.-S. Cross-linked g-C3N4 nanofibers enable thermal stable composite membranes for high-performance loose nanofiltration. Chem. Eng. J. 2024, 494, 153197. [Google Scholar] [CrossRef]
- Chen, S.; Li, K.; Li, X.; Chen, Z.; Su, X.; Zhang, X.; Xie, H.; Zhou, Y.; Wu, W. Degradable polyvinyl alcohol/chitosan@carnauba wax superhydrophobic composite membrane for water-in-oil emulsion separation and heavy metal adsorption. Int. J. Biol. Macromol. 2024, 280, 135603. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, F.; Lu, H.; Li, N.; Peng, B.; Yu, H.; Yuan, Y.; Zhang, H. Improved antifouling performance of a polyamide composite reverse osmosis membrane by surface grafting of dialdehyde carboxymethyl cellulose (DACMC). J. Membr. Sci. 2021, 620, 118843. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, C.; Li, Y.; Qin, Z.; Li, X.; Li, Y.; Zhang, K. Double cross-linked chitosan sponge encapsulated with ZrO2/soy protein isolate amyloid fibrils nanoparticles for the fluoride ion removal from water. Int. J. Biol. Macromol. 2024, 279, 135520. [Google Scholar] [CrossRef] [PubMed]
- Bucatariu, F.; Ghiorghita, C.-A.; Zaharia, M.-M.; Schwarz, S.; Simon, F.; Mihai, M. Removal and Separation of Heavy Metal Ions from Multicomponent Simulated Waters Using Silica/Polyethyleneimine Composite Microparticles. ACS Appl. Mater. Interfaces 2020, 12, 37585–37596. [Google Scholar] [CrossRef] [PubMed]
- de Farias, L.M.S.; Ghislandi, M.G.; de Aguiar, M.F.; Silva, D.B.R.S.; Leal, A.N.R.; de AO Silva, F.; Fraga, T.J.M.; de Melo, C.P.; Alves, K.G.B. Electrospun polystyrene/graphene oxide fibers applied to the remediation of dye wastewater. Mater. Chem. Phys. 2022, 276, 125356. [Google Scholar] [CrossRef]
- Teng, L.; Yue, C.; Zhang, G. Epoxied SiO2 nanoparticles and polyethyleneimine (PEI) coated polyvinylidene fluoride (PVDF) membrane for improved oil water separation, anti-fouling, dye and heavy metal ions removal capabilities. J. Colloid Interface Sci. 2023, 630, 416–429. [Google Scholar] [CrossRef]
- Neville, F.; Murphy, T.; Wanless, E.J. The formation of polyethyleneimine–trimethoxymethylsilane organic–inorganic hybrid particles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 42–50. [Google Scholar] [CrossRef]
- Park, M.J.; Gonzales, R.R.; Abdel-Wahab, A.; Phuntsho, S.; Shon, H.K. Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination 2018, 426, 50–59. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, F.; Zhang, J.; Wang, Y.; Yang, S.; Xing, D. Gradient crosslinking optimization for the selective layer to prepare polyvinyl alcohol (PVA) nanofiltration (NF) membrane: The enhanced filtration performance and potential rejection for EDCs. J. Membr. Sci. 2023, 675, 121548. [Google Scholar] [CrossRef]
- Shan, Z.; Huang, J.; Huang, Y.; Zhou, Y.; Li, Y. Glutaraldehyde crosslinked ternary carboxymethylcellulose/polyvinyl alcohol/polyethyleneimine film with enhanced mechanical properties, water resistance, antibacterial activity, and UV-shielding ability without any UV absorbents. Int. J. Biol. Macromol. 2024, 277, 134563. [Google Scholar] [CrossRef]
- Srinivasa Rao, P.; Smitha, B.; Sridhar, S.; Krishnaiah, A. Preparation and performance of poly(vinyl alcohol)/polyethyleneimine blend membranes for the dehydration of 1,4-dioxane by pervaporation: Comparison with glutaraldehyde cross-linked membranes. Sep. Purif. Technol. 2006, 48, 244–254. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Li, J.; Zhang, P.F.; Xu, P.; Li, Z.; Hu, M.Y.; Mai, Z.H.; Guan, K.C.; Matsuyama, H. Hydrogel membrane composite reduces fouling and retains ammonium efficiently. Environ. Chem. Lett. 2024, 22, 1615–1621. [Google Scholar] [CrossRef]
- Ning, D.; Lu, Z.; Tian, C.; Yan, N.; Hua, L. Hierarchical and superwettable cellulose acetate nanofibrous membranes decorated via 3D flower-like layered double hydroxides for efficient oil/water separation. Sep. Purif. Technol. 2024, 342, 127052. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Wang, S.; Cheng, L.; Matsuyama, H. Engineering of ultrafine polydopamine nanoparticles in-situ assembling on polyketone substrate for highly-efficient oil-water emulsions separation. J. Membr. Sci. 2020, 613, 118501. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Yuan, P.; Xu, X.; Yang, J. Construction of a Covalent Crosslinked Membrane Exhibiting Superhydrophilicity and Underwater Superoleophobicity for the Efficient Separation of High-Viscosity Oil–Water Emulsion Under Gravity. Molecules 2025, 30, 1840. https://doi.org/10.3390/molecules30081840
Zhou M, Yuan P, Xu X, Yang J. Construction of a Covalent Crosslinked Membrane Exhibiting Superhydrophilicity and Underwater Superoleophobicity for the Efficient Separation of High-Viscosity Oil–Water Emulsion Under Gravity. Molecules. 2025; 30(8):1840. https://doi.org/10.3390/molecules30081840
Chicago/Turabian StyleZhou, Mengxi, Peiqing Yuan, Xinru Xu, and Jingyi Yang. 2025. "Construction of a Covalent Crosslinked Membrane Exhibiting Superhydrophilicity and Underwater Superoleophobicity for the Efficient Separation of High-Viscosity Oil–Water Emulsion Under Gravity" Molecules 30, no. 8: 1840. https://doi.org/10.3390/molecules30081840
APA StyleZhou, M., Yuan, P., Xu, X., & Yang, J. (2025). Construction of a Covalent Crosslinked Membrane Exhibiting Superhydrophilicity and Underwater Superoleophobicity for the Efficient Separation of High-Viscosity Oil–Water Emulsion Under Gravity. Molecules, 30(8), 1840. https://doi.org/10.3390/molecules30081840





