New Antioxidant Triphenol-Derived Hydrazide-Hydrazone Thiazole: Formation and Analysis of Inclusion Complex with β-CD Using Experimental and Computational Approaches
Abstract
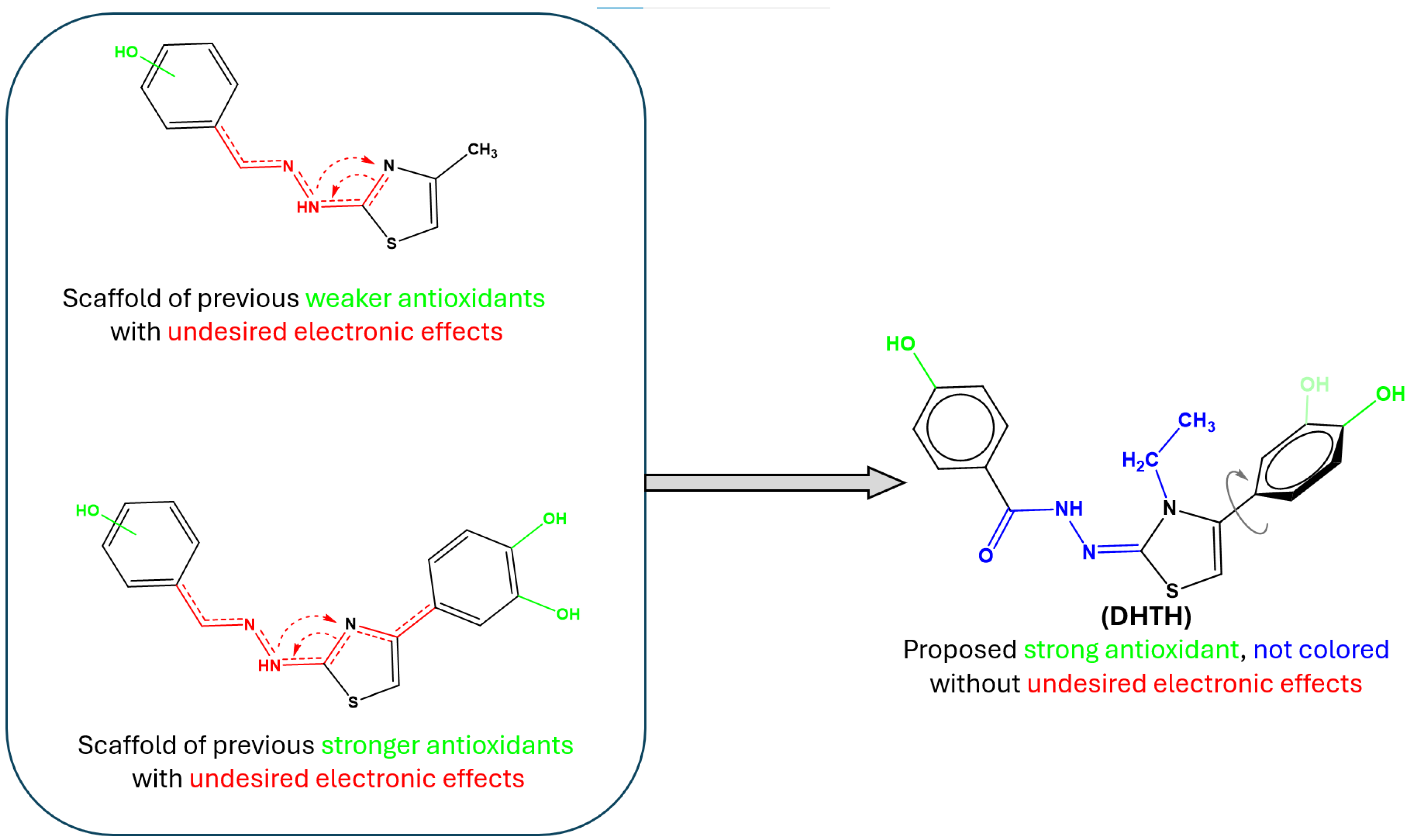
:1. Introduction
2. Results and Discussion
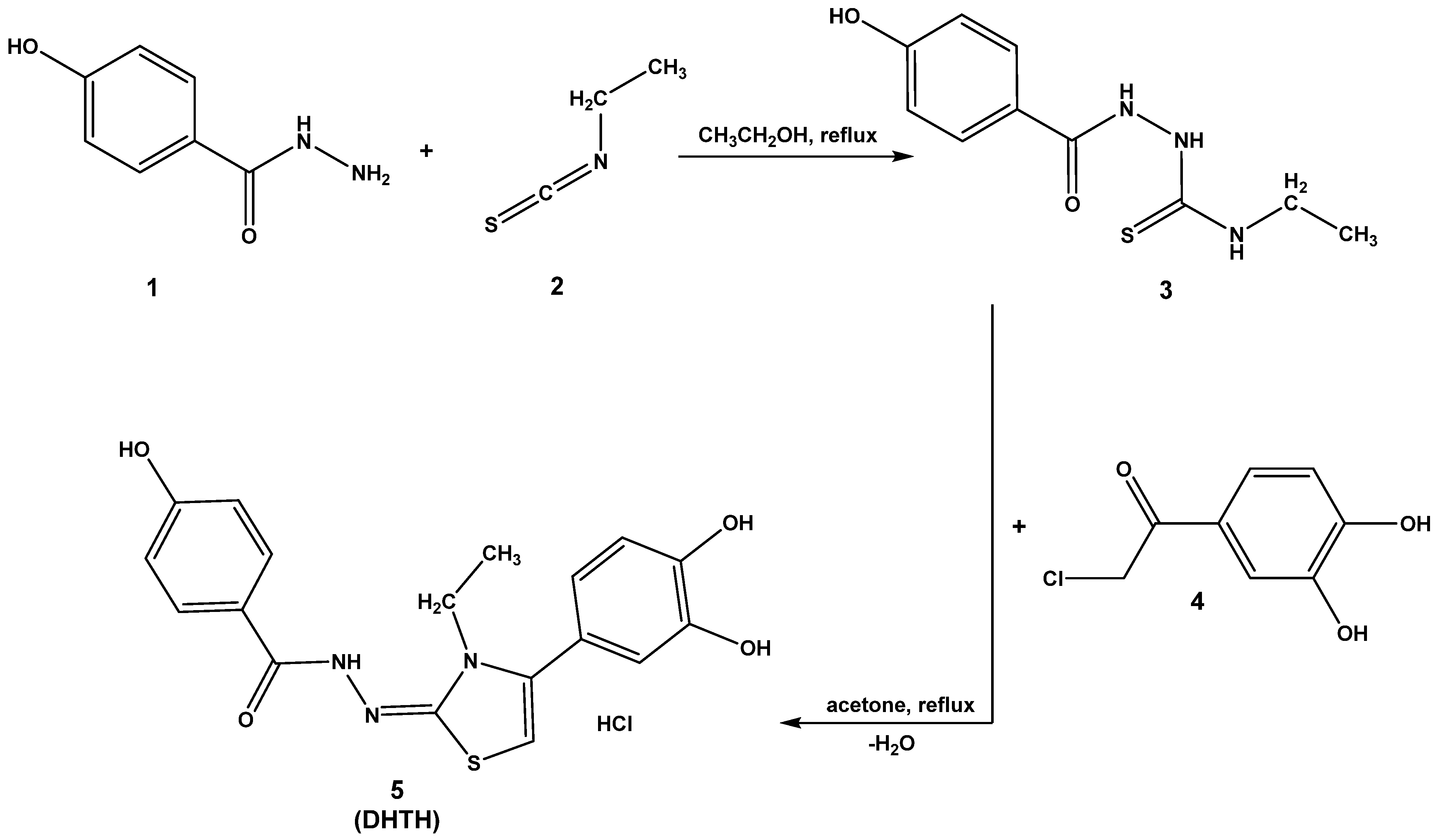
2.1. Chemical Synthesis
2.2. Determination of the Stoichiometry by NMR
2.3. Isothermal Titration Calorimetry
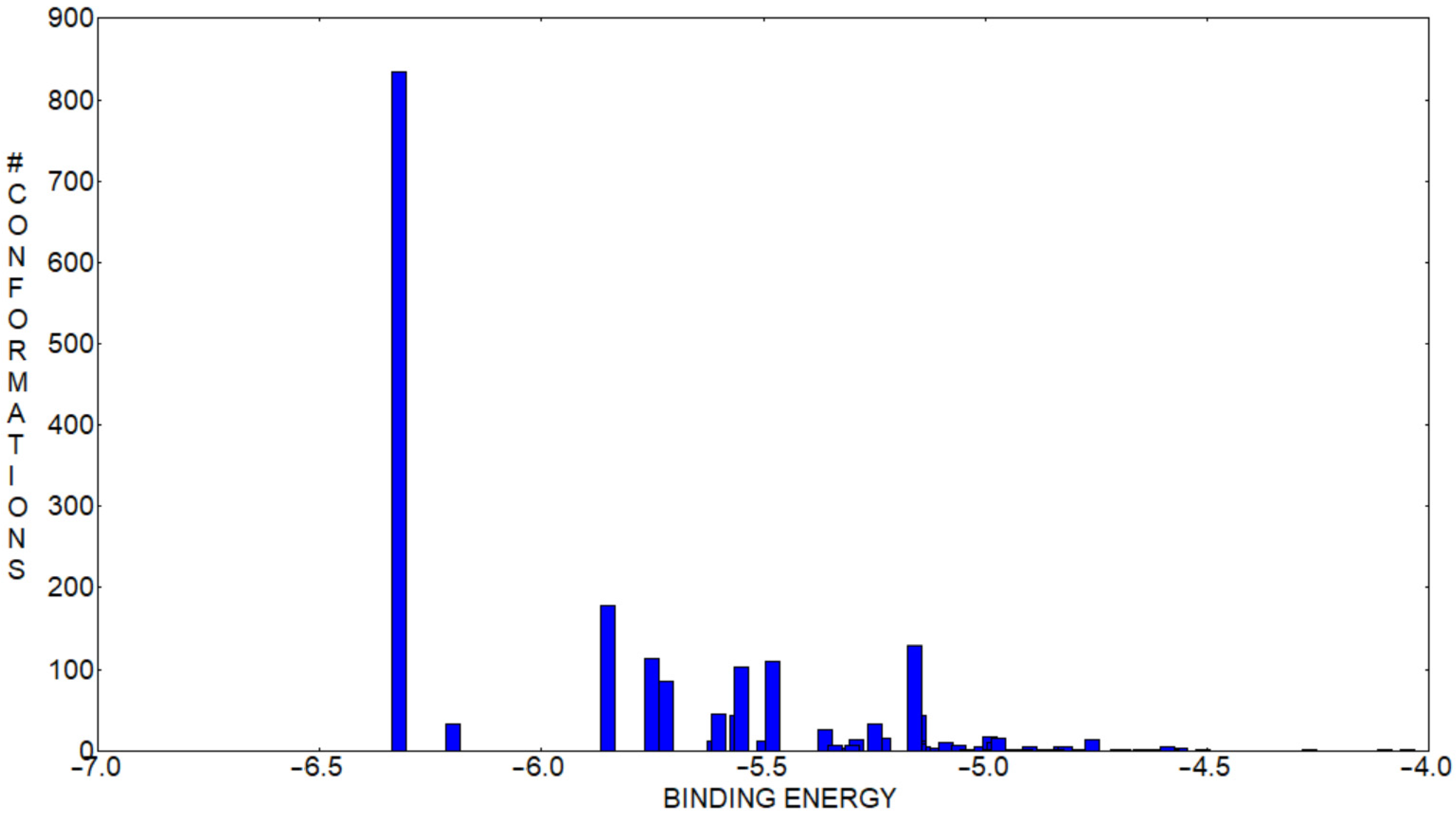
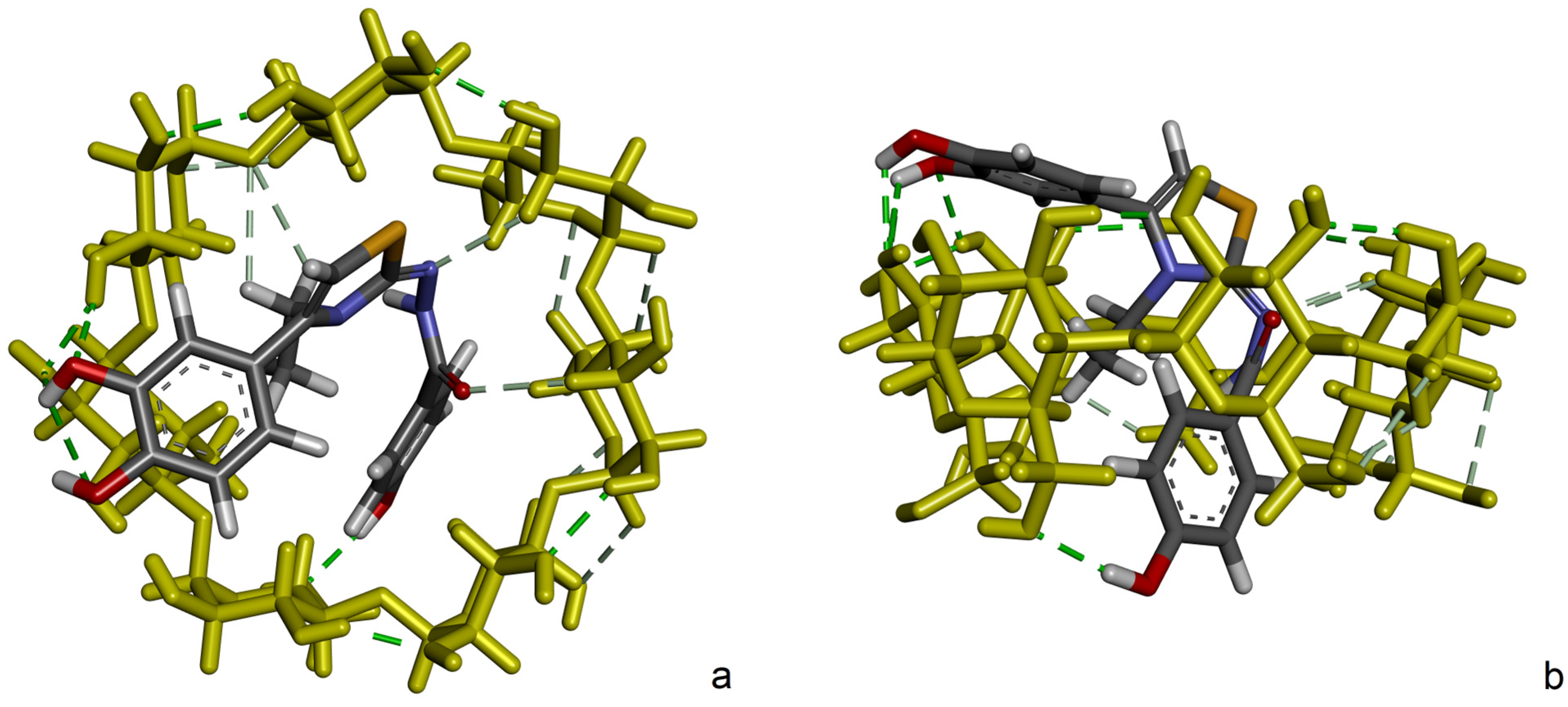
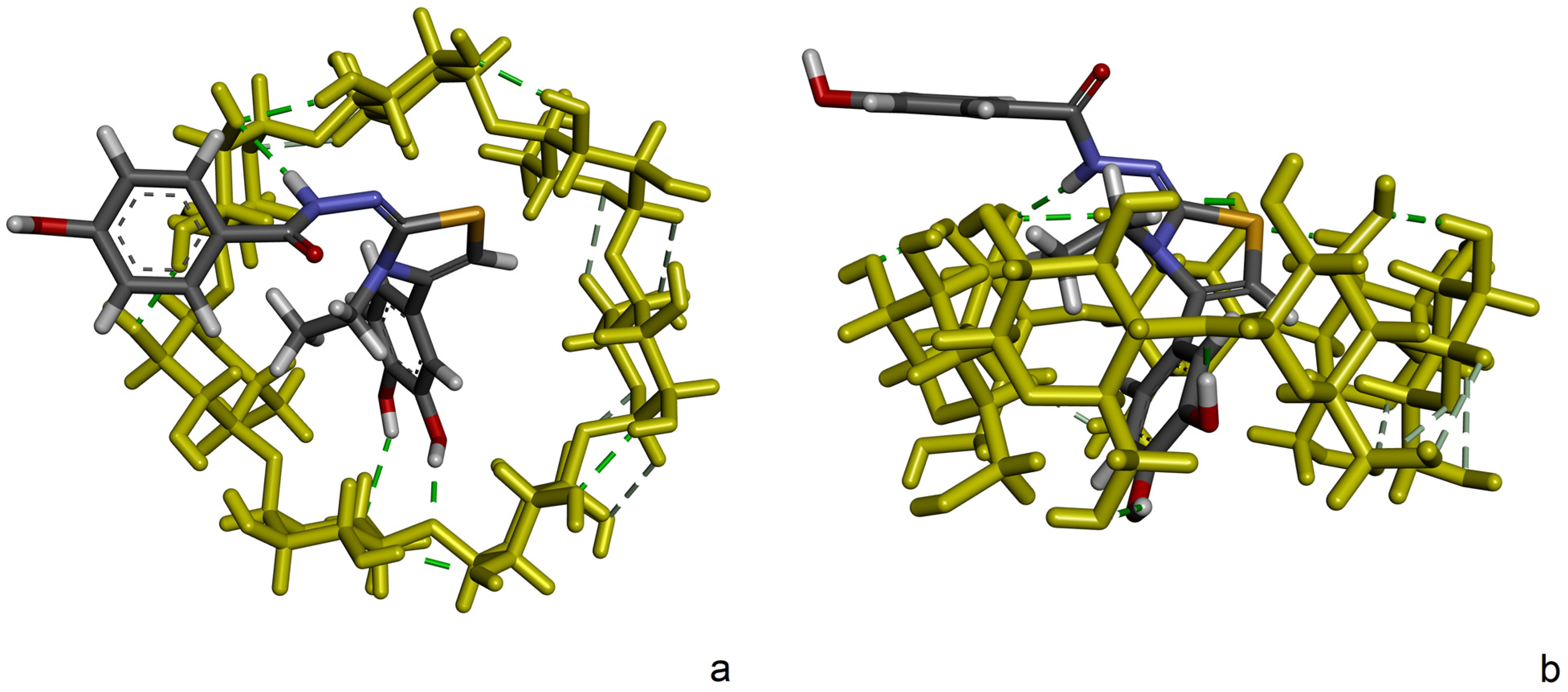
2.4. Molecular Docking
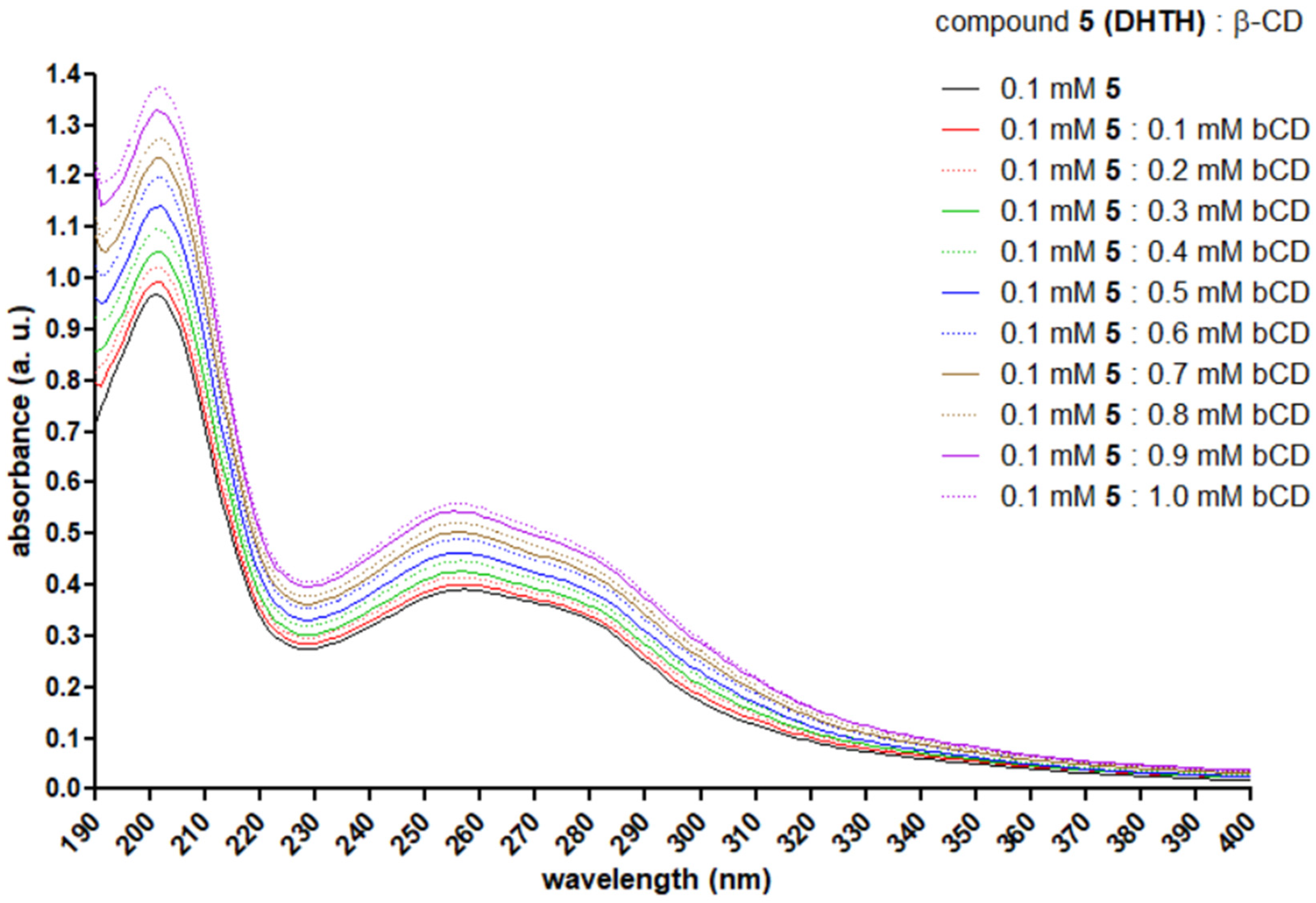
2.5. UV Spectroscopy
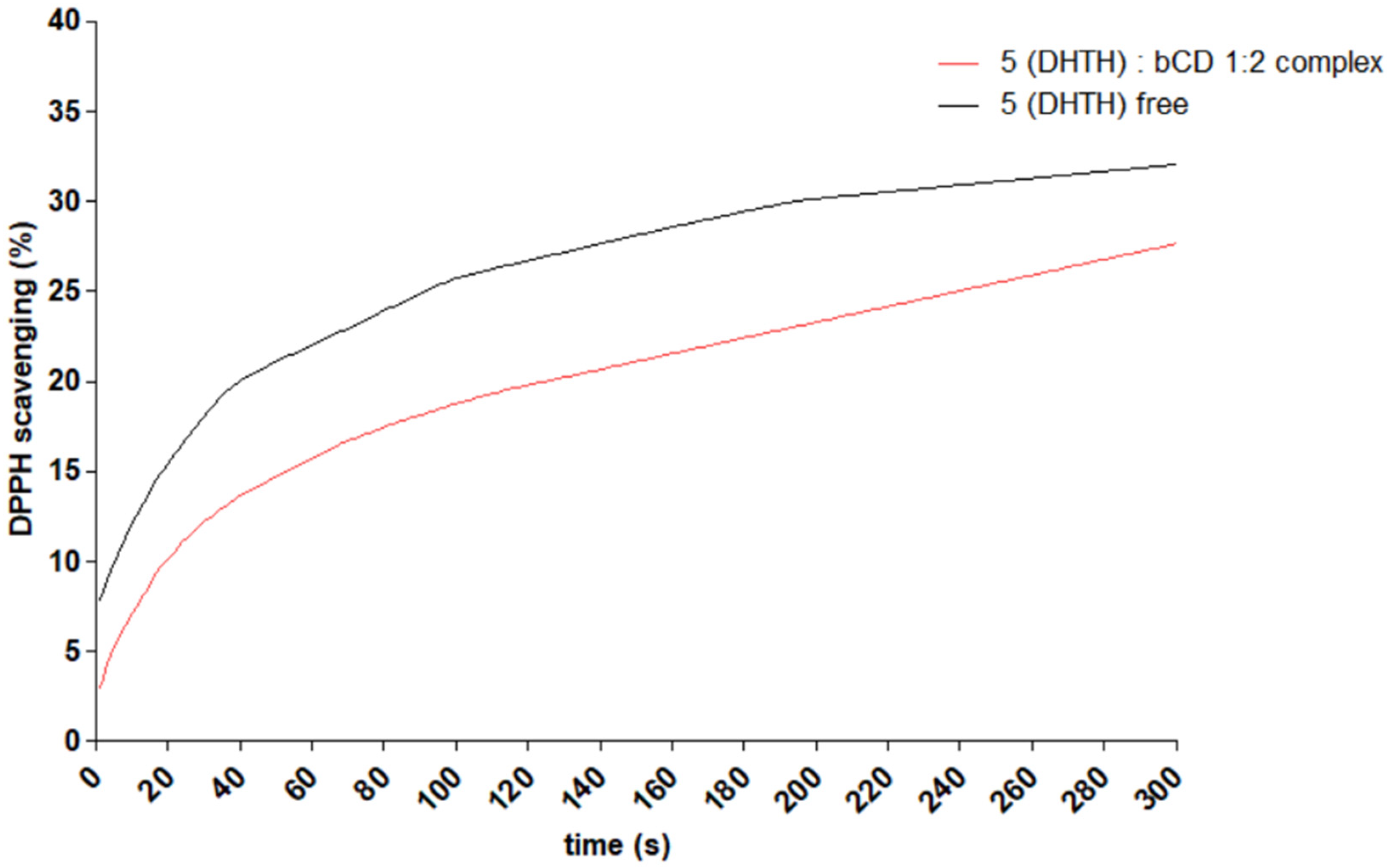
2.6. Antioxidant Activity Evaluation
3. Materials and Methods
3.1. Chemical Synthesis
3.2. NMR Spectroscopy
3.3. Isothermal Titration Calorimetry
3.4. Molecular Docking
3.5. UV Spectroscopy
3.6. Antioxidant Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; An, L.; Dou, Y.; Liu, Y. Density functional theory study of the structure–antioxidant activity of polyphenolic deoxybenzoins. Food Chem. 2014, 151, 198–206. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Rekka, E.A. A Review on Vitamin E Natural Analogues and on the Design of Synthetic Vitamin E Derivatives as Cytoprotective Agents. Mini-Rev. Med. Chem. 2021, 21, 10–22. [Google Scholar] [CrossRef]
- Prakash, S.; Elavarasan, N.; Subashini, K.; Kanaga, S.; Dhandapani, R.; Sivanandam, M.; Kumaradhas, P.; Thirunavukkarasu, C.; Sujatha, V. Isolation of hesperetin—A flavonoid from Cordia sebestena flower extract through antioxidant assay guided method and its antibacterial, anticancer effect on cervical cancer via in vitro and in silico molecular docking studies. J. Mol. Struct. 2020, 1207, 127751. [Google Scholar] [CrossRef]
- Pereira, D.; Valentão, P.; Pereira, J.; Andrade, P. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- López-Yerena, A.; Perez, M.; Vallverdú-Queralt, A.; Escribano-Ferrer, E. Insights into the Binding of Dietary Phenolic Compounds to Human Serum Albumin and Food-Drug Interactions. Pharmaceutics 2020, 12, 1123. [Google Scholar] [CrossRef]
- Latruffe, N.; Menzel, M.; Delmas, D.; Buchet, R.; Lançon, A. Compared Binding Properties between Resveratrol and Other Polyphenols to Plasmatic Albumin: Consequences for the Health Protecting Effect of Dietary Plant Microcomponents. Molecules 2014, 19, 17066–17077. [Google Scholar] [CrossRef]
- Marc, G.; Stana, A.; Tertiş, M.; Cristea, C.; Ciorîţă, A.; Drăgan, Ș.-M.; Toma, V.-A.; Borlan, R.; Focșan, M.; Pîrnău, A.; et al. Discovery of New Hydrazone-Thiazole Polyphenolic Antioxidants through Computer-Aided Design and In Vitro Experimental Validation. Int. J. Mol. Sci. 2023, 24, 13277. [Google Scholar] [CrossRef]
- Zhang, Y.; Takahama, K.; Osawa, Y.; Kuwahara, D.; Yamada, R.; Oyama, K.; Honda, M. Characteristics of LED light-induced geometrical isomerization and degradation of astaxanthin and improvement of the color value and crystallinity of astaxanthin utilizing the photoisomerization. Food Res. Int. 2023, 174, 113553. [Google Scholar] [CrossRef]
- Pinto, C.M.; Clementi, C.; Sabatini, F.; Degano, I.; Romani, A.; Seixas de Melo, J.S. Unveiling the photostability of the molecule of colour shikonin: From solution to the solid state. J. Mol. Struct. 2024, 1316, 138898. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural Food Colorants and Preservatives: A Review, a Demand, and a Challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Phukan, N.; Baruah, J.B. Imine-tautomers of aminothiazole derivatives: Intriguing aspects of chemical reactivities. CrystEngComm 2016, 18, 3877–3890. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Gao, J. Tautomers of 2-aminothiazole molecules in aqueous solutions explored by Raman, SERS and DFT methods. J. Mol. Struct. 2013, 1049, 362–367. [Google Scholar] [CrossRef]
- Batool, A.; Muddassir, M.; Shahid, K. Synthesis of Hydrazide Derivative of Betulinic Acid, Its Organometallic Complexes, Characterization and Bioassay. Chem. Biodivers. 2024, 21, e202301275. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazmi, G.A.A.; El-Metwally, N. A series of nickel(II) complexes derived from hydrazide derivatives, electrochemical, thermal and spectral studies. Arab. J. Chem. 2017, 10, S1003–S1013. [Google Scholar] [CrossRef]
- Paixão, D.A.; Marzano, I.M.; Jaimes, E.H.L.; Pivatto, M.; Campos, D.L.; Pavan, F.R.; Deflon, V.M.; da S. Maia, P.I.; Da Costa Ferreira, A.M.; Uehara, I.A.; et al. Novel copper(II) complexes with hydrazides and heterocyclic bases: Synthesis, structure and biological studies. J. Inorg. Biochem. 2017, 172, 138–146. [Google Scholar] [CrossRef]
- Ribeiro, N.; Correia, I. A review of hydrazide-hydrazone metal complexes’ antitumor potential. Front. Chem. Biol. 2024, 3, 1398873. [Google Scholar] [CrossRef]
- Hippeli, S.; Elstner, E.F. Transition metal ion-catalyzed oxygen activation during pathogenic processes. FEBS Lett. 1999, 443, 1–7. [Google Scholar] [CrossRef]
- Puentes-Díaz, N.; Chaparro, D.; Morales-Morales, D.; Flores-Gaspar, A.; Alí-Torres, J. Role of Metal Cations of Copper, Iron, and Aluminum and Multifunctional Ligands in Alzheimer’s Disease: Experimental and Computational Insights. ACS Omega 2023, 8, 4508–4526. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 1247. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar] [CrossRef]
- Menezes, L.B.; Sampaio, R.M.S.N.; Meurer, L.; Szpoganicz, B.; Cervo, R.; Cargnelutti, R.; Wang, L.; Yang, J.; Prabhakar, R.; Fernandes, C.; et al. A Multipurpose Metallophore and Its Copper Complexes with Diverse Catalytic Antioxidant Properties to Deal with Metal and Oxidative Stress Disorders: A Combined Experimental, Theoretical, and In Vitro Study. Inorg. Chem. 2024, 63, 14827–14850. [Google Scholar] [CrossRef] [PubMed]
- Csire, G.; Canabady-Rochelle, L.; Averlant-Petit, M.-C.; Selmeczi, K.; Stefan, L. Both metal-chelating and free radical-scavenging synthetic pentapeptides as efficient inhibitors of reactive oxygen species generation. Metallomics 2020, 12, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Meunier, B.; Robert, A. Catechol-Based Ligands as Potential Metal Chelators Inhibiting Redox Activity in Alzheimer’s Disease. Eur. J. Inorg. Chem. 2017, 2017, 3198–3204. [Google Scholar] [CrossRef]
- Sánchez, P.; Gálvez, N.; Colacio, E.; Miñones, E.; Domínguez-Vera, J.M. Catechol releases iron(III) from ferritin by direct chelation without iron(II) production. Dalt. Trans. 2005, 811–813. [Google Scholar] [CrossRef]
- Sanjay, L.R.; Ashokbhai, M.K.; Ghatole, S.; Roy, S.; Kashinath, K.P.; Kaity, S. Strategies for beating the bitter taste of pharmaceutical formulations towards better therapeutic outcomes. RSC Pharm. 2025, 2, 59–81. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Bernkop-Schnürch, A. Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products. Carbohydr. Polym. 2024, 324, 121500. [Google Scholar] [CrossRef]
- Rincón-López, J.; Almanza-Arjona, Y.C.; Riascos, A.P.; Rojas-Aguirre, Y. Technological evolution of cyclodextrins in the pharmaceutical field. J. Drug Deliv. Sci. Technol. 2021, 61, 102156. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Latest developments in the application of cyclodextrin host-guest complexes in beverage technology processes. Food Hydrocoll. 2020, 106, 105882. [Google Scholar] [CrossRef]
- Pham, T.L.; Ha Nguyen, T.T.; Nguyen, T.A.; Le-Deygen, I.; Hanh Le, T.M.; Vu, X.M.; Le, H.K.; Van, C.B.; Usacheva, T.R.; Mai, T.T.; et al. Antioxidant activity of an inclusion complex between rutin and β-cyclodextrin: Experimental and quantum chemical studies. RSC Adv. 2024, 14, 18330–18342. [Google Scholar] [CrossRef] [PubMed]
- Lan Pham, T.; Usacheva, T.R.; Alister, D.A.; Thu Ha Nguyen, T.; Tukumova, N.V.; Kuranova, N.N.; Minh Vu, X.; My Hanh Le, T.; Tung Nguyen, Q.; Lam Tran, D. Thermodynamic parameters and quantum chemical calculations of complex formation between rutin and 2-hydroxypropyl-β-cyclodextrin in water-ethanol solvents. J. Mol. Liq. 2022, 366, 120324. [Google Scholar] [CrossRef]
- Antony Muthu Prabhu, A.; Suresh Kumar, G.S.; Fatiha, M.; Sorimuthu, S.; Sundar Raj, M. Encapsulation of phenylalanine and 3,4-dihydroxyphenylalanine into β-cyclodextrin: Spectral and molecular modeling studies. J. Mol. Struct. 2015, 1079, 370–382. [Google Scholar] [CrossRef]
- Alper Öztürk, A.; Başaran, E.; Şenel, B.; Demirel, M.; Sarıca, Ş. Synthesis, characterization, antioxidant activity of Quercetin, Rutin and Quercetin-Rutin incorporated β-cyclodextrin inclusion complexes and determination of their activity in NIH-3T3, MDA-MB-231 and A549 cell lines. J. Mol. Struct. 2023, 1282, 135169. [Google Scholar] [CrossRef]
- Aree, T.; Jongrungruangchok, S. Crystallographic evidence for β-cyclodextrin inclusion complexation facilitating the improvement of antioxidant activity of tea (+)-catechin and (-)-epicatechin. Carbohydr. Polym. 2016, 140, 362–373. [Google Scholar] [CrossRef]
- Hong, J.H.; Kim, M.J.; Oh, W.Y.; Lee, J.H. Evaluation of deodorization techniques using cyclodextrins on the headspace volatiles and antioxidant properties of onion. Food Chem. 2023, 410, 135416. [Google Scholar] [CrossRef]
- Gu, A.; Wheate, N.J. Macrocycles as drug-enhancing excipients in pharmaceutical formulations. J. Incl. Phenom. Macrocycl. Chem. 2021, 100, 55–69. [Google Scholar] [CrossRef]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Alister, D.A.; Kuranova, N.N.; Volynkin, V.A.; Lindt, D.A.; Pham, L.T.; D’Aria, F.; Giancola, C. Effect of water-dimethyl sulfoxide solvents on thermodynamic parameters of complex formation between benzoic acid and 2-hydroxypropyl-β-cyclodextrin. J. Therm. Anal. Calorim. 2024, 149, 12325–12333. [Google Scholar] [CrossRef]
- Usacheva, T.R.; Volynkin, V.A.; Panyushkin, V.T.; Lindt, D.A.; Pham, T.L.; Nguyen, T.T.H.; Le, T.M.H.; Alister, D.A.; Kabirov, D.N.; Kuranova, N.N.; et al. Complexation of Cyclodextrins with Benzoic Acid in Water-Organic Solvents: A Solvation-Thermodynamic Approach. Molecules 2021, 26, 4408. [Google Scholar] [CrossRef] [PubMed]
- Junior, O.V.; Dantas, J.H.; Barão, C.E.; Zanoelo, E.F.; Cardozo-Filho, L.; de Moraes, F.F. Formation of inclusion compounds of (+)catechin with β-cyclodextrin in different complexation media: Spectral, thermal and antioxidant properties. J. Supercrit. Fluids 2017, 121, 10–18. [Google Scholar] [CrossRef]
- Yang, L.J.; Chang, Q.; Zhou, S.Y.; Yang, Y.H.; Xia, F.T.; Chen, W.; Li, M.; Yang, X.D. Host–guest interaction between brazilin and hydroxypropyl-β-cyclodextrin: Preparation, inclusion mode, molecular modelling and characterization. Dye. Pigment. 2018, 150, 193–201. [Google Scholar] [CrossRef]
- Lu, Z.; Cheng, B.; Hu, Y.; Zhang, Y.; Zou, G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009, 113, 17–20. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Fortea, I.; López-Nicolás, J.M.; Núñez-Delicado, E. Cyclodextrins as resveratrol carrier system. Food Chem. 2007, 104, 39–44. [Google Scholar] [CrossRef]
- Mercader-Ros, M.T.; Lucas-Abellán, C.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Effect of HP-β-cyclodextrins complexation on the antioxidant activity of flavonols. Food Chem. 2010, 118, 769–773. [Google Scholar] [CrossRef]
- Liang, Y.; Hou, D.; Ni, Z.; Cao, M.; Cai, L. Preparation, characterization of naringenin, β-cyclodextrin and carbon quantum dot antioxidant nanocomposites. Food Chem. 2022, 375, 131646. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Han, L.; Li, S.; Zhou, W. Antioxidant and antimicrobial polyvinyl alcohol electrospun nanofibers containing baicalein-hydroxypropyl-β-cyclodextrin inclusion complex. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126135. [Google Scholar] [CrossRef]
- Shiozawa, R.; Inoue, Y.; Murata, I.; Kanamoto, I. Effect of antioxidant activity of caffeic acid with cyclodextrins using ground mixture method. Asian J. Pharm. Sci. 2018, 13, 24–33. [Google Scholar] [CrossRef]
- Shah, M.H.; Mhasalkar, M.Y.; Patki, V.M.; Deliwala, C.V.; Sheth, U.K. New 1,2,4(H)-Triazole Derivatives as Diuretic Agents. J. Pharm. Sci. 1969, 58, 1398–1401. [Google Scholar] [CrossRef]
- Mic, M.; Pîrnău, A.; Floare, C.G.; Palage, M.D.; Oniga, O.; Marc, G. Inclusion of a Catechol-Derived Hydrazinyl-Thiazole (CHT) in β-Cyclodextrin Nanocavity and Its Effect on Antioxidant Activity: A Calorimetric, Spectroscopic and Molecular Docking Approach. Antioxidants 2023, 12, 1367. [Google Scholar] [CrossRef] [PubMed]
- Floare, C.G.; Balibanu, F.; Bogdan, M. CONSTEQ—A program for the calculation of the equilibrium constants using spectroscopic data. Stud. Univ. Babes-Bolyai Phys. 2005, 4, 451. [Google Scholar]
- Todorova, N.A.; Schwarz, F.P. The role of water in the thermodynamics of drug binding to cyclodextrin. J. Chem. Thermodyn. 2007, 39, 1038–1048. [Google Scholar] [CrossRef]
- Radulovic, D.M.; Karljikovic-Rajic, K.D.; Lucic, B.M.; Vujic, Z.B. A preliminary study of β-cyclodextrin/metoprolol tartrate inclusion complex for potential enantiomeric separation. J. Pharm. Biomed. Anal. 2001, 24, 871–876. [Google Scholar] [CrossRef]
- Alva-Ensastegui, J.C.; Palomar-Pardavé, M.; Ramírez-Silva, M.T.; Aparicio-Gutierrez, N. Quercetin displacement caused by sodium dodecyl sulphate on inclusion complex quercetin-beta cyclodextrin in an acid environment. J. Photochem. Photobiol. A Chem. 2023, 436, 114392. [Google Scholar] [CrossRef]
- Zagami, R.; Barattucci, A.; Monsù Scolaro, L.; Viale, M.; Raffaini, G.; Maria Bonaccorsi, P.; Mazzaglia, A. Curcumin/amphiphilic cyclodextrin nanoassemblies: Theoretical and spectroscopic studies to address their debut in anticancer therapy. J. Mol. Liq. 2023, 389, 122841. [Google Scholar] [CrossRef]
- Foks, H.; Janowiec, M.; Zwolska, Z.; Augustynowicz-Kopeć, E. Synthesis and Tuberculostatic Activity of Some 2-Piperazinmethylene Derivatives 1,2,4-Triazole-3-Thiones. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 537–543. [Google Scholar] [CrossRef]
- Mic, M.; Pîrnău, A.; Floare, C.; Miclăus, M.; Kacso, I.; Tihăuan, M.; Axinie (Bucos), M.; Marc, G.; Oniga, O.; Crișan, O. Molecular Characterization of Trans Ferulic Acid: Β-Cyclodextrin Inclusion Complex. Experimental and Theoretical Approach. Farmacia 2024, 72, 765–778. [Google Scholar] [CrossRef]
- Bouchemal, K.; Mazzaferro, S. How to conduct and interpret ITC experiments accurately for cyclodextrin–guest interactions. Drug Discov. Today 2012, 17, 623–629. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Caira, M.R.; Miller, J.L.; Nassimbeni, L.R. β-Cyclodextrin Inclusion Complexes of Mg2+ and Ca2+ Salts of Meclofenamic Acid: Preparation and Structural Characterisation. Supramol. Chem. 2006, 18, 553–559. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Fuhrmann, J.; Rurainski, A.; Lenhof, H.-P.; Neumann, D. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J. Comput. Chem. 2010, 31, 1911–1918. [Google Scholar] [CrossRef]
- Mic, M.; Pîrnău, A.; Floare, C.G.; Marc, G.; Franchini, A.H.; Oniga, O.; Vlase, L.; Bogdan, M. Synthesis and molecular interaction study of a diphenolic hidrazinyl-thiazole compound with strong antioxidant and antiradical activity with HSA. J. Mol. Struct. 2021, 1244, 131278. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- BLOIS, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
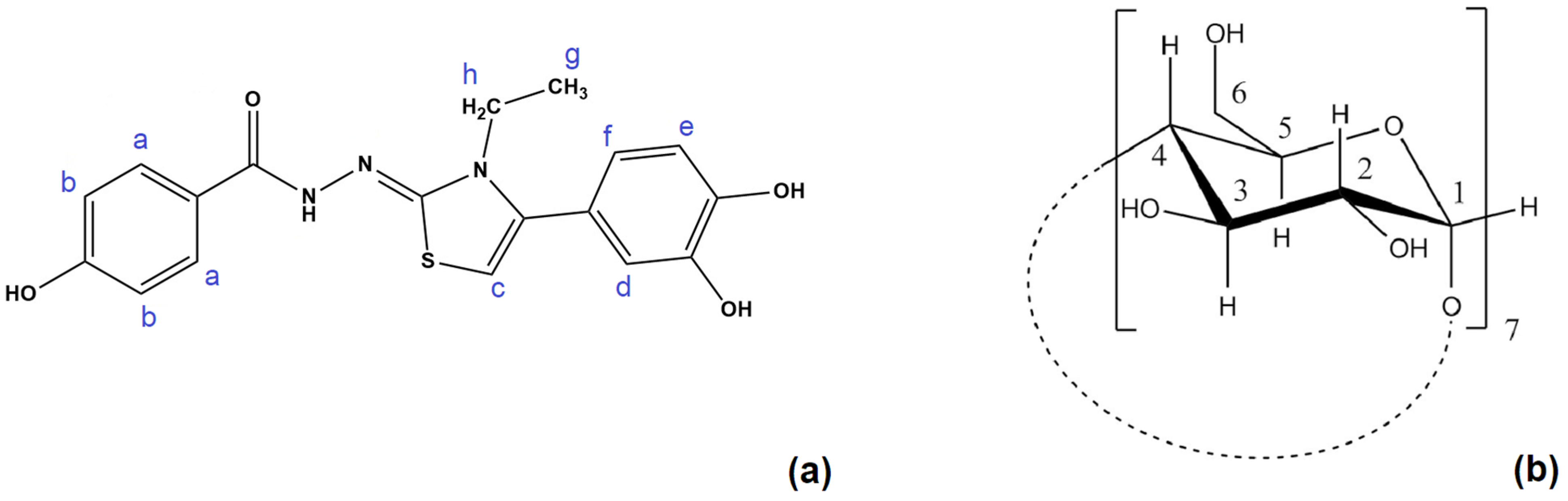
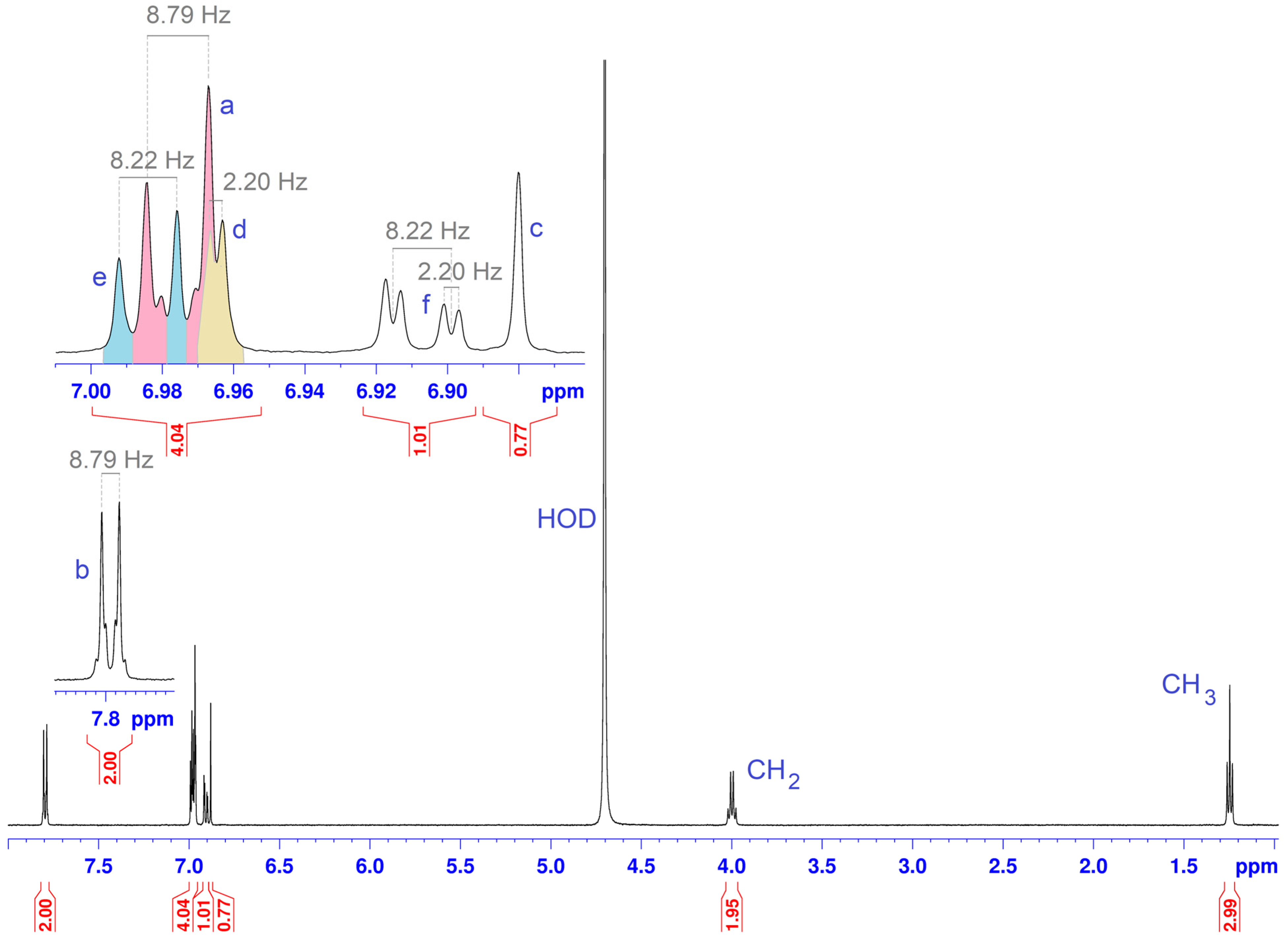
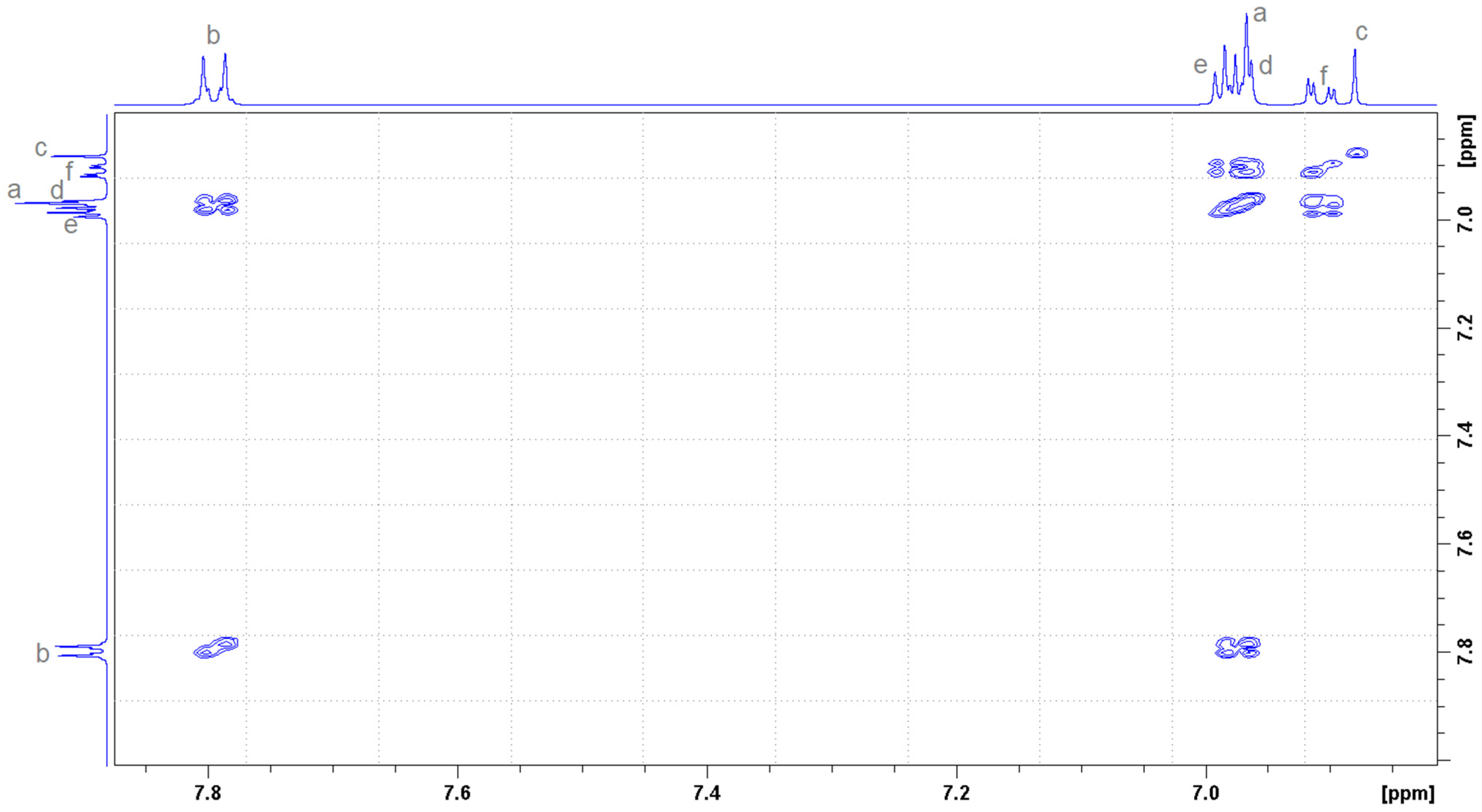



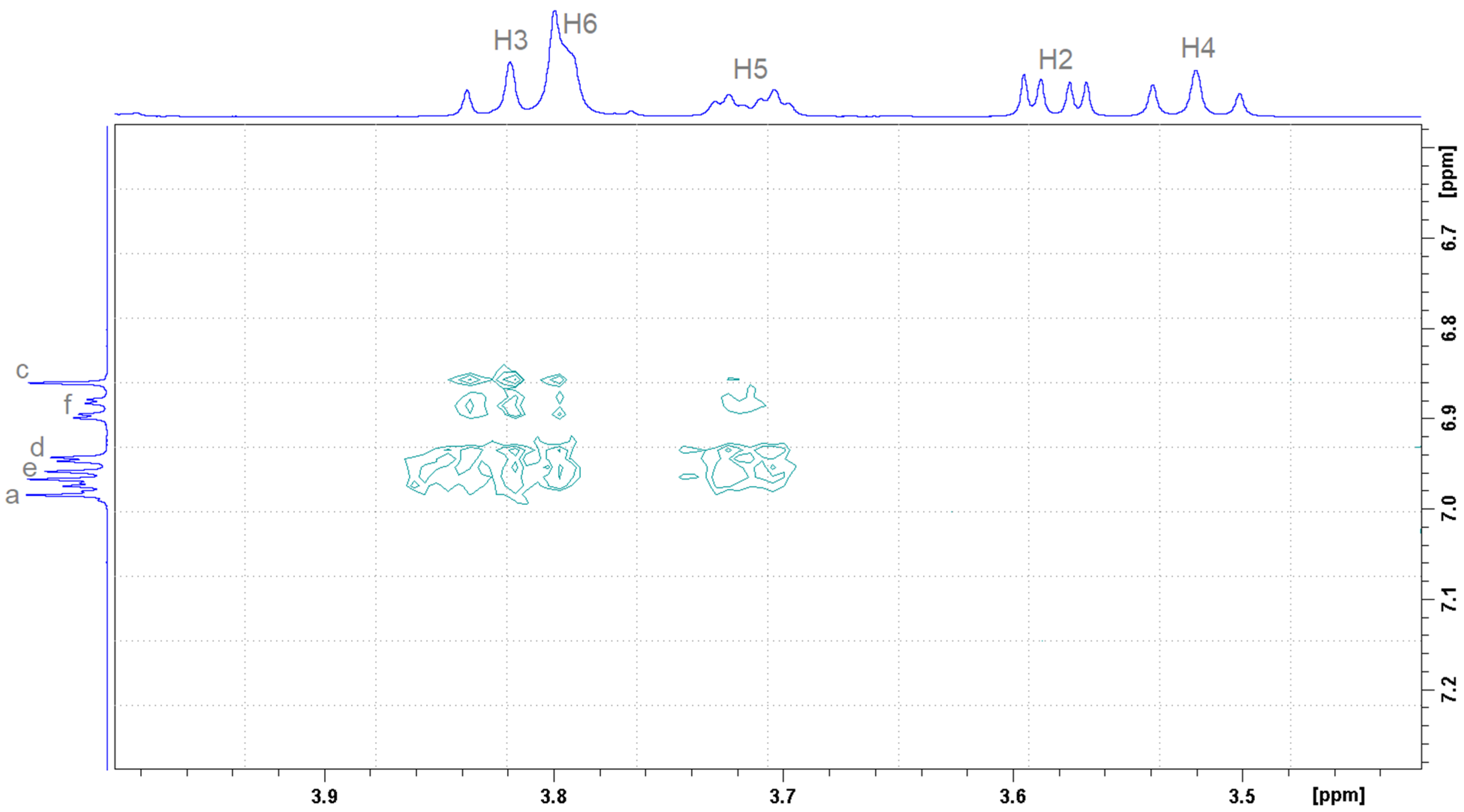

| Proton Position | Number of Protons | Multiplicity | Coupling Constant (Hz) | Chemical Shift in Free State (ppm) | Type |
|---|---|---|---|---|---|
| a | 2 | d | Ja-b = 8.79 | 6.97 | aromatic |
| b | 2 | d | Jb-a = 8.79 | 7.79 | aromatic |
| c | 1 | s | - | 6.88 | thiazole |
| d | 1 | d | Jd-f = 2.20 | 6.96 | aromatic |
| e | 1 | d | Je-f = 8.22 | 6.98 | aromatic |
| f | 1 | dd | Jf-e = 8.22 Jf-d = 2.20 | 6.90 | aromatic |
| g | 3 | t | Jg-h = 7.33 | 1.24 | methyl |
| h | 2 | q | Jh-g = 7.33 | 4.00 | methylene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pîrnău, A.; Mic, M.; Floare, C.G.; Oniga, O.; Oniga, S.D.; Crișan, O.; Vlase, L.; Marc, G. New Antioxidant Triphenol-Derived Hydrazide-Hydrazone Thiazole: Formation and Analysis of Inclusion Complex with β-CD Using Experimental and Computational Approaches. Molecules 2025, 30, 1842. https://doi.org/10.3390/molecules30081842
Pîrnău A, Mic M, Floare CG, Oniga O, Oniga SD, Crișan O, Vlase L, Marc G. New Antioxidant Triphenol-Derived Hydrazide-Hydrazone Thiazole: Formation and Analysis of Inclusion Complex with β-CD Using Experimental and Computational Approaches. Molecules. 2025; 30(8):1842. https://doi.org/10.3390/molecules30081842
Chicago/Turabian StylePîrnău, Adrian, Mihaela Mic, Călin G. Floare, Ovidiu Oniga, Smaranda Dafina Oniga, Ovidiu Crișan, Laurian Vlase, and Gabriel Marc. 2025. "New Antioxidant Triphenol-Derived Hydrazide-Hydrazone Thiazole: Formation and Analysis of Inclusion Complex with β-CD Using Experimental and Computational Approaches" Molecules 30, no. 8: 1842. https://doi.org/10.3390/molecules30081842
APA StylePîrnău, A., Mic, M., Floare, C. G., Oniga, O., Oniga, S. D., Crișan, O., Vlase, L., & Marc, G. (2025). New Antioxidant Triphenol-Derived Hydrazide-Hydrazone Thiazole: Formation and Analysis of Inclusion Complex with β-CD Using Experimental and Computational Approaches. Molecules, 30(8), 1842. https://doi.org/10.3390/molecules30081842








