Explosives Analysis Using Thin-Layer Chromatography–Quantum Cascade Laser Spectroscopy
Abstract
1. Introduction
2. Results
2.1. Separation of TNT and PETN from a Pentolite Formulation
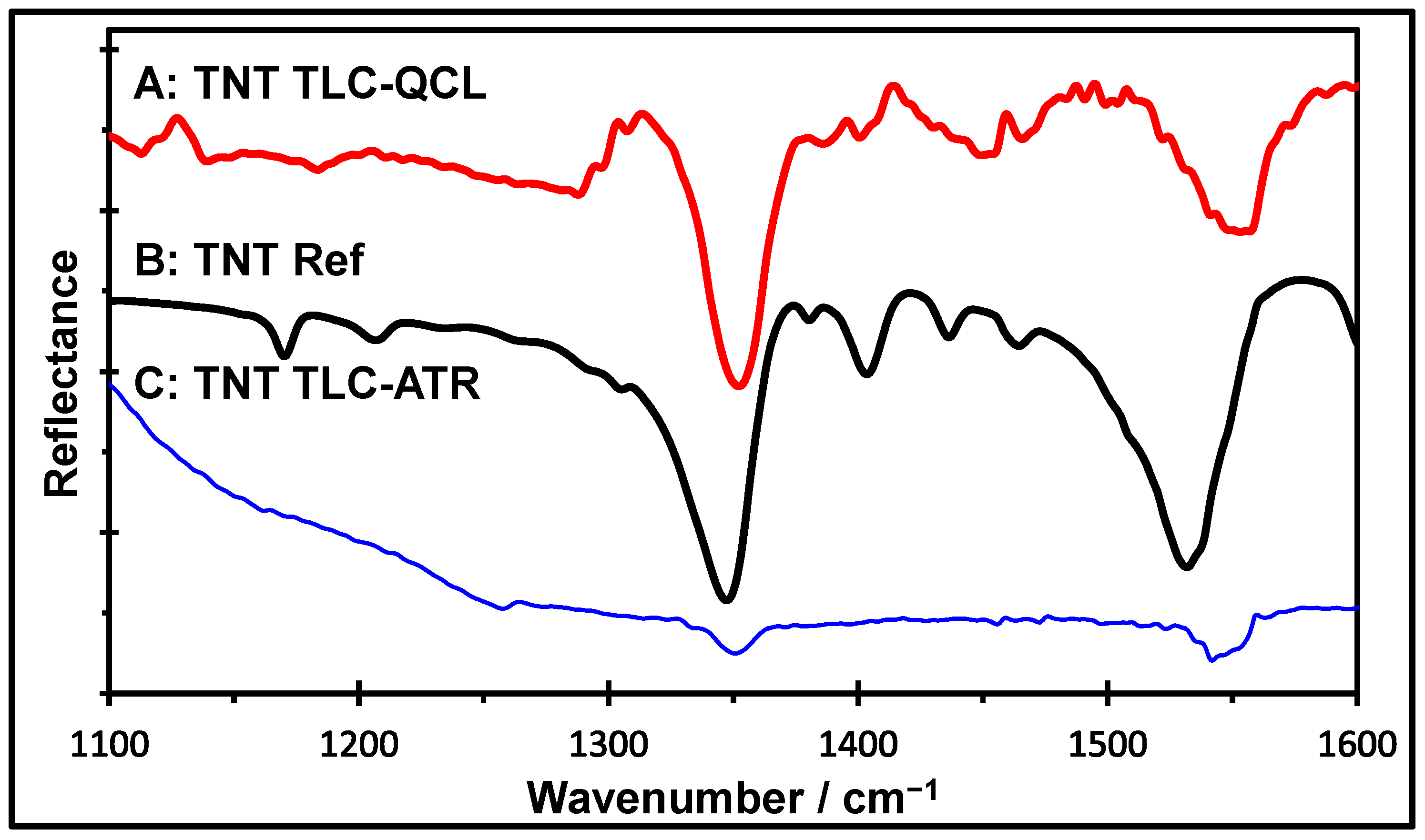
2.2. Spectral Profiles of TNT
2.3. Comparison Between TLC-QCL and TLC-FTIR Methods
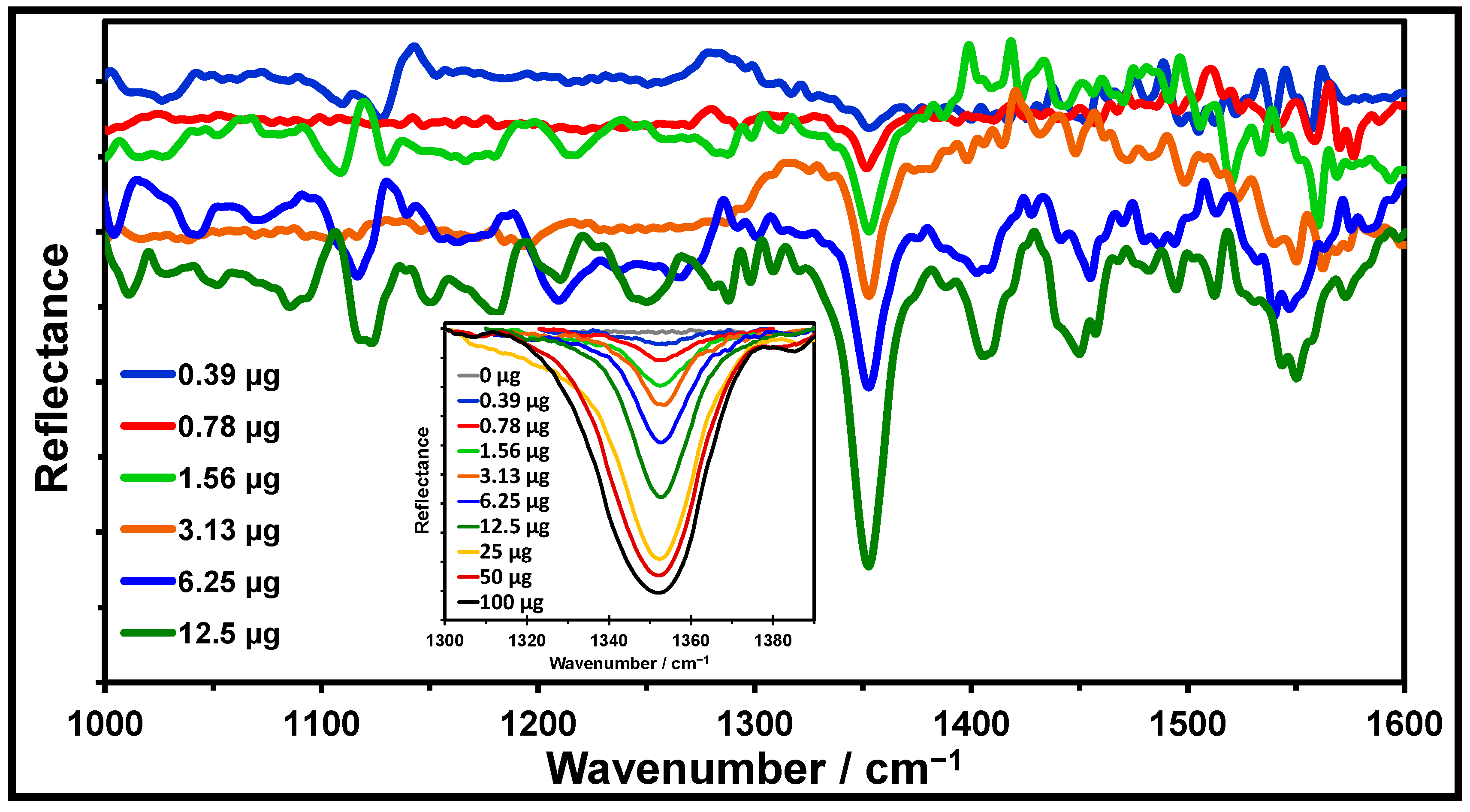
2.4. Quantification Profiles of TNT on Silicagel TLC Plaes
2.5. Quantification by Univariate Analysis
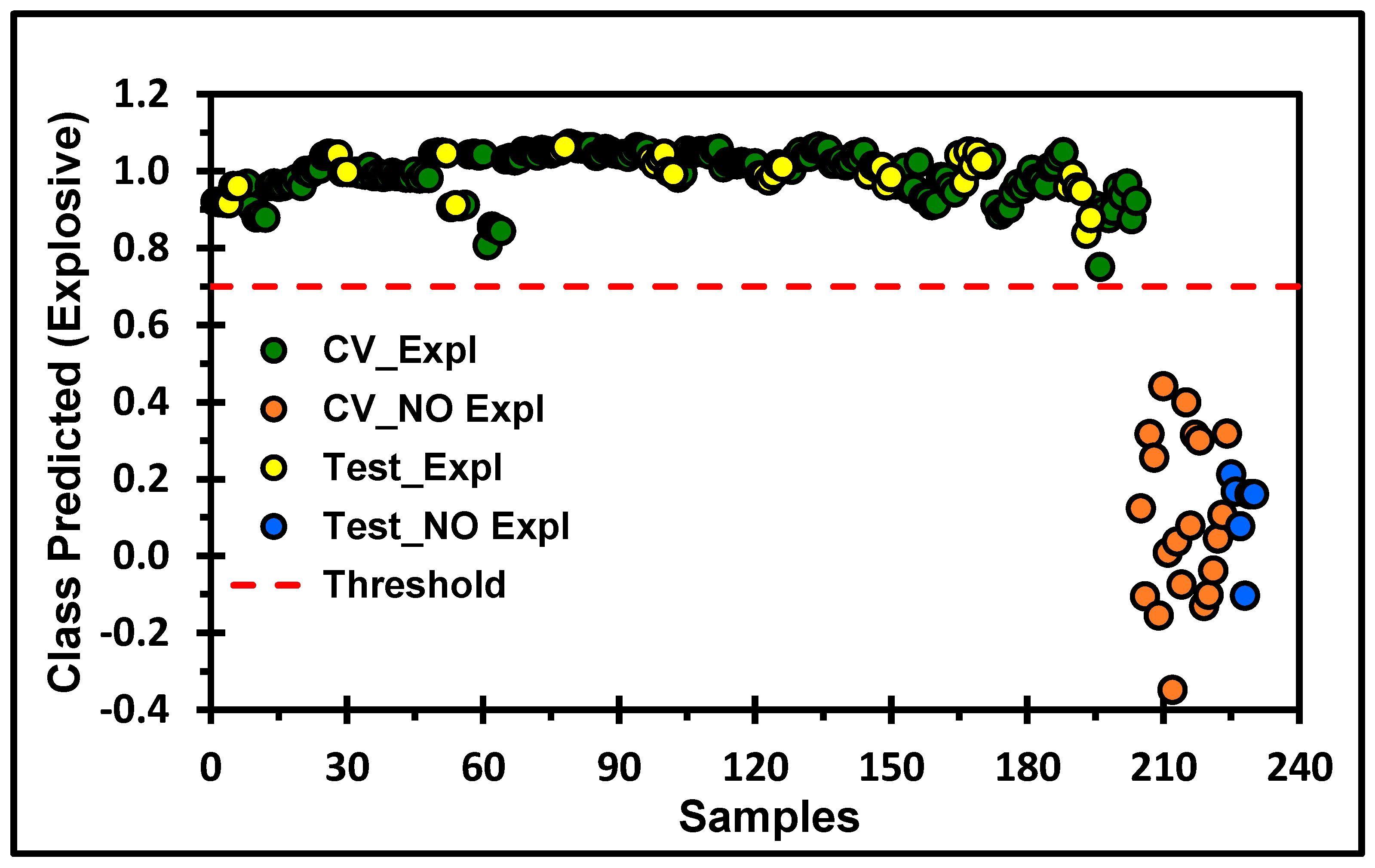
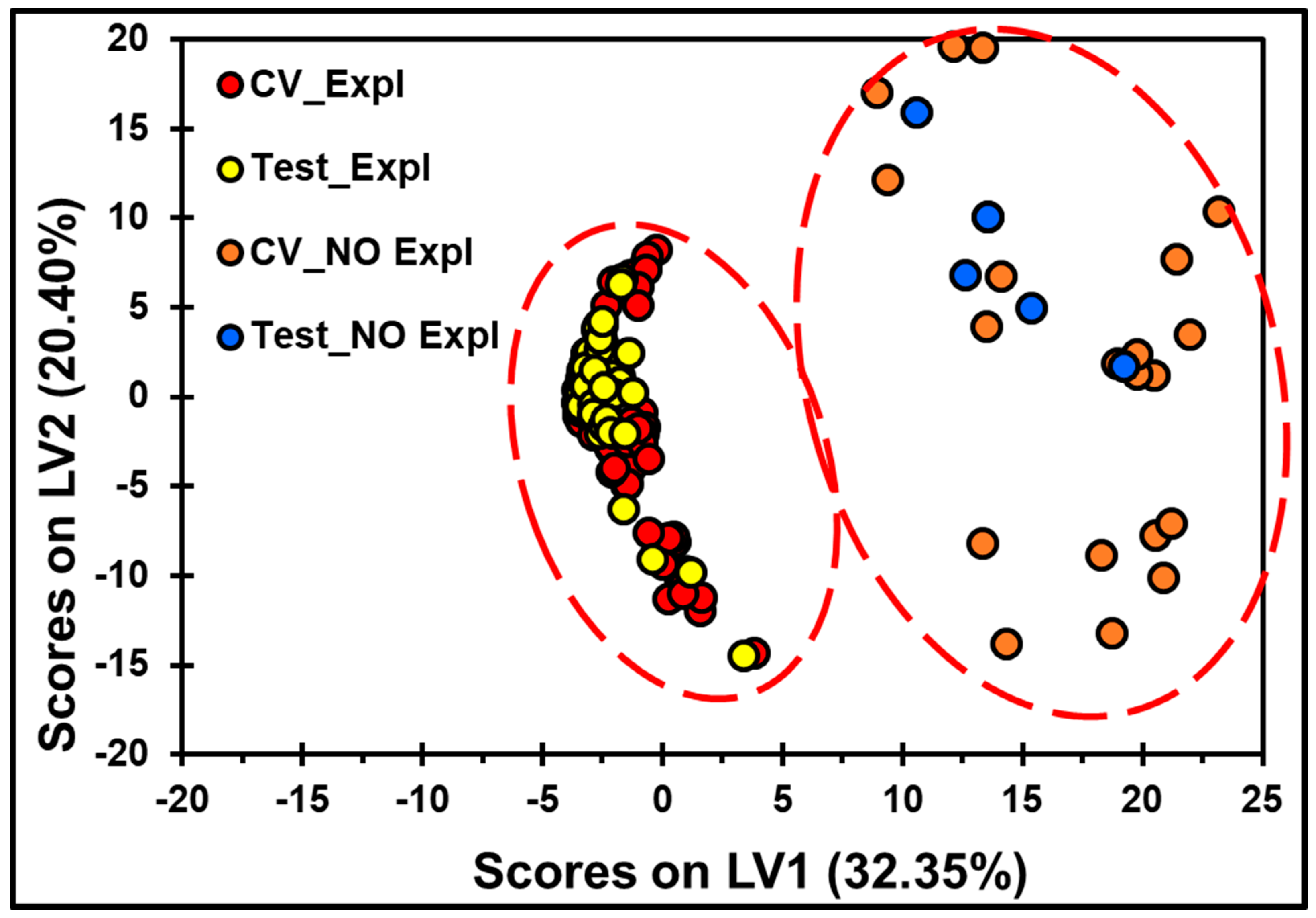
2.6. MVA of the TLC-QCL-Based Methodology
3. Materials and Methods
3.1. Materials and Reagents
3.2. TLC Protocol
3.3. MIR Laser Spectroscopy Instrumentation and Spectra Acquisition
3.4. Univariate Analysis
3.5. Multivariate Analysis (MVA)
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez-Cedeño, M.L.; Gaensbauer, N.; Félix-Rivera, H.; Ortiz-Rivera, W.; Pacheco-Londoño, L.; Hernández-Rivera, S.P. Fiber Optic Coupled Raman Based Detection of Hazardous Liquids Concealed in Commercial Products. Int. J. Spectrosc. 2012, 2012, 463731. [Google Scholar] [CrossRef]
- Caron, T.; Guillemot, M.; Montméat, P.; Veignal, F.; Perraut, F.; Prené, P.; Serein-Spirau, F. Ultra Trace Detection of Explosives in Air: Development of a Portable Fluorescent Detector. Talanta 2010, 81, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, A.; Luong, J.H.T. Micromachined Electrophoresis Chips with Electrochemical Detectors for Analysis of Explosive Compounds in Soil and Groundwater. Environ. Sci. Technol. 2000, 34, 3046–3050. [Google Scholar] [CrossRef]
- Yinon, J. Trace Analysis of Explosives in Water by Gas Chromatography-Mass Spectrometry with a Temperature-Programmed Injector. J. Chromatogr. A 1996, 742, 205–209. [Google Scholar] [CrossRef]
- Szakal, C.; Brewer, T.M. Analysis and Mechanisms of Cyclotrimethylenetrinitramine Ion Formation in Desorption Electrospray Ionization. Anal. Chem. 2009, 81, 5257–5266. [Google Scholar] [CrossRef]
- Miller, C.J.; Yoder, T.S. Explosive Contamination from Substrate Surfaces: Differences and Similarities in Contamination Techniques Using RDX and C-4. Sens. Imaging Int. J. 2010, 11, 77–87. [Google Scholar] [CrossRef]
- Ortiz-Rivera, W.; Pacheco-Londoño, L.C.; Hernández-Rivera, S.P. Remote Continuous Wave and Pulsed Laser Raman Detection of Chemical Warfare Agents Simulants and Toxic Industrial Compounds. Sens. Imaging Int. J. 2010, 11, 131–145. [Google Scholar] [CrossRef]
- Ortiz-Rivera, W.; Pacheco-Londoño, L.C.; Castro-Suarez, J.R.; Felix-Rivera, H.; Hernandez-Rivera, S.P. Vibrational Spectroscopy Standoff Detection of Threat Chemicals. In Micro-and Nanotechnology Sensors, Systems, and Applications III, Proceedings of the SPIE Defense, Security, and Sensing, Orlando, FL, USA, 25–29 April 2011; SPIE: Bellingham, WA USA, 2011. [Google Scholar] [CrossRef]
- Pacheco-Londoño, L.C.; Ortiz-Rivera, W.; Primera-Pedrozo, O.M.; Hernández-Rivera, S.P. Vibrational Spectroscopy Standoff Detection of Explosives. Anal. Bioanal. Chem. 2009, 395, 323–335. [Google Scholar] [CrossRef]
- Banas, A.; Banas, K.; Bahou, M.; Moser, H.O.; Wen, L.; Yang, P.; Li, Z.J.; Cholewa, M.; Lim, S.K.; Lim, C.H. Post-Blast Detection of Traces of Explosives by Means of Fourier Transform Infrared Spectroscopy. Vib. Spectrosc. 2009, 51, 168–176. [Google Scholar] [CrossRef]
- Castro-Suarez, J.R.; Pacheco-Londoño, L.C.; Ortiz-Rivera, W.; Vélez-Reyes, M.; Diem, M.; Hernandez-Rivera, S.P. Open Path FTIR Detection of Threat Chemicals in Air and on Surfaces. In Infrared Technology and Applications Xxxvii, Proceedings of the SPIE Defense, Security, and Sensing, Orlando, FL, USA, 25–29 April 2011; SPIE: Bellingham, WA USA, 2011. [Google Scholar] [CrossRef]
- Banas, K.; Banas, A.; Moser, H.O.; Bahou, M.; Li, W.; Yang, P.; Cholewa, M.; Lim, S.K. Multivariate Analysis Techniques in the Forensics Investigation of the Postblast Residues by Means of Fourier Transform-Infrared Spectroscopy. Anal. Chem. 2010, 82, 3038–3044. [Google Scholar] [CrossRef]
- Van Neste, C.W.; Senesac, L.R.; Thundat, T. Standoff Spectroscopy of Surface Adsorbed Chemicals. Anal. Chem. 2009, 81, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, J.; Herbst, J.; Wöllenstein, J.; Lambrecht, A. Explosive Detection Using Infrared Laser Spectroscopy. In Quantum sensing and nanophotonic devices VI, Proceedings of the SPIE OPTO: Integrated Optoelectronic Devices, San Jose, CA, USA, 28–29 January 2009; SPIE: Bellingham, WA USA, 2009. [Google Scholar] [CrossRef]
- Castro-Suarez, J.R.; Pacheco Londoño, L.C.; Vélez-Reyes, M.; Diem, M.; Tague, T.J.; Hernandez-Rivera, S.P. Open-Path FTIR Detection of Explosives on Metallic Surfaces. In Fourier Transforms: New Analytical Approaches and FTIR Strategies; InTech Open: Rijeka, Croatia, 2011; pp. 431–458. [Google Scholar]
- Castro-Suarez, J.R.; Pacheco-Londoño, L.C.; Vélez-Reyes, M.; Diem, M.; Tague, T.J.; Hernandez-Rivera, S.P. FT-IR Standoff Detection of Thermally Excited Emissions of Trinitrotoluene (TNT) Deposited on Aluminum Substrates. Appl. Spectrosc. 2013, 67, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Gunzler, H.; Gremlich, H.-U. IR Spectroscopy: An Introduction; Wiley-Vch: Weinheim, Germania, 2002. [Google Scholar]
- Faist, J.; Capasso, F.; Sivco, D.L.; Sirtori, C.; Hutchinson, A.L.; Cho, A.Y. Quantum Cascade Laser. Science 1994, 264, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Faist, J.; Capasso, F.; Sirtori, C.; Sivco, D.L.; Baillargeon, J.N.; Hutchinson, A.L.; Chu, S.G.; Cho, A.Y. High Power Mid-Infrared (λ∼5 Μm) Quantum Cascade Lasers Operating above Room Temperature. Appl. Phys. Lett. 1996, 68, 3680–3682. [Google Scholar] [CrossRef]
- Pushkarsky, M.B.; Dunayevskiy, I.G.; Prasanna, M.; Tsekoun, A.G.; Go, R.; Patel CK, N. High-Sensitivity Detection of TNT. Proc. Natl. Acad. Sci. USA 2006, 103, 19630–19634. [Google Scholar] [CrossRef]
- Patel, C.K.N. Laser Based In-Situ and Standoff Detection of Chemical Warfare Agents and Explosives. In Optically Based Biological and Chemical Detection for Defence V, Proceedings of the SPIE Security + Defence, Berlin, Germany, 31 August–3 September 2009; SPIE: Bellingham, WA USA, 2009. [Google Scholar] [CrossRef]
- Willer, U. Use of Quantum Cascade Lasers for Detection of Explosives: Progress and Challenges. Opt. Eng. 2010, 49, 111126. [Google Scholar] [CrossRef]
- Pacheco-Londoño, L.C.; Castro-Suarez, J.R.; Hernández-Rivera, S.P. Detection of Nitroaromatic and Peroxide Explosives in Air Using Infrared Spectroscopy: QCL and FTIR. Adv. Opt. Technol. 2013, 2013, 532670. [Google Scholar] [CrossRef]
- Castro-Suarez, J.R.; Hidalgo-Santiago, M.; Hernández-Rivera, S.P. Detection of Highly Energetic Materials on Non-Reflective Substrates Using Quantum Cascade Laser Spectroscopy. Appl. Spectrosc. 2015, 69, 1023–1035. [Google Scholar] [CrossRef]
- Percival, C.J.; Griffiths, P.R. Direct Measurement of the Infrared Spectra of Compounds Separated by Thin-Layer Chromatography. Anal. Chem. 1975, 47, 154–156. [Google Scholar] [CrossRef]
- Gomez-Taylor, M.M.; Kuehl, D.; Griffiths, P.R. Vibrational Spectrometry of Pesticides and Related Materials on Thin Layer Chromatography Adsorbents. Appl. Spectrosc. 1976, 30, 447–452. [Google Scholar] [CrossRef]
- Gomez-Taylor, M.M.; Griffiths, P.R. Improved Sensitivity for in Situ Infrared Identification of Species Separated by Thin-Layer Chromatography Using Programmed Multiple Development. Appl. Spectrosc. 1977, 31, 528–530. [Google Scholar] [CrossRef]
- Fuller, M.P.; Griffiths, P.R. Diffuse Reflectance Measurements by Infrared Fourier Transform Spectrometry. Anal. Chem. 1978, 50, 1906–1910. [Google Scholar] [CrossRef]
- Zuber, G.E.; Warren, R.J.; Begosh, P.P.; O’Donnell, E.L. Direct Analysis of Thin-Layer Chromatography Spots by Diffuse Reflectance Fourier Transform Infrared Spectrometry. Anal. Chem. 1984, 56, 2935–2939. [Google Scholar] [CrossRef]
- Otto, A.; Bode, U.; Heise, H.M. Experiences with Infrared Microsampling for Thin-Layer and High-Performance Liquid Chromatography. Fresenius Z. Für Anal. Chem. 1988, 331, 376–382. [Google Scholar] [CrossRef]
- Bush, S.G.; Breaux, A.J. The Application of Diffuse and Specular Reflectance to TLC-FTIR and Other Microsampling Applications. Mikrochim. Acta 1988, 94, 17–20. [Google Scholar] [CrossRef]
- Beauchemin, B.T.; Brown, P.R. Quantitative Analysis of Diazonaphthoquinones by Thin-Layer Chromatographic/Diffuse Reflectance Infrared Fourier-Transform Spectrometry. Anal. Chem. 1989, 61, 615–618. [Google Scholar] [CrossRef]
- Brown, P.R.; Beauchemin, B.T. Thin-Layer Chromatographic-Diffuse Reflectance Infrared Fourier Transform Spectroscopic Analysis. J. Liq. Chromatogr. 1988, 11, 1001–1014. [Google Scholar] [CrossRef]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kumar, R.; Sharma, V. Chemometrics in Forensic Science. TrAC Trends Anal. Chem. 2018, 105, 191–201. [Google Scholar] [CrossRef]
- Brereton, R.G. Applied Chemometrics for Scientists; John Wiley & Sons Incorporated: Hoboken, NJ, USA, 2007. [Google Scholar]
- Barker, M.; Rayens, W. Partial Least Squares for Discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Brereton, R.G. Chemometrics for Pattern Recognition; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ballabio, D.; Consonni, V. Classification Tools in Chemistry. Part 1: Linear Models. PLS-DA. Anal. Methods 2013, 5, 3790. [Google Scholar] [CrossRef]
- Satcher, J.H.; Pagoria Jr Philip, F.; Whipple, R.E.; Carman, M.L. Rapid Identification of Explosives Using Thin-Layer Chromatography and Colorimetric Techniques. U.S. Patent 2011/02397.45A1, 2011. [Google Scholar]
- Satcher, J.H.; Maienschein, J.L.; Pagoria, P.F.; Racoveanu, A.; Carman, M.L.; Whipple, R.E.; Reynolds, J.G. Portable Thin Layer Chromatography for Field Detection of Explosives and Propellants. In Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XIII, Proceedings of the SPIE Defense, Security, and Sensing, Baltimore, MD, USA, 23–27 April 2012; SPIE: Bellingham, WA USA, 2012. [Google Scholar] [CrossRef]
- Somsen, G.W.; Morden, W.; Wilson, I.D. Planar Chromatography Coupled with Spectroscopic Techniques. J. Chromatogr. A 1995, 703, 613–665. [Google Scholar] [CrossRef]
- Griffiths, P.R.; De, J.A. Fourier Transform Infrared Spectrometry; Wiley-Interscience: Hoboken, NJ, USA, 2007. [Google Scholar]
- Musial, B.A.; Sommer, A.J.; Danielson, N.D. Microchannel Thin Layer Chromatography with in situ Plate Scanning Micro-DRIFTS Detection Using Plain and Polybutadiene Modified Zirconia Stationary Phases. Appl. Spectrosc. 2002, 56, 1059–1066. [Google Scholar] [CrossRef]
- Clarkson, J.; Smith, W.E.; Batchelder, D.N.; Smith, D.A.; Coats, A.M. A Theoretical Study of the Structure and Vibrations of 2,4,6-Trinitrotolune. J. Mol. Struct. 2003, 648, 203–214. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland. 2005. Available online: https://www.gmp-compliance.org/guidelines/gmp-guideline/ich-q2r1-validation-of-analytical-procedures-text-and-methodology (accessed on 1 April 2025).

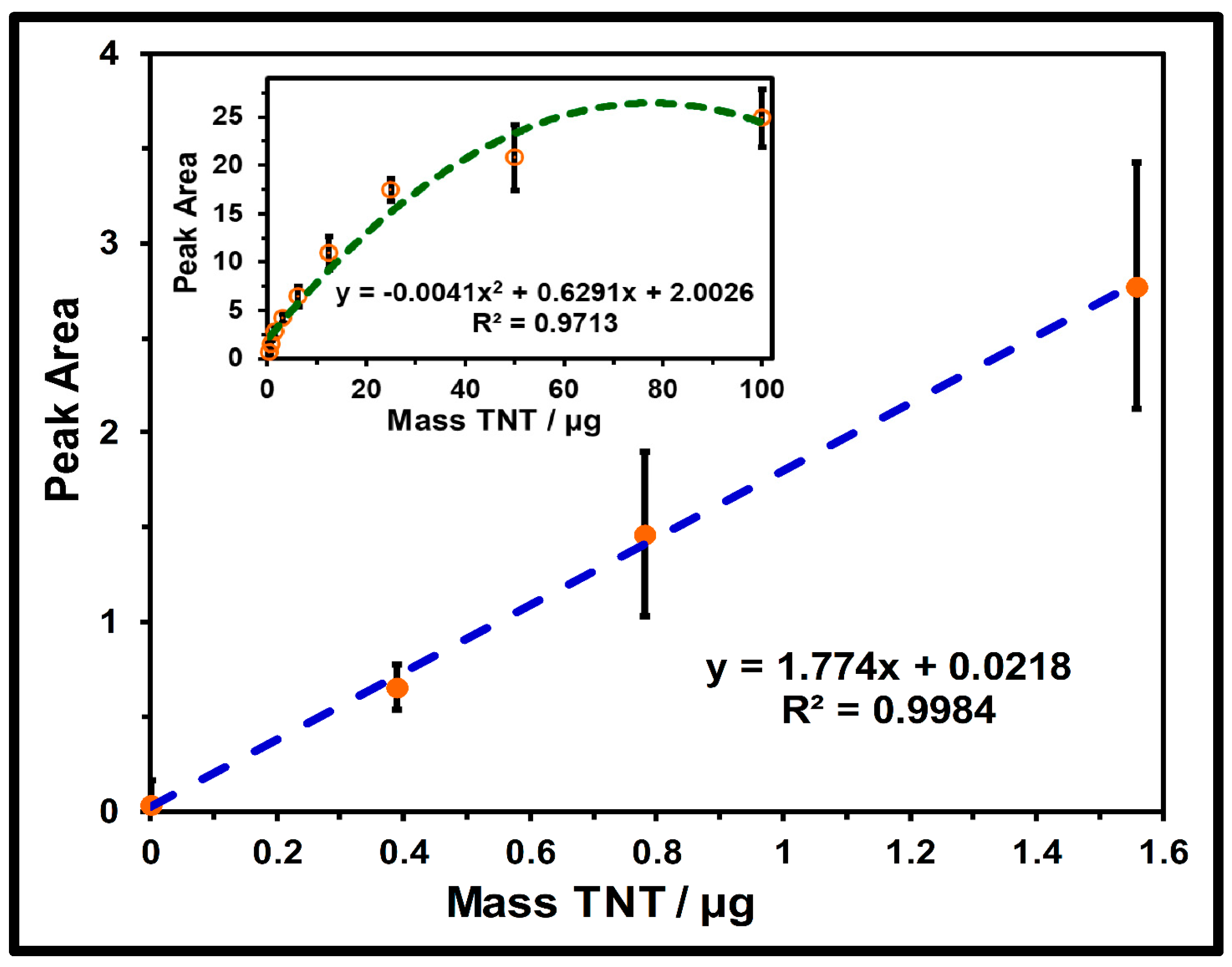


| Parameters | Value |
|---|---|
| Linearity range (µg spot−1) | 0–1.56 |
| Slope (m) | 1.774 |
| Standard deviation of slope | 0.050 |
| Intercept | 0.022 |
| Standard deviation of intercept (σ) | 0.045 |
| Regression equation | Peak area: |
| 1.774 × (Mass TNT) + 0.022 | |
| Determination coefficient (R2) | 0.998 |
| LOD (µg) | 0.084 |
| LOQ (µg) | 0.253 |
| Precision inter-day (CV, %) | |
| for 100 µg spot−1 (n = 6) | 1.090 |
| for 25 µg spot−1 (n = 6) | 1.369 |
| for 1.56 µg spot−1 (n = 6) | 3.492 |
| Repeatability (CV, %) | |
| for 100 µg spot−1 (n = 4) | 0.809 |
| for 25 µg spot−1 (n = 4) | 1.005 |
| for 1.56 µg spot−1 (n = 4) | 2.083 |
| Parameters | Data Set | |
|---|---|---|
| Explosive | No Explosive | |
| Sensitivity (Cal) | 1.000 | 1.000 |
| Specificity (Cal) | 1.000 | 1.000 |
| Sensitivity (CV) | 1.000 | 1.000 |
| Specificity (CV) | 1.000 | 1.000 |
| Sensitivity (Pred) | 1.000 | 1.000 |
| Specificity (Pred) | 1.000 | 1.000 |
| Classification error (Cal) | 0.000 | 0.000 |
| Classification error (CV) | 0.000 | 0.000 |
| Classification error (Pred) | 0.000 | 0.000 |
| RMSEC | 0.076 | 0.076 |
| RMSECV | 0.096 | 0.096 |
| RMSEP | 0.069 | 0.069 |
| R2 Cal | 0.944 | 0.944 |
| R2 CV | 0.911 | 0.911 |
| R2 Pred | 0.953 | 0.953 |
| Number of LVs | 3 | |
| Variance captured in Y (%) | 94.37 | |
| Parameters | Value |
|---|---|
| Conc. Range (µg spot−1) | 0–3.125 |
| Wavenumber Range (cm−1) | 1302.4–1388.4 |
| Number of LVs | 3 |
| Variance Captured (%) | 98.63 |
| RMSEC | 0.135 |
| RMSECV | 0.155 |
| RMSEP | 0.210 |
| R2 CV | 0.982 |
| R2 Pred | 0.978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Suarez, J.R.; Pérez-Almodóvar, L.A.; Laguer-Martínez, D.M.; Ruiz-Caballero, J.L.; Centeno-Ortiz, J.A.; Felix-Massa, T.; Pacheco-Londoño, L.C.; Hernández-Rivera, S.P. Explosives Analysis Using Thin-Layer Chromatography–Quantum Cascade Laser Spectroscopy. Molecules 2025, 30, 1844. https://doi.org/10.3390/molecules30081844
Castro-Suarez JR, Pérez-Almodóvar LA, Laguer-Martínez DM, Ruiz-Caballero JL, Centeno-Ortiz JA, Felix-Massa T, Pacheco-Londoño LC, Hernández-Rivera SP. Explosives Analysis Using Thin-Layer Chromatography–Quantum Cascade Laser Spectroscopy. Molecules. 2025; 30(8):1844. https://doi.org/10.3390/molecules30081844
Chicago/Turabian StyleCastro-Suarez, John R., Luis A. Pérez-Almodóvar, Doris M. Laguer-Martínez, José L. Ruiz-Caballero, José A. Centeno-Ortiz, Tamara Felix-Massa, Leonardo C. Pacheco-Londoño, and Samuel P. Hernández-Rivera. 2025. "Explosives Analysis Using Thin-Layer Chromatography–Quantum Cascade Laser Spectroscopy" Molecules 30, no. 8: 1844. https://doi.org/10.3390/molecules30081844
APA StyleCastro-Suarez, J. R., Pérez-Almodóvar, L. A., Laguer-Martínez, D. M., Ruiz-Caballero, J. L., Centeno-Ortiz, J. A., Felix-Massa, T., Pacheco-Londoño, L. C., & Hernández-Rivera, S. P. (2025). Explosives Analysis Using Thin-Layer Chromatography–Quantum Cascade Laser Spectroscopy. Molecules, 30(8), 1844. https://doi.org/10.3390/molecules30081844









