Tuning 2,3-Bis(arylimino)butane-nickel Precatalysts for High-Molecular-Weight Polyethylene Elastomers
Abstract
:1. Introduction
2. Results and Discussion
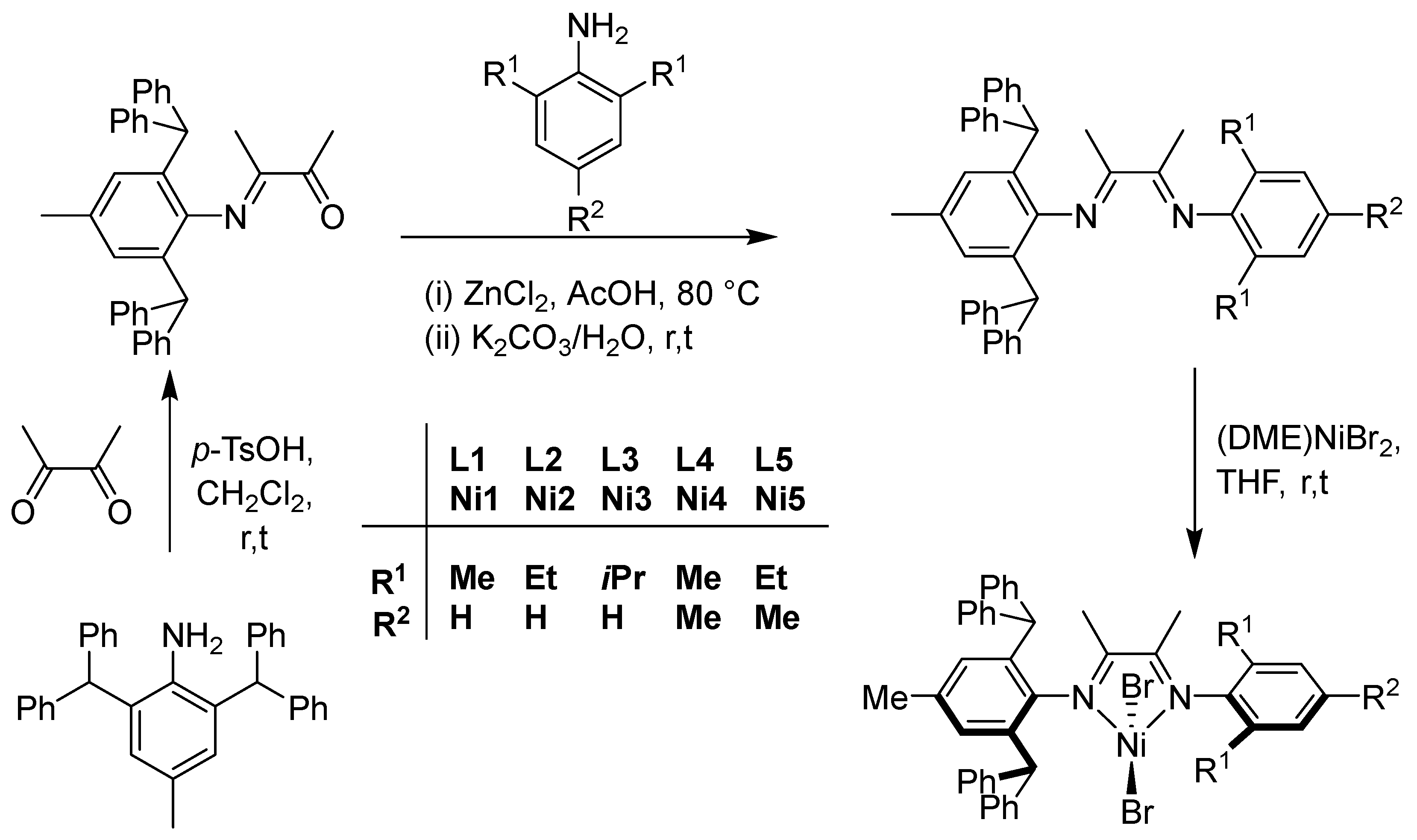
2.1. Synthesis and Characterization of Ligands and Their Nickel Complexes
2.2. Ethylene Polymerization Investigation
2.2.1. Optimization of Alkyl Aluminum Cocatalyst Selection
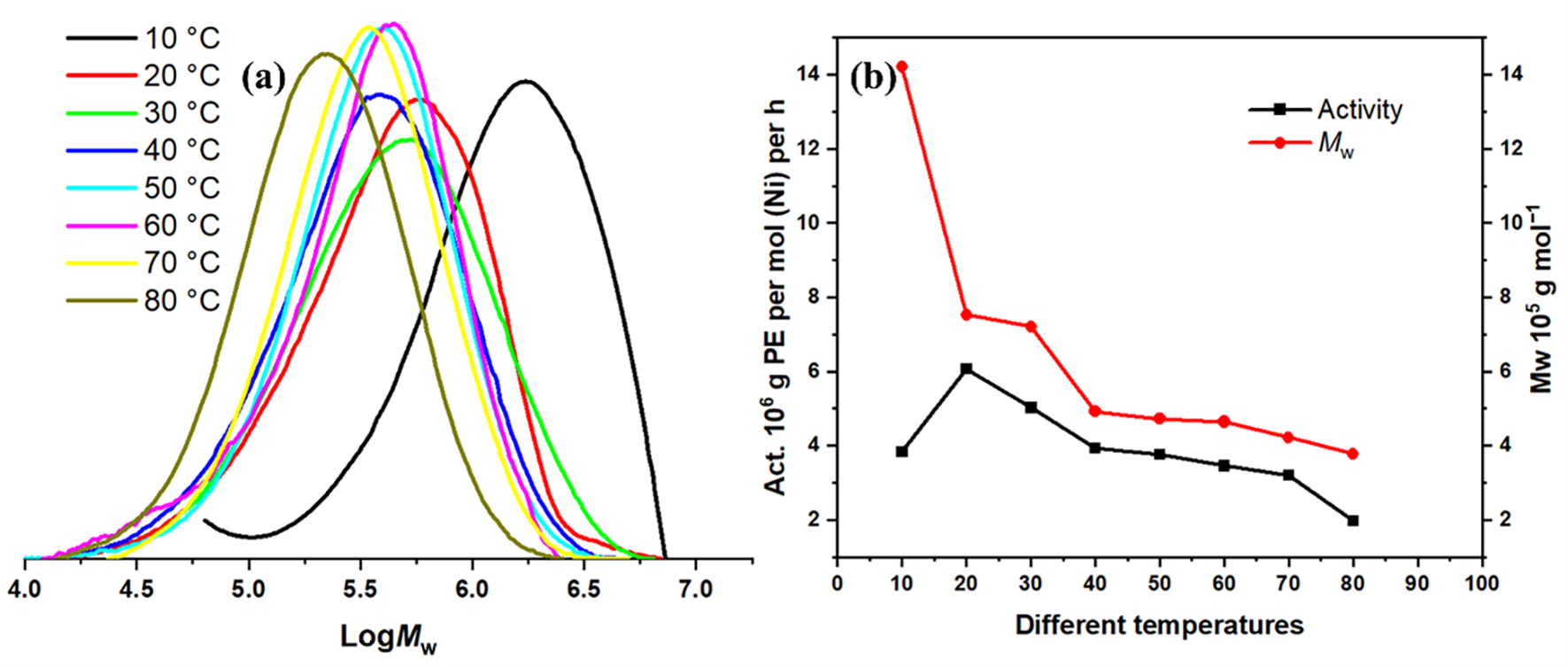
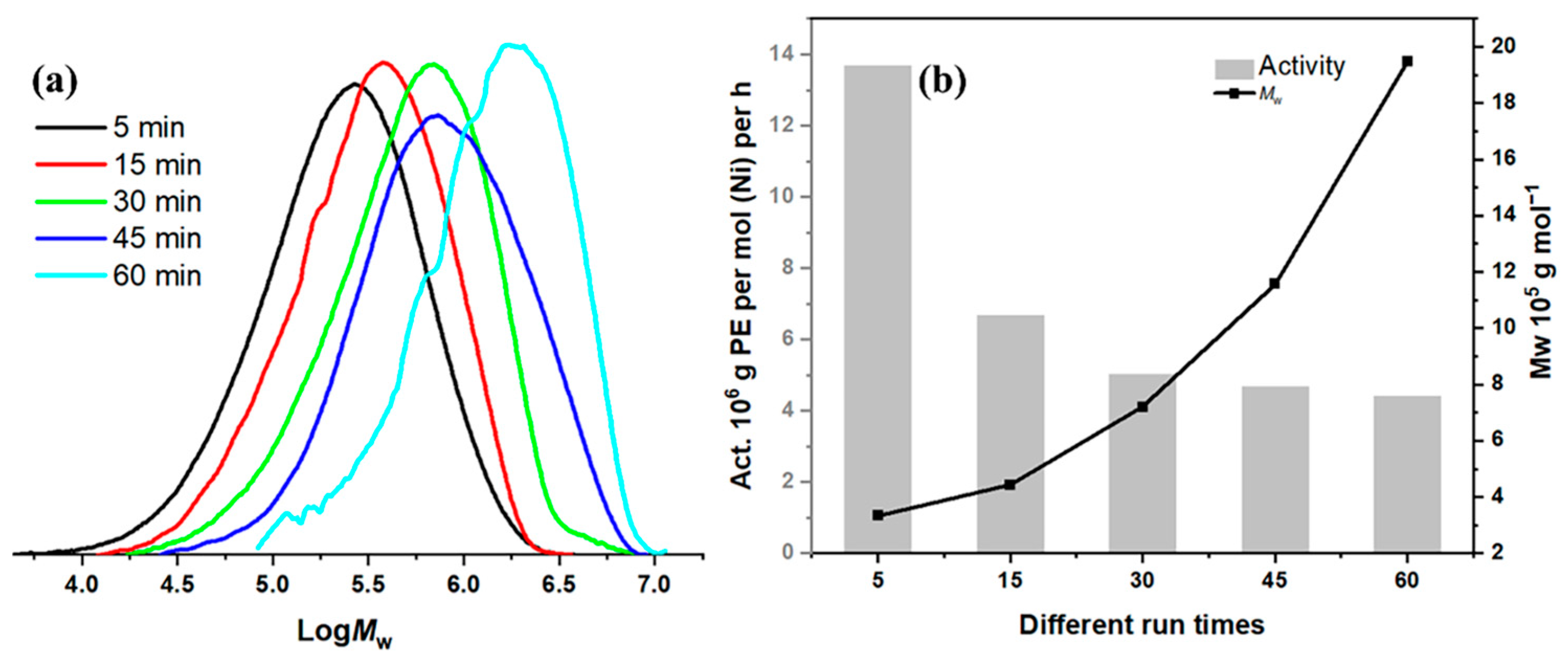
2.2.2. Optimization of Polymerization Conditions Using Ni1 with Et2AlCl as Cocatalyst
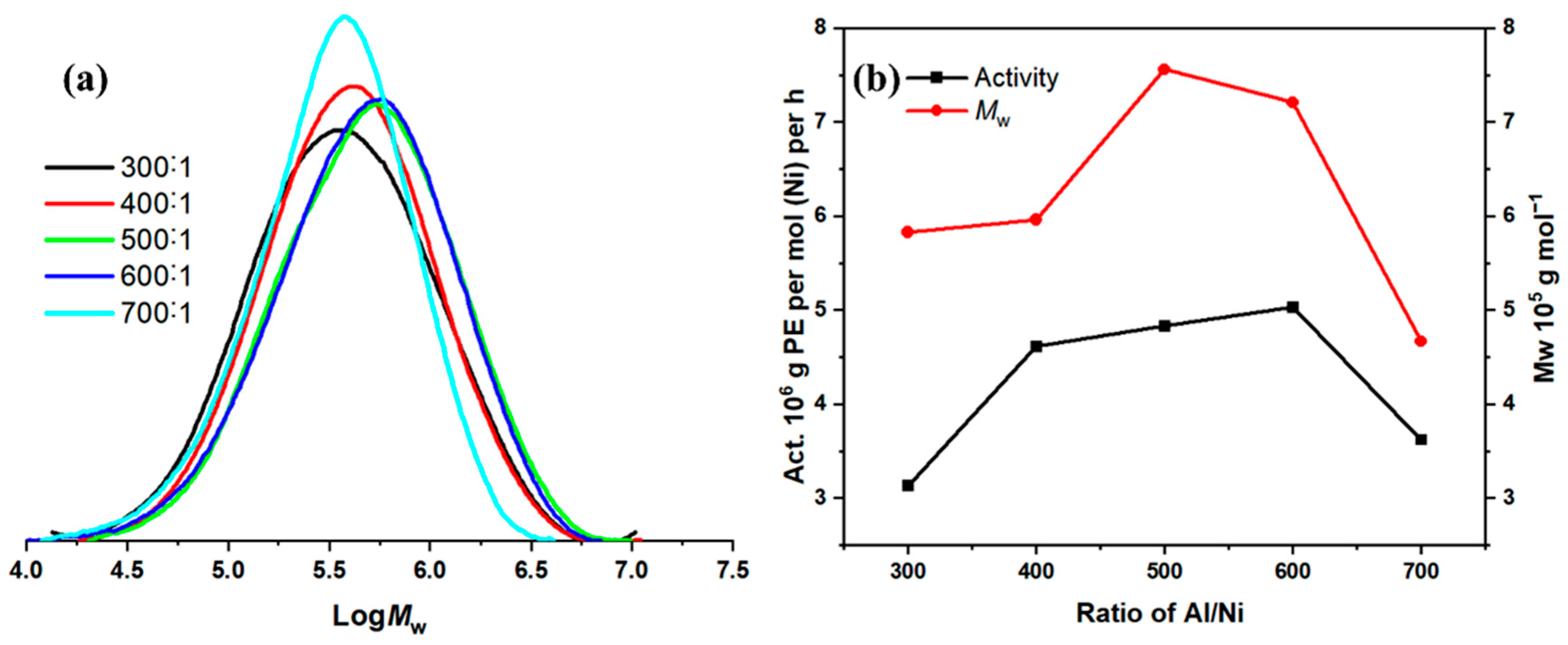
2.2.3. Ligand Structure Screening Under Optimal Conditions
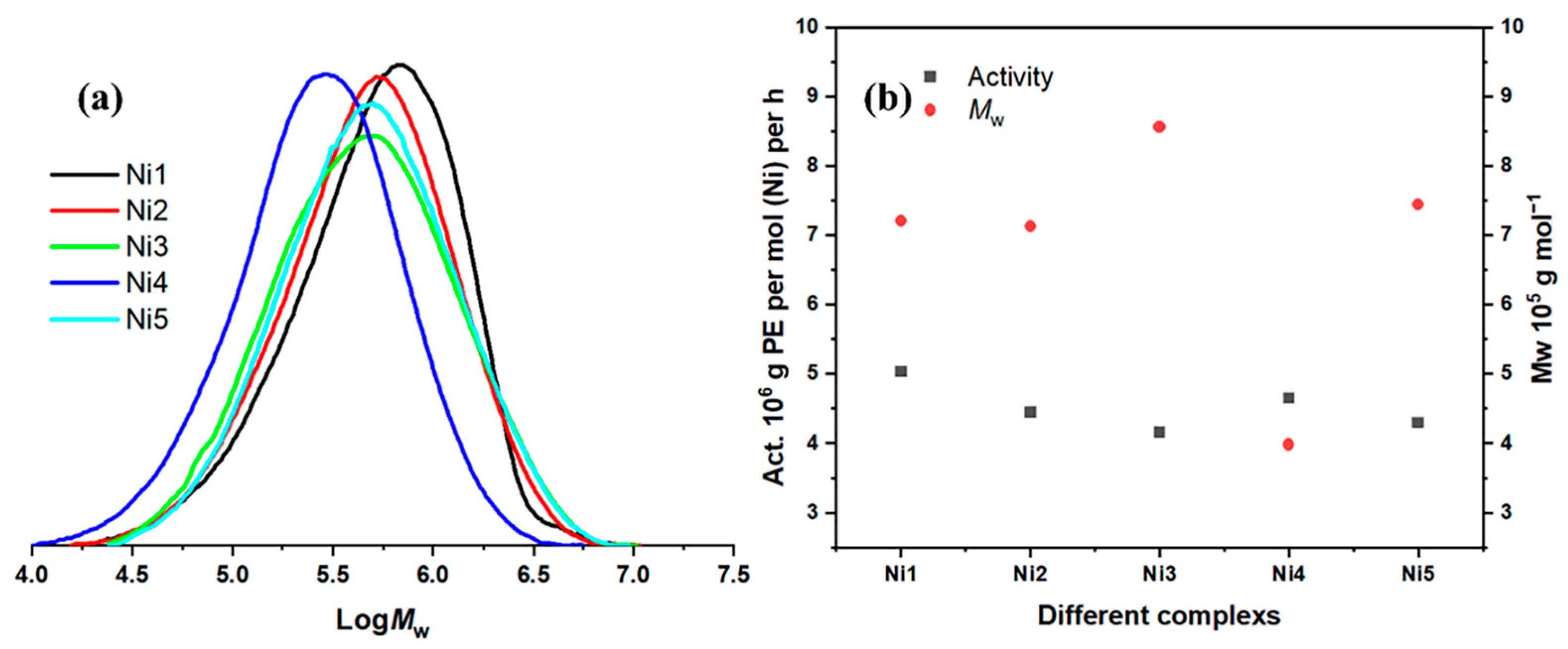
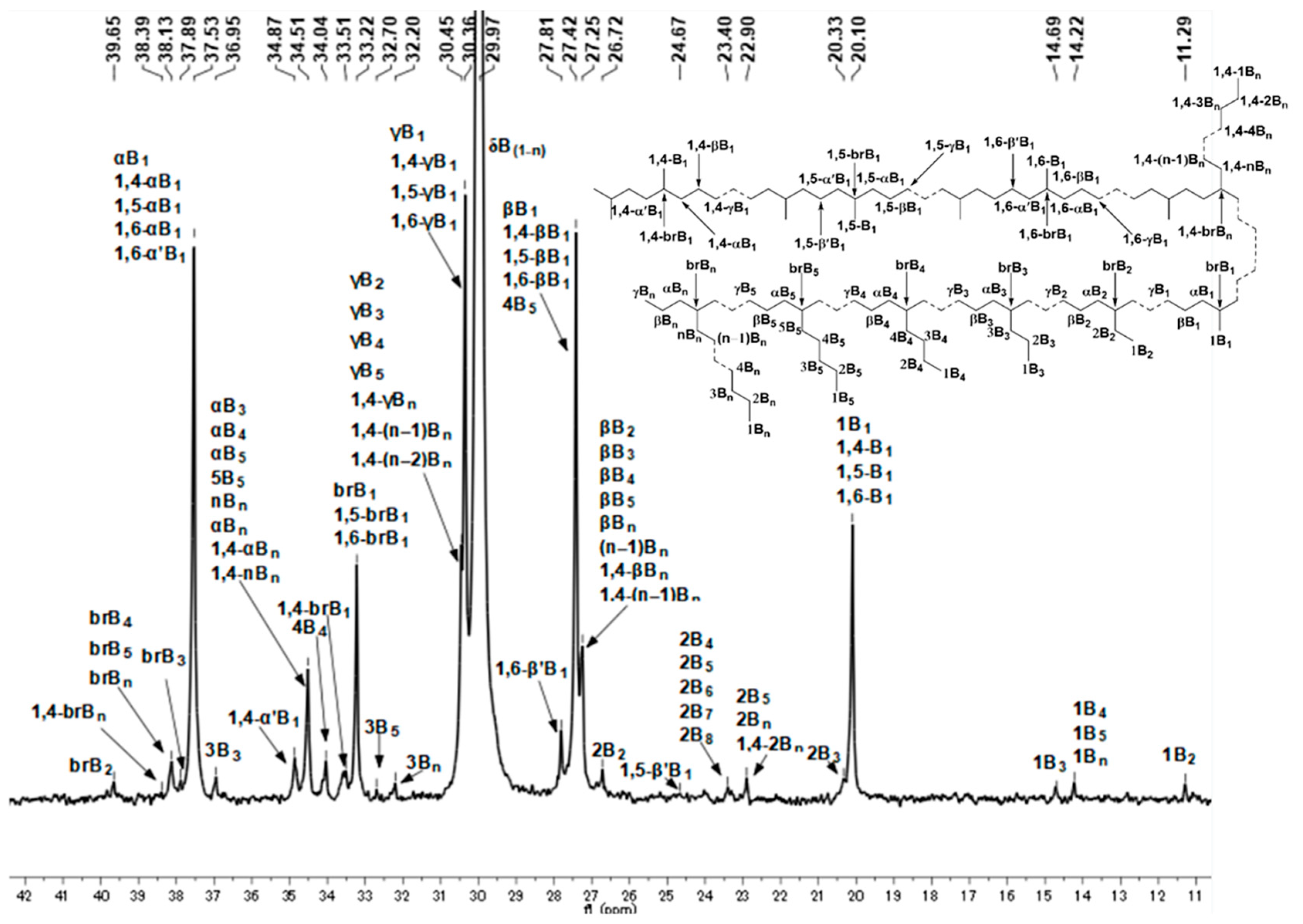
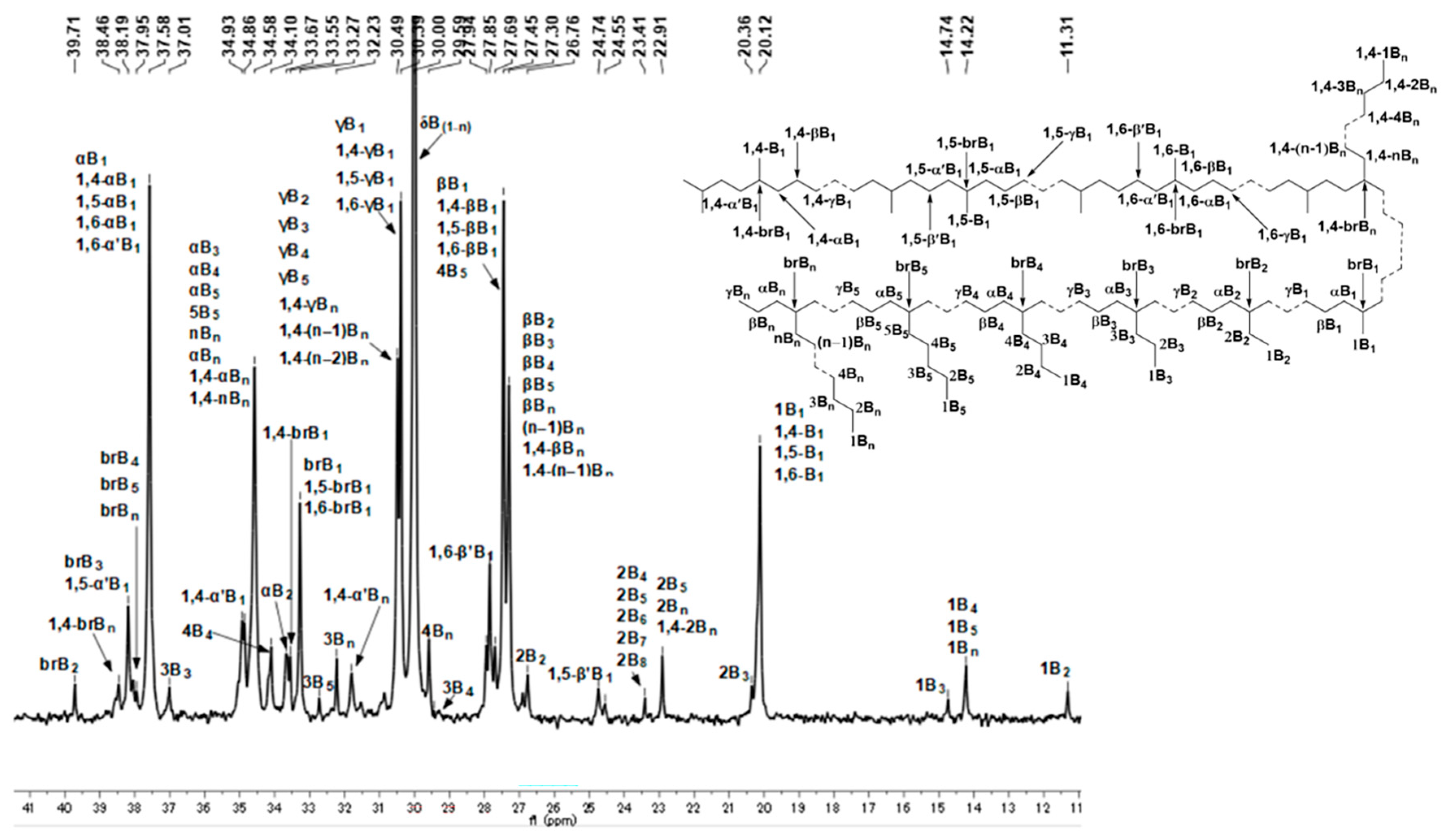
2.3. Microstructure Analysis of Polyethylenes
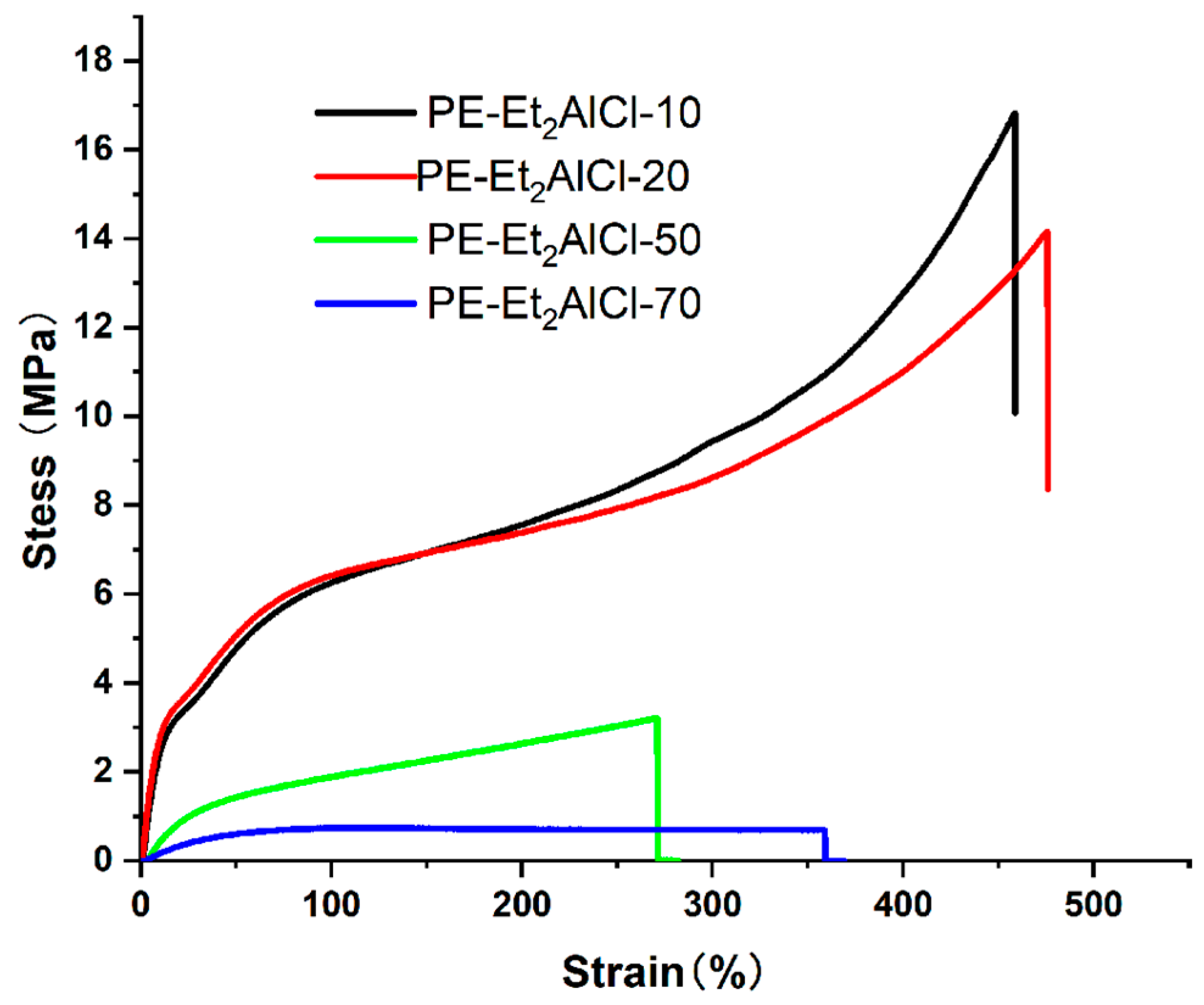
2.4. Evaluation of Mechanical Performance in Polyethylenes
3. Materials and Methods
3.1. Synthesis of Monoketone and Ligands (L1–L5)
- A solution of 2,6-diphenylmethyl-4-methylaniline (8.79 g, 20.0 mmol), 2,3-butanedione (1.72 g, 20.0 mmol), and a catalytic amount of p-toluenesulfonic acid (0.879 g) in dichloromethane (300 mL) was stirred at ambient temperature for 4 h. Afterward, the solvent was removed under reduced pressure, and the crude product was purified by recrystallization from methanol, affording 6.60 g of yellow solid with 65% yield. 1H NMR (400 MHz, CDCl3, TMS): δ 7.26–7.14 (m, 16H, Py–H), 7.01 (t, J = 2.0 Hz, 4H, Py–H), 6.64 (s, 2H, Py–H), 5.09 (s, 2H, Ar–CH(Ph)2), 2.31 (s, 3H, O=C–CH3), 2.15 (s, 3H, Ar–CH3), 0.68 (s, 3H, N=C–CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 199.3 (O=C–CH3), 168.5 (N=C–CH3), 144.3, 142.5, 142.0, 132.3, 130.8, 129.4, 129.1, 128.3, 127.0, 126.2, 126.0, 52.1, 24.7, 21.1, 14.3. FT-IR (cm–1): 3059 (w), 3026 (w), 2921 (w), 1702 (s), 1649 (m), 1599 (w), 1572 (w), 1494 (m), 1439 (m), 1356 (m), 1262 (m), 1183 (m), 1119 (m), 1077 (m) 1031(m), 924 (w), 892 (w), 768 (m), 742 (m), 697 (s), 684 (m). Anal. calcd for C37H33NO (507.68): C, 87.54; H, 6.55; N, 2.79%. Found: C, 87.29; H, 6.59; N, 2.73%.
- To a 25 mL round-bottomed flask, equipped with a stir bar, was added zinc(II) chloride (0.20 g, 1.5 mmol), 2-(2,6-dibenzhydryl-4-methylphenylimino)butanone (0.76 g, 1.5 mmol), 2,6-dimethylaniline (0.18 g, 1.5 mmol), and acetic acid (1 mL). The reaction mixture was stirred and heated for 4 h at 80 °C. Once cooled to room temperature, diethyl ether (10 mL) was added and the resulting yellow precipitate was filtered. This intermediate zinc(II) chloride complex was then dissolved in dichloromethane and a saturated aqueous solution of potassium carbonate (K2CO3) was added and the stirred at room temperature for 1.5 h [58,59,60]. Using a separating funnel, the organic layer was extracted and washed with water three times and dried over anhydrous magnesium sulfate (MgSO4). After removing the volatiles by rotary evaporation, the product was recrystallized from hexane to yield L1 as a yellow powder (0.52 g, 57%). 1H NMR (400 MHz, CDCl3, TMS): δ 7.27–7.22 (m, 8H, Py–H), 7.20–7.15 (m, 4H, Py–H), 7.09–7.03 (m, 10H, Py–H), 6.91 (t, J = 8.0 Hz, 1H, Py–H), 6.65 (s, 2H, Py–H), 5.23 (s, 2H, Ar–CH(Ph)2), 2.17 (s, 3H, Ar–CH3), 1.81 (s, 3H, N=C–CH3), 1.09 (s, 6H, Py–CH3), 0.85 (s, 3H, N=C–CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 170.0 (N=C–CH3), 167.8 (N=C–CH3), 148.6, 145.8, 143.7, 142.6, 131.9, 131.6, 129.9, 129.6, 128.8, 128.6, 128.2, 128.0, 126.5, 126.2, 124.6, 123.2, 52.5, 21.5, 18.1, 16.1, 16.0. FT-IR (cm–1): 3024 (w, vPy–H), 2968 (w, vN=C–C–H), 1635 (m, vC=N), 1597 (w, vPy), 1493 (m, vPy), 1447 (m, vPy), 1419 (m, vPy), 1351 (m, vPy), 1257 (m, vC–N), 1199 (m, vC–N), 1122 (m, vPy), 1081 (m, vPy), 1028 (m, vPy), 924 (w, vPy ), 915 (w, vPy), 885 (m, vPy), 859 (m, vAr–C–H), 803 (s, vAr–C–H), 746 (m, vAr-C-H), 698 (s, vAr–CH(Ph-H)). Anal. calcd for C45H42N2 (610.85): C, 88.48; H, 6.93; N, 4.59%. Found: C, 88.33; H, 6.82; N, 4.66%.
- Using a similar procedure as described for L1 but using 2,6-diethylaniline as the aniline gave L2 as a yellow solid (0.48 g, 50%). 1H NMR (400 MHz, CDCl3, TMS): δ 7.28–7.17 (m, 11H, Py–H), 7.11–6.99 (m, 12H, Py–H), 6.67 (s, 2H, Py–H), 5.25 (s, 2H, Ar–CH(Ph)2), 2.31 (t, J = 8.0 Hz, 4H, Py–CH2CH3), 2.18 (s, 3H, Ar–CH3), 1.84 (s, 3H, N=C–CH3), 1.18 (s, J = 8.0 Hz, 6H, Py–CH2CH3), 0.89 (s, 3H, N=C–CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 170.0 (N=C–CH3), 167.7 (N=C–CH3), 147.5, 145.7, 143.8, 142.6, 131.9, 131.6, 130.4, 129.9, 129.6, 128.9, 128.6, 128.2, 126.5, 126.2, 126.0, 123.6, 52.4, 24.6, 21.5, 16.4, 16.1, 14.0. FT-IR (cm–1): 3024 (w), 2963 (w), 1640 (m, vC=N), 1597 (m), 1493 (m), 1446 (m), 1419 (m), 1356 (m), 1255 (m), 1194 (m), 1121 (m), 1076 (m), 1029 (m), 1008 (w), 913 (w), 859 (m), 801 (m), 748 (m), 697 (s). Anal. calcd for C47H46N2 (638.90): C, 88.36; H, 7.26; N, 4.38%. Found: C, 88.10; H, 7.38; N, 4.31%.
- Using a similar procedure as described for L1 but using 2,6-diisopropylaniline as the aniline gave L3 as a yellow solid (0.42 g, 42%). 1H NMR (400 MHz, CDCl3, TMS): δ 7.28–7.24 (m, 8H, Py–H), 7.20–7.17 (m, 4H, Py–H), 7.15–7.09 (m, 6H, Py–H), 7.08–7.04 (m, 5H, Py–H), 6.66 (s, 2H, Py–H), 5.25 (s, 2H, Ar–CH(Ph)2), 2.61 (m, 2H, Py–CH(CH3)2), 2.18 (s, 3H, Ar–CH3), 1.85 (s, 3H, N=C–CH3), 1.22 (d, J = 6.8 Hz, 6H, Py–CH(CH3)2), 1.17 (d, J = 6.8 Hz, 6H, Py–CH(CH3)2), 0.88 (s, 3H, N=C–CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 170.0 (N=C–CH3), 168.0 (N=C–CH3), 146.2, 145.8, 143.9, 142.7, 135.1, 131.9, 131.6, 129.9, 129.6, 128.9, 128.6, 128.2, 126.5, 126.2, 123.9, 123.1, 52.4, 28.3, 23.5, 23.3, 21.5, 16.8, 16.4. FT-IR (cm–1): 3025 (w), 2961 (w), 2921 (w), 1641 (m, vC=N), 1597 (w), 1597 (m), 1493 (m), 1443 (m), 1357 (m), 1326 (m), 1250 (m), 1186 (m), 1117 (m), 1075 (m), 1021 (m), 997 (w), 914 (w), 857 (w), 794 (m), 767 (m), 697 (s). Anal. calcd for C49H50N2 (666.95): C, 88.24; H, 7.56; N, 4.20%. Found: C, 88.01; H, 7.70; N, 4.29%.
- Using a similar procedure as described for L1 but using 2,4,6-trimethylaniline as the aniline gave L4 as a yellow solid (0.45 g, 48%). 1H NMR (400 MHz, CDCl3, TMS): δ 7.29–7.24 (m, 8H, Py–H), 7.22–7.17 (m, 4H, Py–H), 7.12–7.06 (m, 8H, Py–H), 6.88 (s, 2H, Py–H), 6.68 (s, 2H, Py–H), 5.25 (s, 2H, Ar–CH(Ph)2), 2.29 (s, 3H, Py–CH3), 2.19 (s, 3H, Ar–CH3), 1.83 (s, 3H, N=C–CH3), 1.98 (s, 6H, Py–CH3), 0.88 (s, 3H, N=C–CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 170.1 (N=C–CH3), 168.0 (N=C–CH3), 146.2, 145.8, 143.8, 142.6, 132.4, 131.8, 131.6, 129.9, 128.8, 128.6, 128.2, 126.5, 126.2, 124.5, 52.5, 21.5, 20.8, 18.0, 16.2, 15.9. FT-IR (cm–1): 3022 (w), 2920 (w), 1634 (m, vC=N), 1598 (m), 1570 (w), 1492 (m), 1359 (m), 1252 (m), 1201 (w), 1124 (m), 1075 (m), 1029 (m), 913 (w), 854 (m), 768 (w), 741 (m), 696 (s). Anal. calcd for C46H44N2 (624.87): C, 88.42; H, 7.10; N, 4.48%. Found: C, 88.59; H, 7.00; N, 4.42%.
- Using a similar procedure as described for L1 but using 2,6-diethyl-4-methylaniline as the aniline gave L5 as a yellow solid (0.43 g, 44%). 1H NMR (400 MHz, CDCl3, TMS): δ 7.28–7.17 (m, 10H, Py–H), 7.12–6.98 (m, 10H, Py–H), 6.91 (s, 2H, Py–H), 6.67 (s, 2H, Py–H), 5.26 (s, 2H, Ar–CH(Ph)2), 2.31 (s, 3H, Py–CH3), 2.30–2.25 (m, 4H, Py–CH2CH3), 2.19 (s, 3H, Ar–CH3), 1.84 (s, 3H, N=C–CH3), 1.17 (t, J = 7.6 Hz, 6H, Py–CH2CH3), 0.90 (s, 3H, N=C–CH3). 13C NMR (100 MHz, CDCl3, TMS): δ 170.1 (N=C–CH3), 168.0 (N=C–CH3), 145.8, 145.1, 143.1, 142.7, 132.7, 131.6, 131.6, 130.3, 129.9, 129.5, 128.2, 126.5, 126.2, 124.5, 52.4, 24.6, 28.8, 21.5, 16.4, 14.3, 14.1. FT-IR (cm–1): 3023 (w), 3010 (w), 2959 (w), 1642 (m, vC=N), 1599 (m), 1572 (w), 1493 (m), 1419 (w), 1359 (m), 1249 (m), 1203 (m), 1124 (m), 1075 (m), 1030 (m), 913 (w), 859 (w), 768 (w), 741 (m), 696 (s). Anal. calcd for C48H48N2 (652.93): C, 88.30; H, 7.41; N, 4.29%. Found: C, 88.11; H, 7.53; N, 4.20%.
3.2. Synthesis of Nickel Complexes (Ni1–Ni5)
- The complexes Ni1–Ni5 were prepared by the treatment of (DME)NiBr2 with the corresponding ligands (L1–L5) in THF. The preparation of Ni1 is outlined as follows. L1 (0.1 g, 0.16 mmol) and (DME)NiBr2 (0.05 g, 0.15 mmol) were added to a Schlenk tube, along with 10 mL of dichloromethane. The reaction mixture was stirred for 12 h at room temperature, followed by the addition of absolute diethyl ether (10 mL) to precipitate the complex. The precipitate was washed with diethyl ether and dried under vacuum, yielding a brick-red powder of Ni1 (70%, 0.093 g). FT-IR (cm–1): 3027 (w), 2917 (w), 2923 (w), 2866 (w), 1636 (m, vC=N), 1599 (m), 1494 (w), 1472 (w), 1441 (m), 1376 (m), 1301 (m), 1219 (m), 1152 (m), 1118 (m), 1075 (m), 1033 (m), 1003 (w), 916 (m), 828 (w), 800 (w), 770 (m), 751 (m), 698 (s). Anal calcd for C45H42Br2N2Ni (829.35): C, 65.17; H, 5.10; N, 3.38%. Found C, 64.99; H, 5.22; N, 3.10%.
- A similar approach to that used for Ni1 was employed, replacing L1 with L2 as the ligand. Ni2 was isolated as a brick red complex (0.099 g, 72%). FT-IR (cm–1): 3329 (w), 2969 (w), 2933 (w), 1637 (m, vC=N), 1602 (m), 1543 (w), 1491 (m), 1445 (m), 1400 (w), 1380 (w), 1340 (w), 1073 (w), 990 (m), 980 (w), 970 (w), 940 (w), 900 (w), 864 (m), 829 (m), 791 (m), 767 (m), 700 (s). Anal calcd for C47H46Br2N2Ni (857.40): C, 65.84; H, 5.41; N, 3.27%. Found: C, 65.55; H, 5.20; N, 3.10%.
- A similar approach to that used for Ni1 was employed, replacing L1 with L3 as the ligand. Ni3 was isolated as a brick red complex (0.120 g, 85%). FT-IR (cm–1): 3224 (w), 3024 (w), 2960 (m), 2923 (w), 2790 (w), 1634 (m, vC=N), 1600 (m), 1576 (w), 1494 (m), 1447 (m), 1413 (m), 1378 (s), 1323 (w), 1302 (w), 1252 (w), 1214 (m), 1153 (m), 1123 (m), 1033 (m), 995 (m), 912 (m), 861 (m), 830 (m), 791 (m), 763 (m), 741 (m), 698 (s). Anal calcd for C49H50Br2N2Ni (885.45): C, 66.47; H, 5.69; N, 3.16%. Found: C, 66.21; H, 5.82; N, 3.29%.
- A similar approach to that used for Ni1 was employed, replacing L1 with L4 as the ligand. Ni4 was isolated as a brick red complex (0.106 g, 79%). FT-IR (cm–1): 3120 (w), 2916 (m), 2762 (w), 1639 (m, vC=N), 1601 (m), 1491 (m), 1413 (m), 1380 (m), 1361 (m), 1302 (m), 1226 (m), 1167 (m), 1124 (m), 1074 (m), 1036 (m), 997 (w), 974 (w), 912 (w), 859 (m), 828 (m), 800 (m), 753 (m), 710 (s). Anal calcd for C46H44Br2N2Ni (843.37): C, 65.51; H, 5.26; N, 3.32%. Found: C, 65.21; H, 5.00; N, 3.11%.
- A similar approach to that used for Ni1 was employed, replacing L1 with L5 as the ligand. Ni5 was isolated as a brick red complex (0.100 g, 72%). FT-IR (cm–1): 3112 (w), 2960 (m), 2731 (w), 1637 (m, vC=N), 1574 (m), 1494 (m), 1449 (m), 1392 (m), 1380 (m), 1359 (w), 1330 (w), 1311 (w), 1250 (m), 1219 (m), 1173 (m), 1125 (m), 1032 (m), 1000 (m), 914 (w), 866 (m), 828 (m), 780 (w), 751 (m), 691 (s). Anal calcd for C47H45Br2N2Ni (871.43): C, 66.16; H, 5.55; N, 3.21%. Found: C, 66.41; H, 5.10; N, 3.45%.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, A.L.N.D.; Tavares, M.I.B.; Politano, D.P.; Coutinho, F.M.B.; Rocha, M.C.G. Polymer blends based on polyolefin elastomer and polypropylene. J. Appl. Polym. Sci. 2015, 66, 2005–2014. [Google Scholar] [CrossRef]
- Hotta, A.; Cochran, E.; Ruokolainen, J.; Khanna, V.; Fredrickson, G.; Kramer, E.; Shin, Y.; Shimizu, F.; Cherian, A.; Hustad, P. Semicrystalline thermoplastic elastomeric polyolefins: Advances through catalyst development and macromolecular design. Proc. Natl. Acad. Sci. USA 2006, 103, 15327–15332. [Google Scholar] [CrossRef] [PubMed]
- Giller, C.; Gururajan, G.; Wei, J.; Zhang, W.; Hwang, W.; Chase, D.B.; Rabolt, J.F.; Sita, L.R. Synthesis, characterization, and electrospinning of architecturally-discrete isotacticatacticisotactic triblock stereoblock polypropene elastomers. Macromolecules 2011, 44, 471–482. [Google Scholar] [CrossRef]
- Chapleski, R.C.; Kern, J.L.; Anderson, W.C.; Long, B.K.; Roy, S. A mechanistic study of microstructure modulation in olefin polymerizations using a redox-active Ni(ii) α-diimine catalyst. Catal. Sci. Technol. 2020, 10, 2029–2039. [Google Scholar] [CrossRef]
- Souza, R.F.; Casagrande, O.L. Recent advances in olefin polymerization using binary catalyst systems. Macromol. Rapid Commun. 2001, 22, 1293–1301. [Google Scholar] [CrossRef]
- Hustad, P.D. Frontiers in olefin polymerization: Reinventing the world’s most common synthetic polymers. Science 2009, 325, 704–707. [Google Scholar] [CrossRef]
- Ohtaki, H.; Deplace, F.; Vo, G.D.; LaPointe, A.M.; Shimizu, F.; Sugano, T.; Kramer, E.J.; Fredrickson, G.H.; Coates, G.W. Allyl-terminated polypropylene macromonomers: A route to polyolefin elastomers with excellent elastic behavior. Macromolecules 2015, 48, 7489–7494. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Controlled Isospecific polymerization of α-olefins by hafnium complex incorporating with a trans-cyclooctanediyl-bridged [OSSO]-type bis(phenolate) ligand. Macromolecules 2013, 46, 6758–6764. [Google Scholar] [CrossRef]
- Collina, G.; Braga, V.; Sartori, F. New thermoplastic polyolefins elastomers from the novel Multicatalysts Reactor Granule Technology: Their relevant physical-mechanical properties after crosslinking. Polym. Bull. 1997, 38, 701–705. [Google Scholar] [CrossRef]
- Aluas, M.; Filip, C. Solid-state NMR characterization of cross-linking in EPDM/PP blends from 1H–13C polarization transfer dynamics—ScienceDirect. Solid State Nucl. Magn. Reson. 2005, 27, 165–173. [Google Scholar] [CrossRef]
- Montoya, M.; Tomba, J.P.; Carella, J.M.; Gobernado-Mitre, M.I. Physical characterization of commercial polyolefinic thermoplastic elastomers. Eur. Polym. J. 2004, 40, 2757–2766. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Poon, B.C.; Dias, P.; Ansems, P.; Chum, S.P.; Hiltner, A.; Baer, E. Structure and deformation of an elastomeric propylene–ethylene copolymer. J. Appl. Polym. Sci. 2010, 104, 489–499. [Google Scholar] [CrossRef]
- Schmalz, H.; Abetz, V.; Lange, R. Thermoplastic elastomers based on semicrystalline block copolymers. Compos. Sci. Technol. 2003, 63, 1179–1186. [Google Scholar] [CrossRef]
- Mehrabzadeh, M.; Delfan, N. Thermoplastic elastomers of butadiene-acrylonitrile copolymer and polyamide. VI. Dynamic crosslinking by different systems. J. Appl. Polym. Sci. 2000, 77, 2057–2066. [Google Scholar] [CrossRef]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From multisite polymerization catalysis to sustainable materials and all-polyolefin composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef]
- Wu, C.; Ren, M.; Hou, L.; Qu, S.; Li, X.; Zheng, C.; Chen, J.; Wang, W. Ethylene copolymerization with linear and end-cyclized olefins via a metallocene catalyst: Polymerization behavior and thermal properties of copolymers. Engineering 2023, 93–99. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.; Zhu, S. Polyolefin thermoplastics for multiple shape and reversible shape memory. ACS Appl. Mater. Interfaces 2017, 9, 4882–4889. [Google Scholar] [CrossRef]
- Mcnally, T.; Mcshane, P.; Nally, G.M.; Murphy, W.R.; Cook, M.; Miller, A. Rheology, phase morphology, mechanical, impact and thermal properties of polypropylene/metallocene catalysed ethylene 1-octene copolymer blends. Polymer 2002, 43, 3785–3793. [Google Scholar] [CrossRef]
- Silva, A.L.N.D.; Rocha, M.C.G.; Coutinho, F.M.B.; Bretas, R.; Scuracchio, C. Rheological, mechanical, thermal, and morphological properties of polypropylene/ethylene-octene copolymer blends. J. Appl. Polym. Sci. 2015, 75, 692–704. [Google Scholar] [CrossRef]
- Anderson, W.C., Jr.; Long, B.K. Modulating polyolefin copolymer composition via redox-active olefin polymerization catalysts. ACS Macro. Lett. 2016, 5, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Pan, R.-C.; Liu, Y.; Lu, X.-B. Bulky o-phenylene-bridged bimetallic α-diimine Ni(II) and Pd(II) catalysts in ethylene (co)polymerization. Organometallics 2021, 40, 3703–3711. [Google Scholar] [CrossRef]
- Sakuma, A.; Weiser, M.-S.; Fujita, T. Living olefin polymerization and block copolymer formation with FI catalysts. Polym. J. 2007, 39, 193–207. [Google Scholar] [CrossRef]
- Makio, H.; Fujita, T. Development and application of FI catalysts for olefin polymerization: Unique catalysis and distinctive polymer formation. Acc. Chem. Res. 2009, 42, 1532–1544. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-based catalysts for polymerization of ethylene and .alpha.-Olefins. J. Am. Chem. Soc 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Killian, C.M.; Tempel, D.J.; Johnson, L.K.; Brookhart, M. Living polymerization of α-olefins using NiII−α-diimine catalysts. Synthesis of new block polymers based on α-olefins. J. Am. Chem. Soc. 1996, 118, 11664–11665. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef]
- Cherian, A.E.; Rose, J.M.; Lobkovsky, E.B.; Coates, G.W. A C-2-Symmetric, living α-diimine Ni(II) catalyst: Regioblock copolymers from propylene. J. Am. Chem. Soc. 2005, 127, 13770–13771. [Google Scholar] [CrossRef]
- Anderson, W.C.; Rhinehart, J.L.; Tennyson, A.G.; Long, B.K. Redox-active ligands: An advanced tool to modulate polyethylene microstructure. J. Am. Chem. Soc. 2016, 138, 774–777. [Google Scholar] [CrossRef]
- Camacho, D.H.; Guan, Z. Designing late-transition metal catalysts for olefin insertion polymerization and copolymerization. Chem. Commun. 2010, 46, 7879–7893. [Google Scholar] [CrossRef]
- Li, X.; Hu, Z.; Mahmood, Q.; Wang, Y.; Sohail, S.; Zou, S.; Liang, T.; Sun, W.-H. Tunable C2-symmetric α-diimine nickel catalysts mediated ethylene polymerization for PEE synthesis: In-depth insights from ligand and nickel halide variations. Dalton Trans. 2024, 53, 18193–18206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Q.; Wang, Y.; Zou, S.; Ma, Y.; Liang, T.; Sun, W.-H. Axial-phenyl constrained bis(imino)acenaphthene-nickel precatalysts enhancing ethylene polymerization. Polym. Chem. 2024, 15, 4993–5006. [Google Scholar]
- Zheng, Y.; Jiang, S.; Liu, M.; Yu, Z.; Ma, Y.; Solan, G.A.; Zhang, W.; Liang, T.; Sun, W.-H. High molecular weight PE elastomers through 4,4-difluorobenzhydryl substitution in symmetrical α-diimino-nickel ethylene polymerization catalysts. RSC Adv. 2022, 12, 24037–24049. [Google Scholar] [CrossRef]
- Sa, S.; Jeon, M.; Kim, S.Y. Controlling branch distribution of polyethylenes by steric tuning of Ni α-diimine complexes based on phenanthrenequinone. J. Mol. Catal. A Chem. 2014, 393, 263–271. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Yuan, Y.; Du, S.; Sun, Y.; Solan, G.A.; Guo, C.-Y.; Sun, W.-H. Raising the n-aryl fluoride content in unsymmetrical diaryliminoacenaphthylenes as a route to highly active nickel(ii) catalysts in ethylene polymerization. Dalton Trans. 2016, 45, 18313–18323. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Ma, Y.; Guo, C.Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. Elastomeric polyethylenes accessible via ethylene homo-polymerization using an unsymmetrical α-diimino-nickel catalyst. Polym. Chem. 2017, 8, 2785. [Google Scholar] [CrossRef]
- Mahmood, Q.; Zeng, Y.; Yue, E.; Solan, G.A.; Liang, T.; Sun, W.-H. Ultra-high molecular weight elastomeric polyethylene using an electronically and sterically enhanced nickel catalyst. Polym. Chem. 2017, 8, 6416–6430. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, R.; Ma, Y.; Han, M.; Solan, G.A.; Yang, W.; Liang, T.; Sun, W.-H. Trifluoromethoxy-substituted nickel catalysts for producing highly branched polyethylenes: Impact of solvent, activator and N,N′-ligand on polymer properties. Polym. chem. 2022, 13, 1040–1058. [Google Scholar] [CrossRef]
- Wu, R.; Wang, Y.; Zhang, R.; Guo, C.-Y.; Flisak, Z.; Sun, Y.; Sun, W.-H. Finely tuned nickel complexes as highly active catalysts affording branched polyethylene of high molecular weight: 1-(2,6-dibenzhydryl-4-methoxyphenylimino)-2-(arylimino) acenaphthylenenickel halides. Polymer 2018, 153, 574–586. [Google Scholar] [CrossRef]
- Kong, S.; Guo, C.-Y.; Yang, W.; Wang, L.; Sun, W.-H.; Glaser, R. 2,6-Dibenzhydryl-n-(2-phenyliminoacenaphthylenylidene)-4-chloro-aniline nickel dihalides: Synthesis, characterization and ethylene polymerization for polyethylenes with high molecular weights. J. Organomet. Chem. 2013, 725, 37–45. [Google Scholar] [CrossRef]
- Mahmood, Q.; Zeng, Y.; Wang, X.; Sun, Y.; Sun, W.-H. Advancing polyethylene properties by incorporating NO2 moiety in 1,2-bis(arylimino)acenaphthylnickel precatalysts: Synthesis, characterization and ethylene polymerization. Dalton Trans. 2017, 46, 6934–6947. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, W.; Hao, X.; Redshaw, C.; Sun, W.-H. 2,6-Dibenzhydryl-N-(2-phenyliminoacenaphthylenylidene)-4-methylbenzenamine nickel dibromides: Synthesis, characterization, and ethylene polymerization. Organometallics 2011, 30, 2418–2424. [Google Scholar] [CrossRef]
- Fan, L.; Du, S.Z.; Guo, C.Y.; Hao, X.; Sun, W.-H. 1-(2,6-dibenzhydryl-4-fluorophenylimino)-2-aryliminoacenaphthylylnickel halides highly polymerizing ethylene for the polyethylenes with high branches and molecular weights. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1369–1378. [Google Scholar] [CrossRef]
- Yang, W.; Ma, Z.; Yi, J.; Sun, W.-H. Quantitative structure-thermostability relationship of late transition metal catalysts in ethylene oligo/polymerization. Catalysts 2017, 7, 120. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A robust Ni(II) α-diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef]
- Matsko, M.A.; Semikolenova, N.V.; Zakharov, V.A.; Soshnikov, I.E.; Shundrina, I.K.; Sun, W.-H. Formation of branched polyethylenes by ethylene homopolymerization using LNiBr2 homoand heterogeneous precatalysts: Interpretation of the polymer structures in comparison with commercial LLDPE. J. Appl. Polym. Sci. 2021, 138, 50436. [Google Scholar] [CrossRef]
- Wu, R.; Wu, W.K.; Stieglitz, L.; Gaan, S.; Rieger, B.; Heuberger, M. Recent advances on α-diimine Ni and Pd complexes for catalyzed ethylene (co)polymerization: A comprehensive review. Coord. Chem. Rev. 2023, 474, 214844. [Google Scholar] [CrossRef]
- Yan, X.; Fu, Z.; Li, Y.; Shi, Y. Homo- and copolymerizations of ethylene and α-olefins in n -hexane catalyzed by Ni(ii)-α-diimine catalyst. Polym. Bull. 2012, 68, 327–339. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Antonov, A.A.; Sun, W.-H.; Talsi, E.P. Activation of an alpha-diimine Ni(II) precatalyst with AlMe3 and (AlBu3)-Bu-i: Catalytic and NMR and EPR spectroscopy studies. Organometallics 2020, 39, 3034–3040. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.; Jia, D.; Hao, X.; Redshaw, C.; Sun, W.-H. 2-{2,6-Bis[bis(4-fluorophenyl)methyl]-4-chlorophenylimino}-3-aryliminobutylnickel(II) bromide complexes: Synthesis, characterization, and investigation of their catalytic behavior. Appl. Catal. A Gen. 2014, 475, 195–202. [Google Scholar] [CrossRef]
- Jiang, S.; Zheng, Y.; Oleynik, I.V.; Yu, Z.; Solan, G.A.; Oleynik, I.I.; Liu, M.; Ma, Y.; Liang, T.; Sun, W.-H. N,N-Bis(2,4-Dibenzhydryl-6-cycloalkylphenyl)butane-2,3-diimine–nickel complexes as tunable and effective catalysts for high-molecular-weight PE elastomers. Molecules 2023, 28, 4852. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Zhang, W.; Liu, W.; Wang, L.; Redshaw, C.; Sun, W.-H. Unsymmetrical α-diiminonickel bromide complexes: Synthesis, characterization and their catalytic behavior toward ethylene. Catal. Sci. Technol. 2013, 3, 2737–2745. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, S.Y. Ethylene polymerizations with unsymmetrical (α-diimine)nickel(II) catalysts. Polym. J. 2008, 40, 409–413. [Google Scholar] [CrossRef]
- Du, S.; Kong, S.; Shi, Q.; Mao, J.; Guo, C.; Yi, J.; Liang, T.; Sun, W.-H. Enhancing the activity and thermal stability of nickel complex precatalysts using 1-[2,6-Bis(bis(4-fluorophenyl)methyl)-4-methyl phenylimino]-2-aryliminoacenaphthylene derivatives. Organometallics 2015, 34, 582–590. [Google Scholar] [CrossRef]
- Saeed, H.; Mahmood, Q.; Yuan, R.; Wang, Y.; Zou, S.; Tahir, K.F.; Ma, Y.; Liang, T.; Sun, W.-H. High-performance polyethylene elastomers using a hybrid steric approach in α-diimine nickel precatalysts. Polym. Chem. 2024, 15, 1437–1452. [Google Scholar] [CrossRef]
- Galland, G.B.; Souza, R.F.D.; Mauler, R.S.; Nunes, F.F. 13C NMR determination of the composition of linear low-density polyethylene obtained with [η 3 -methallyl-nickel-diimine]PF 6 complex. Macromolecules 2016, 32, 1620–1625. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Mahmood, Q.; Yuan, S.; Li, X.; Qin, L.; Zou, S.; Liang, T.; Sun, W.-H. Enhancing the thermal stability of α-diimine Ni(II) catalysts for ethylene polymerization via symmetrical axial shielding strategy. Eur. Polym. J. 2023, 194, 112112. [Google Scholar] [CrossRef]
- Rosa, V.; Avilés, T.; Aullon, G.; Covelo, B.; Lodeiro, C. A new bis(1-naphthylimino)acenaphthene compound and its Pd(II) and Zn(II) complexes: Synthesis, characterization, solid-state structures and sensity sunctional theory studies on the syn and anti isomers. Inorg. Chem. 2008, 47, 7734–7744. [Google Scholar] [CrossRef]
- Zhang, D.; Nadres, E.T.; Brookhart, M.; Daugulis, O. Synthesis of highly branched polyethylene using “sandwich” (8-p-tolyl naphthyl α-diimine)nickel(II) catalysts. Organometallics 2013, 32, 5136–5143. [Google Scholar] [CrossRef]
- Vasudevan, K.V.; Findlater, M.; Cowley, A.H. Synthesis and reactivity of tetrakis(imino)pyracene (TIP) ligands; bifunctional analogues of the BIAN ligand class. Chem. Commun. 2008, 16, 1918–1919. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]


| Nickel Complexes | Ni4 | Ni5 |
|---|---|---|
| Bond length (Å) | ||
| Ni(1)–N(1) | 1.999(3) | 2.007(2) |
| Ni(1)–N(2) | 2.016(3) | 2.020(2) |
| Ni(1)–Br(1) | 2.3063(9) | 2.3387(8) |
| Ni(1)–Br(2) | 2.3425(7) | 2.3159(9) |
| N(1)–C(2) | 1.286(4) | 1.276(4) |
| N(1)–C(14) | 1.448(4) | 1.445(4) |
| N(2)–C(3) | 1.289(4) | 1.288(4) |
| N(2)–C(5) | 1.450(4) | 1.447(3) |
| Bond angles (°) | ||
| Br(1)–Ni(1)–Br(2) | 119.36(3) | 119.77(3) |
| N(1)–Ni(1)–Br(1) | 122.20(8) | 113.32(7) |
| N(1)–Ni(1)–Br(2) | 101.83(8) | 110.88(7) |
| N(1)–Ni(1)–N(2) | 80.84(10) | 80.99(10) |
| N(2)–Ni(1)–Br(1) | 117.25(7) | 111.20(7) |
| N(2)–Ni(1)–Br(2) | 108.47(7) | 114.34(7) |
| Entry | Cocat. | T/°C | t/min | Al/Ni | Act. b | Mw c | Mw/Mn c | Tm d |
|---|---|---|---|---|---|---|---|---|
| 1 | MAO | 30 | 30 | 2000 | 4.11 | 4.23 | 2.15 | 85.11 |
| 2 | MMAO | 30 | 30 | 2000 | 2.81 | 3.52 | 3.99 | 66.01 |
| 3 | EASC | 30 | 30 | 400 | 0.47 | 5.12 | 2.22 | 61.61 |
| 4 | Et2AlCl | 30 | 30 | 400 | 4.61 | 5.96 | 2.35 | 49.01 |
| Entry | Precat. | T/°C | t/min | Al/Ni | Activity b | Mw c | Mw/Mn c | Tm d |
|---|---|---|---|---|---|---|---|---|
| 1 | Ni1 | 30 | 30 | 300 | 3.13 | 5.83 | 2.67 | 60.75 |
| 2 | Ni1 | 30 | 30 | 400 | 4.61 | 5.96 | 2.35 | 49.01 |
| 3 | Ni1 | 30 | 30 | 500 | 4.83 | 7.56 | 2.54 | 47.40 |
| 4 | Ni1 | 30 | 30 | 600 | 5.03 | 7.21 | 2.60 | 45.92 |
| 5 | Ni1 | 30 | 30 | 700 | 3.62 | 4.67 | 2.15 | 45.05 |
| 6 | Ni1 | 10 | 30 | 600 | 3.84 | 14.22 | 2.23 | 88.99 |
| 7 | Ni1 | 20 | 30 | 600 | 6.07 | 7.53 | 2.37 | 81.58 |
| 8 | Ni1 | 40 | 30 | 600 | 3.94 | 4.92 | 2.26 | 41.04 |
| 9 | Ni1 | 50 | 30 | 600 | 3.77 | 4.72 | 1.97 | 15.47 |
| 10 | Ni1 | 60 | 30 | 600 | 3.46 | 4.64 | 2.22 | – g |
| 11 | Ni1 | 70 | 30 | 600 | 3.20 | 4.22 | 1.85 | – g |
| 12 | Ni1 | 80 | 30 | 600 | 1.97 | 3.78 | 1.95 | – g |
| 13 | Ni1 | 20 | 5 | 600 | 13.67 | 3.33 | 2.48 | 80.38 |
| 14 | Ni1 | 20 | 15 | 600 | 6.68 | 4.44 | 2.21 | 54.99 |
| 15 | Ni1 | 20 | 45 | 600 | 4.67 | 11.58 | 2.47 | 74.42 |
| 16 | Ni1 | 20 | 60 | 600 | 4.42 | 19.47 | 2.15 | 75.81 |
| 17 e | Ni1 | 20 | 30 | 600 | 0.31 | 3.78 | 1.95 | 51.50 |
| 18 f | Ni1 | 20 | 30 | 600 | 1.43 | 5.81 | 2.31 | 52.45 |
| 19 | Ni2 | 20 | 30 | 600 | 4.45 | 7.13 | 2.44 | 53.88 |
| 20 | Ni3 | 20 | 30 | 600 | 4.16 | 8.56 | 2.29 | 69.80 |
| 21 | Ni4 | 20 | 30 | 600 | 4.65 | 3.98 | 2.35 | 78.64 |
| 22 | Ni5 | 20 | 30 | 600 | 4.3 | 7.44 | 2.50 | 76.73 |
| PE Sample | Branches /1000Cs | Branching Composition (%) | |||||
|---|---|---|---|---|---|---|---|
| Me | Et | Pr | Bu | Amyl | Longer Branch | ||
| PE-Et2AlCl-20 | 84 | 84.8 | 3.1 | 4.0 | 4.9 | 0.8 | 2.4 |
| PE-Et2AlCl-70 | 217 | 71.4 | 5.3 | 6.1 | 8.8 | 2.0 | 6.4 |
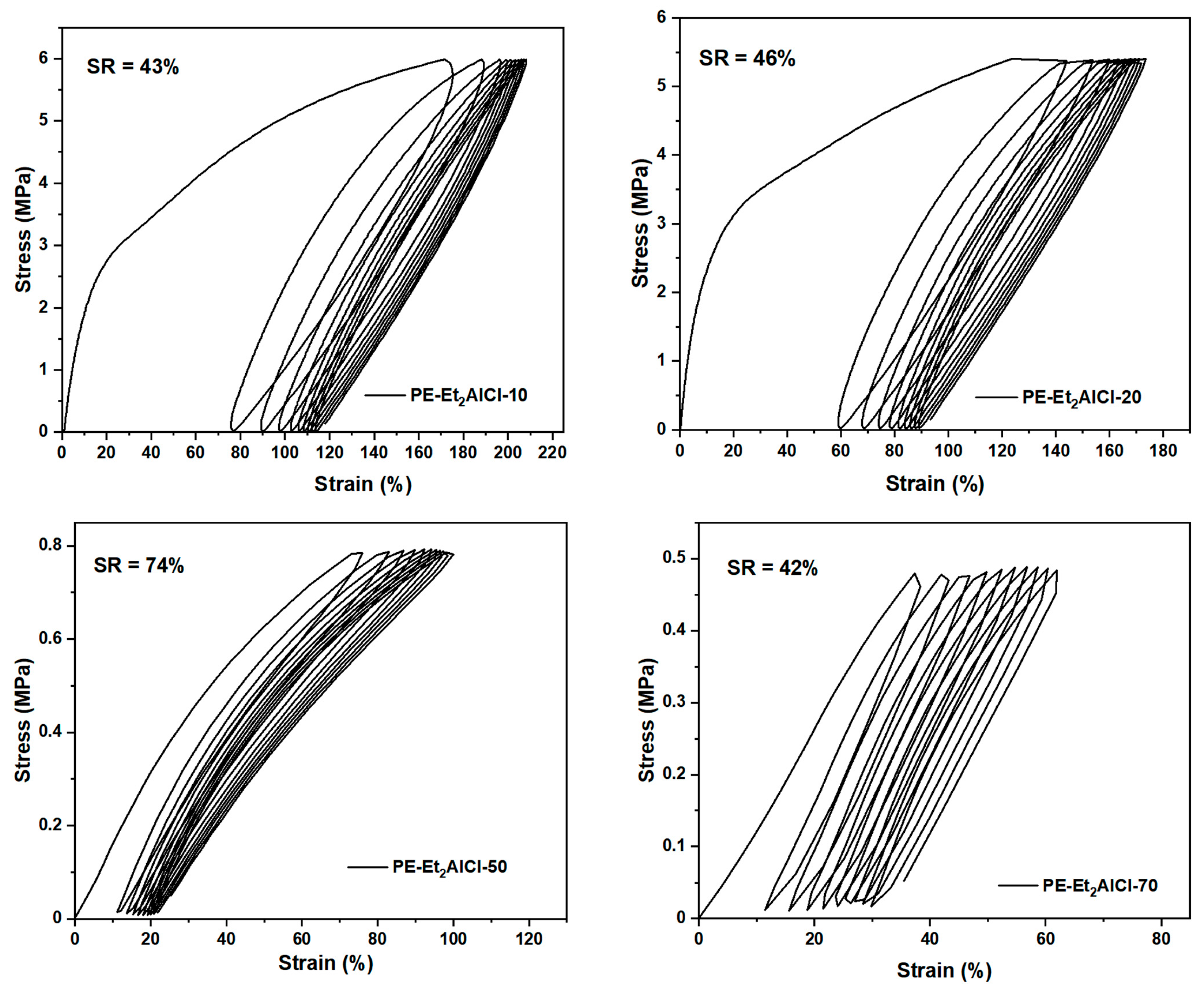
| PE Sample | T (°C) | Mw (105) a | Tm (°C) b | Xc (%) b | σb (MPa) c | εb (%) c | SR (%) d |
|---|---|---|---|---|---|---|---|
| PE-Et2AlCl-10 | 10 | 14.22 | 88.99 | 35.78 | 16.83 | 458 | 43 |
| PE-EtAlCl2-20 | 20 | 7.53 | 81.58 | 29.76 | 14.16 | 475 | 46 |
| PE-Et2AlCl-50 | 50 | 4.72 | 15.47 | 8.19 | 3.21 | 271 | 74 |
| PE-Et2AlCl-70 | 70 | 4.22 | – e | – | 0.74 | 358 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Jia, D.; Zhang, Q.; Ma, Y.; Mahmood, Q.; Sun, W.-H. Tuning 2,3-Bis(arylimino)butane-nickel Precatalysts for High-Molecular-Weight Polyethylene Elastomers. Molecules 2025, 30, 1847. https://doi.org/10.3390/molecules30081847
Zhu D, Jia D, Zhang Q, Ma Y, Mahmood Q, Sun W-H. Tuning 2,3-Bis(arylimino)butane-nickel Precatalysts for High-Molecular-Weight Polyethylene Elastomers. Molecules. 2025; 30(8):1847. https://doi.org/10.3390/molecules30081847
Chicago/Turabian StyleZhu, Dongzhi, Dedong Jia, Qiuyue Zhang, Yanping Ma, Qaiser Mahmood, and Wen-Hua Sun. 2025. "Tuning 2,3-Bis(arylimino)butane-nickel Precatalysts for High-Molecular-Weight Polyethylene Elastomers" Molecules 30, no. 8: 1847. https://doi.org/10.3390/molecules30081847
APA StyleZhu, D., Jia, D., Zhang, Q., Ma, Y., Mahmood, Q., & Sun, W.-H. (2025). Tuning 2,3-Bis(arylimino)butane-nickel Precatalysts for High-Molecular-Weight Polyethylene Elastomers. Molecules, 30(8), 1847. https://doi.org/10.3390/molecules30081847










