Synthesis, Evaluation of Biological Activity, and Structure–Activity Relationships of New Amidrazone Derivatives Containing Cyclohex-1-ene-1-Carboxylic Acid
Abstract
:1. Introduction
2. Results
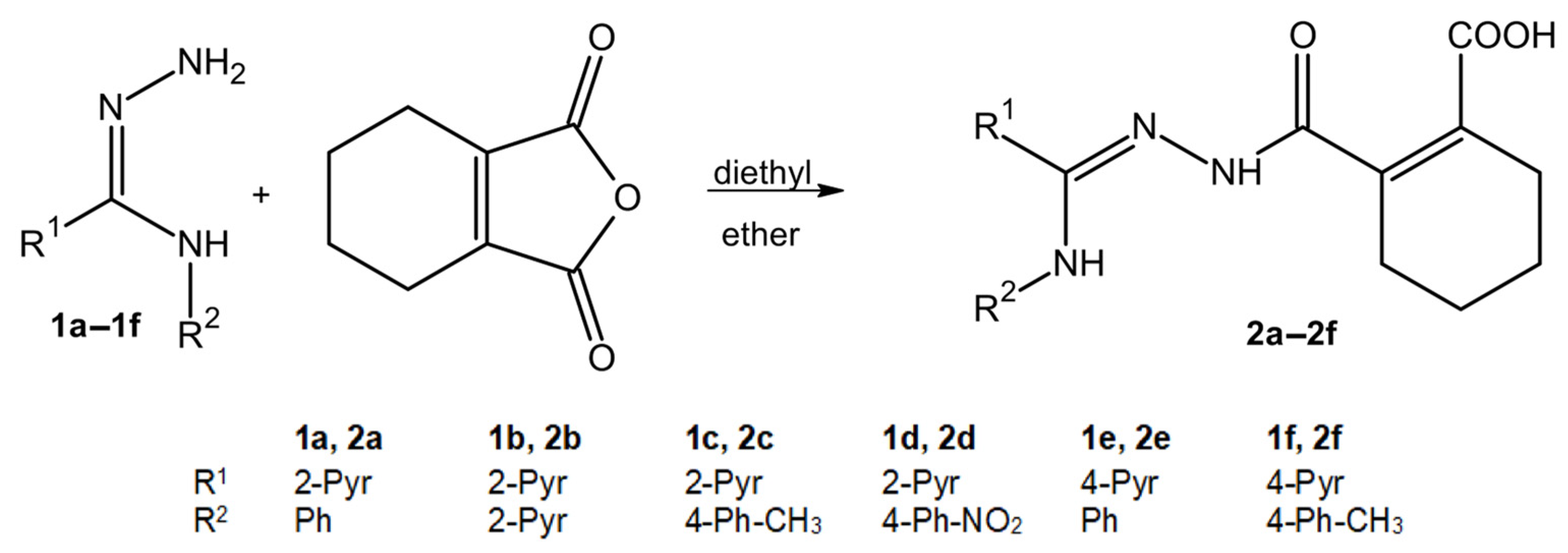
2.1. The Synthesis of Compounds 2a–2f
2.2. Toxicity of Compounds 2a–2f
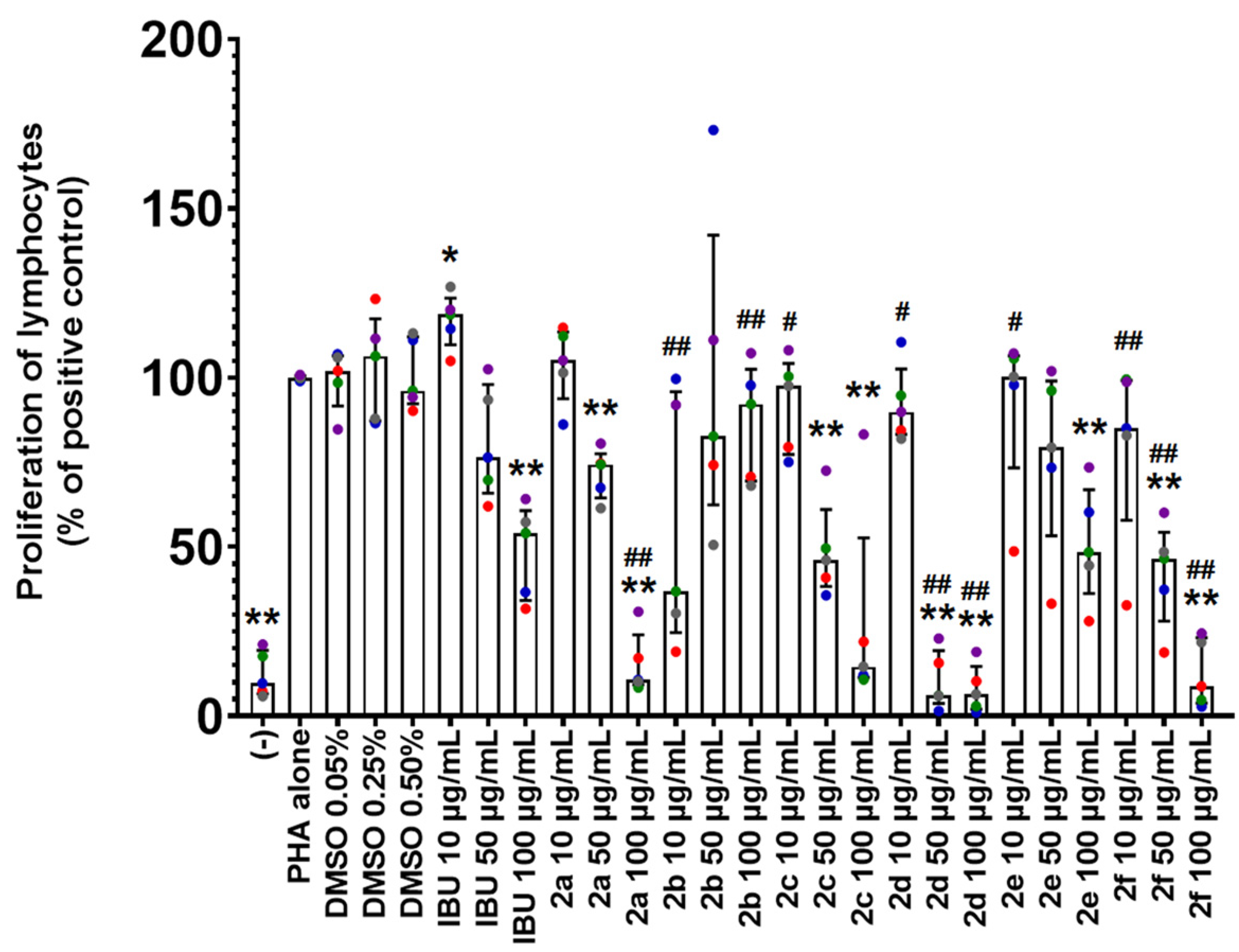
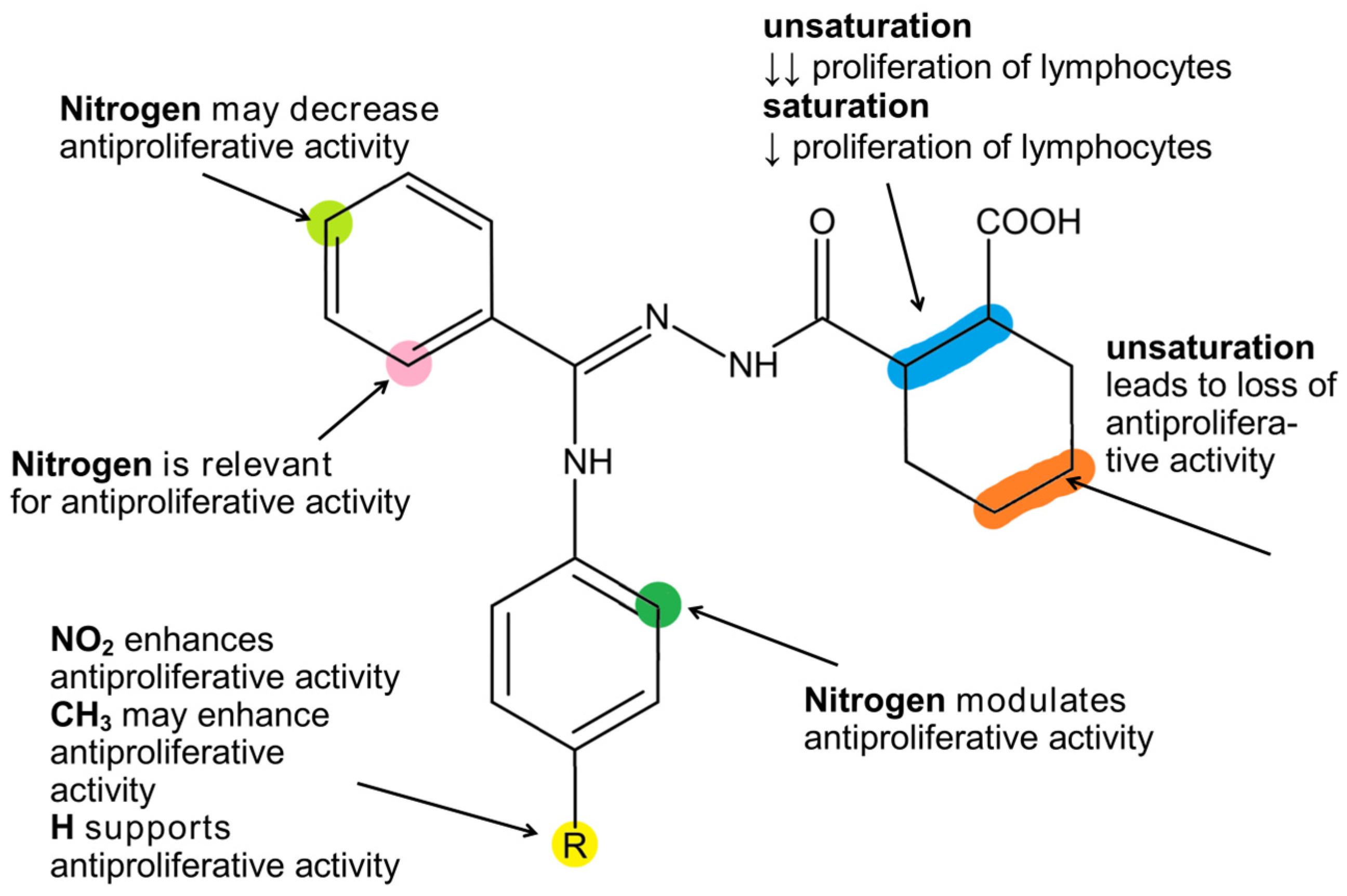
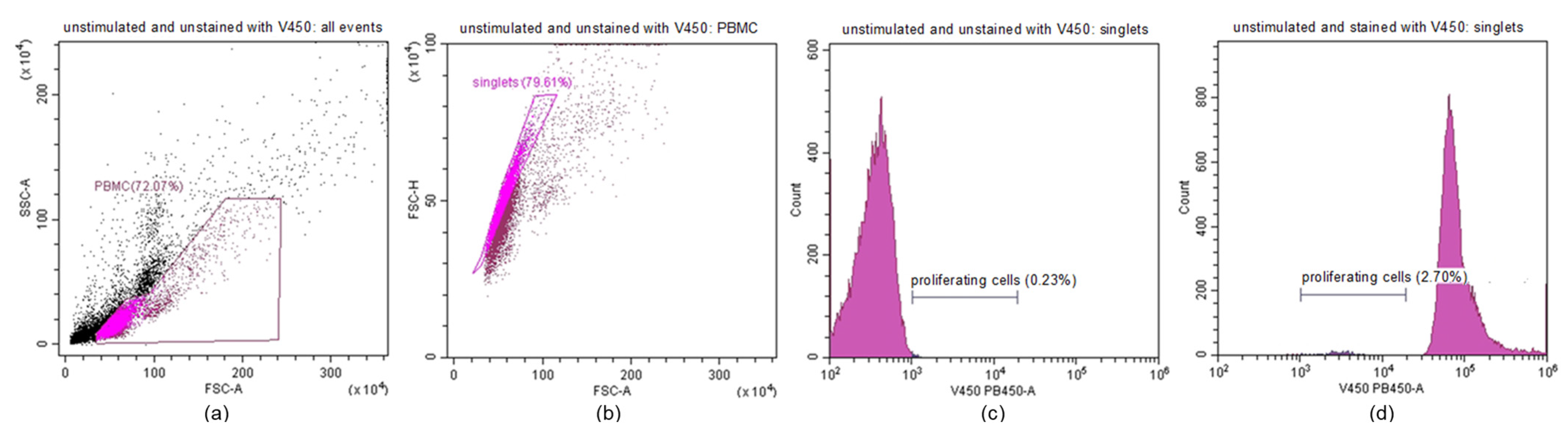
2.3. Antiproliferative Activity of Compounds 2a–2f
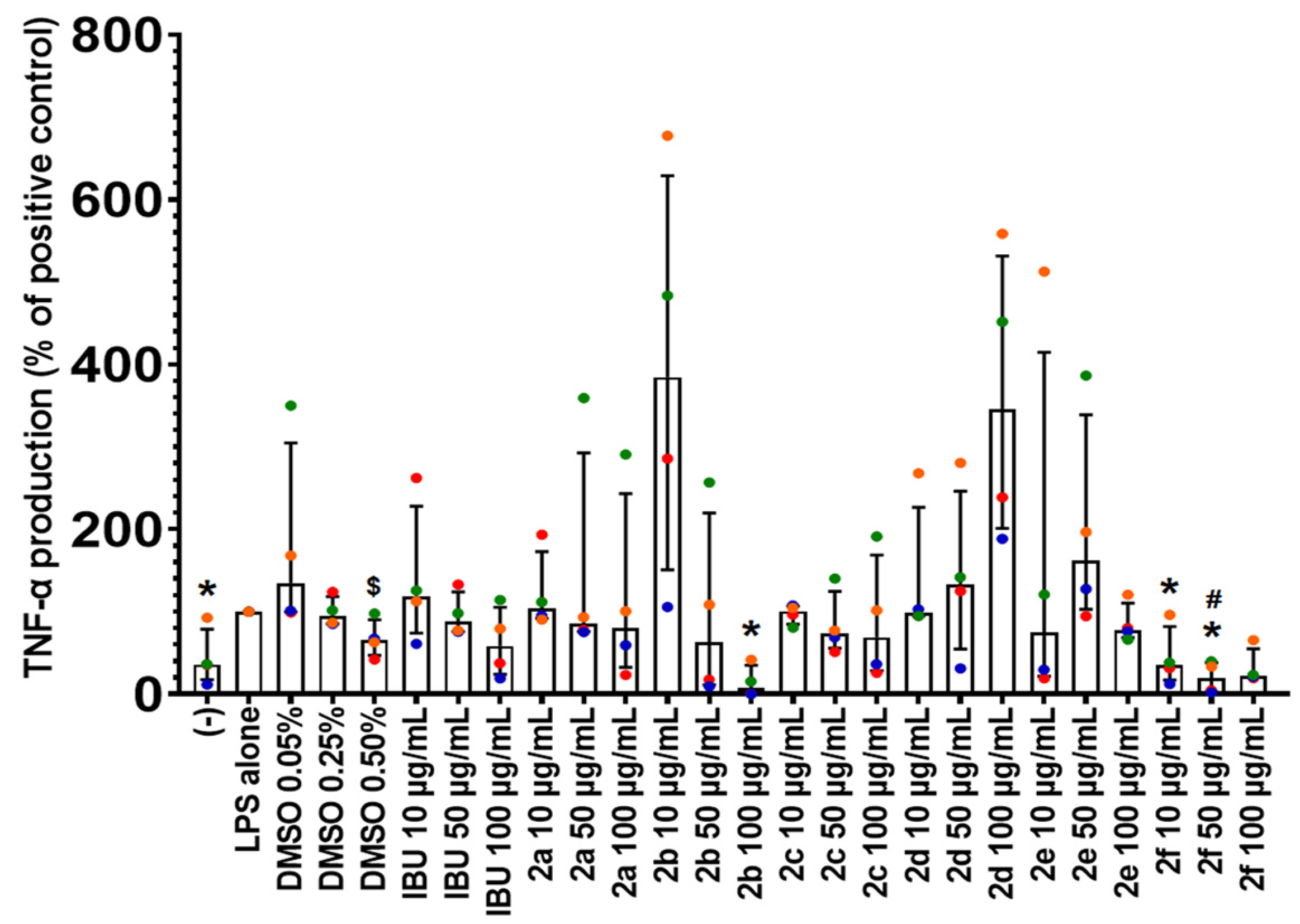
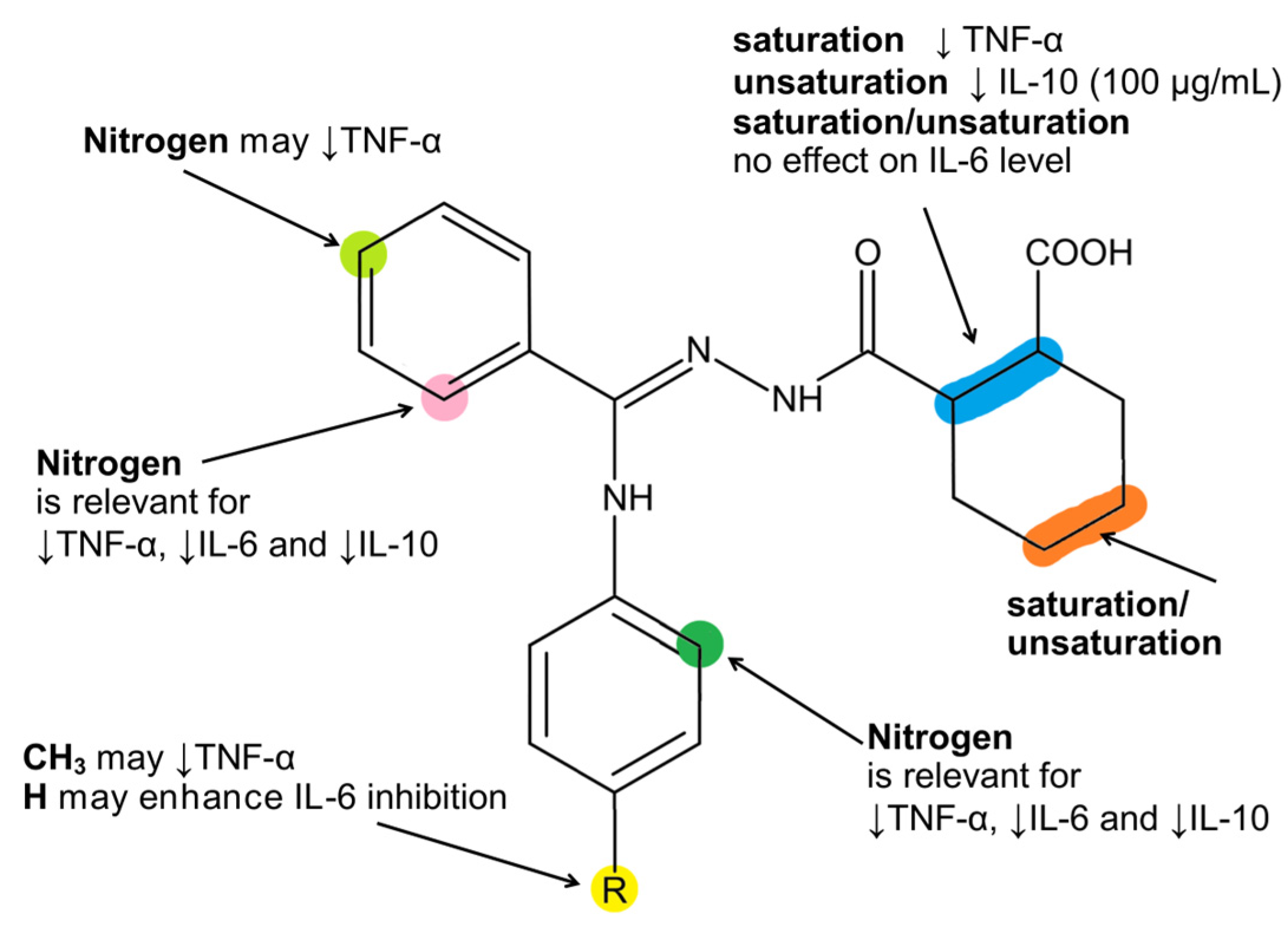
2.4. Effect of Compounds 2a–2f on TNF-α Production
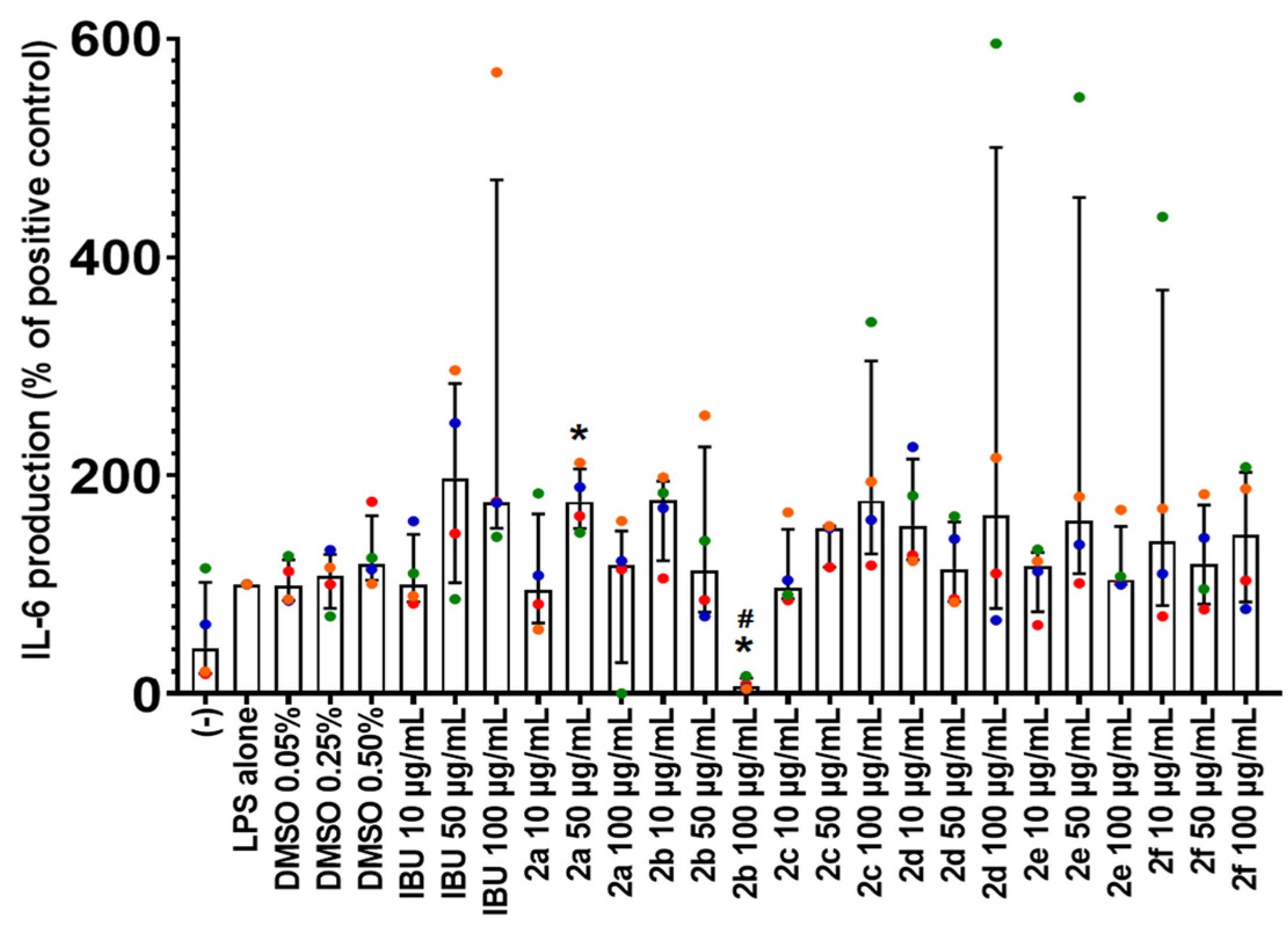
2.5. Effect of Compounds 2a–2f on IL-6 Production
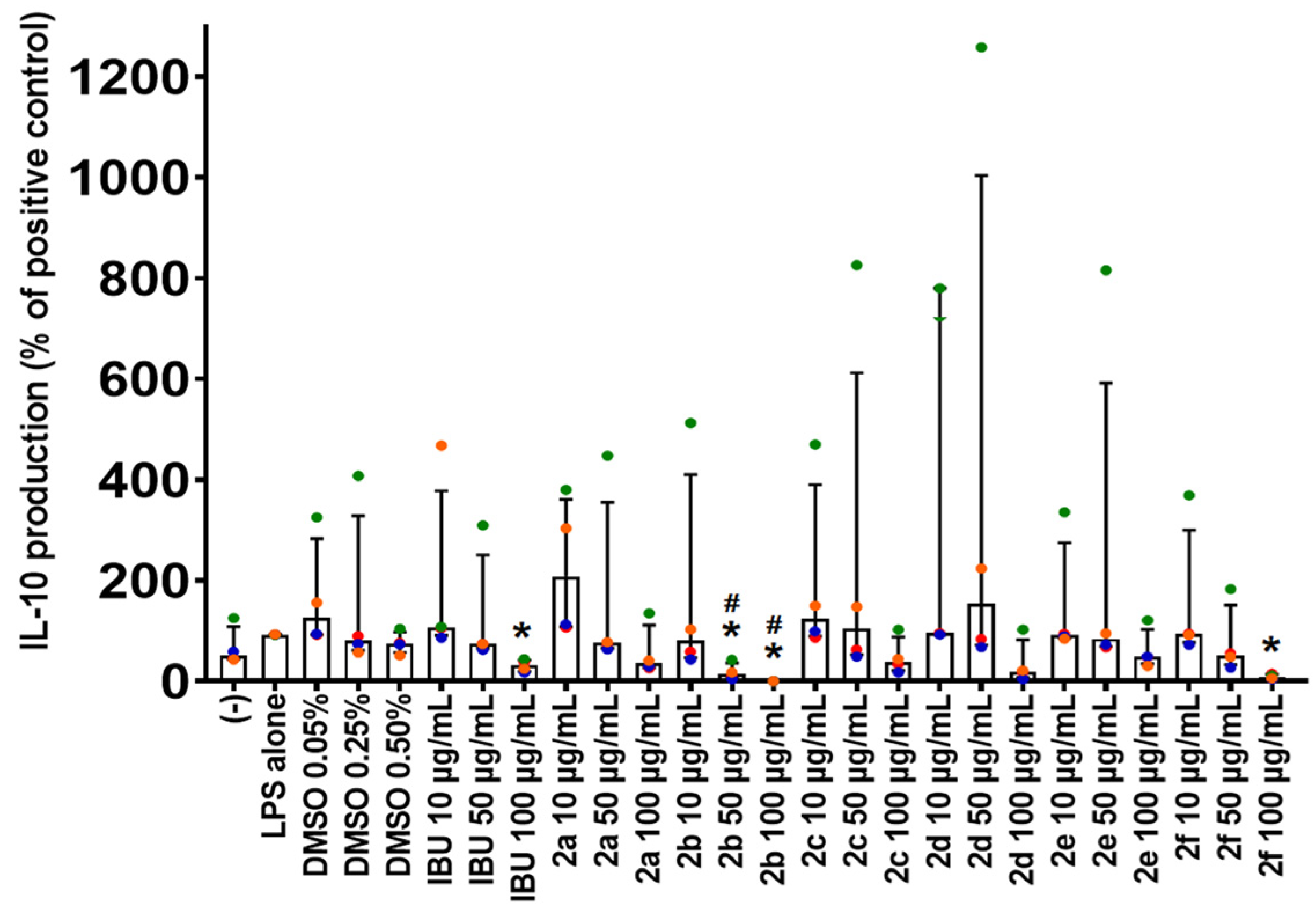
2.6. Effect of Compounds 2a–2fon IL-10 Production
2.7. The Influence of Compounds 2a–2fon IL-1β Production
2.8. Antimicrobial Activity of Compounds 2a–2f
3. Discussion
- (1)
- Increased activity of compound 2b against Y. enterocolitica;
- (2)
- Reduced bacteriostatic effect of compound 2a against S. aureus;
- (3)
- Reduced bacteriostatic effect of compound 2e against M. smegmatis.
4. Materials and Methods
4.1. General
4.2. General Method for the Synthesis of Compounds 2a–2f
4.3. Peripheral Blood Mononuclear Cell Cultures
4.4. Cell Toxicity Analysis Protocol
4.5. Lymphocyte Proliferation Assay Protocol
4.6. Cytokine Assay Protocol
4.7. Antimicrobial Activity Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| DAMP | danger/damage-associated molecular pattern |
| DMSO | dimethyl sulfoxide |
| IBU | ibuprofen |
| IL | interleukin |
| LPS | lipopolysaccharide |
| MIC | minimal inhibitory concentration |
| NET | neutrophil extracellular traps |
| PAMP | pathogen-associated molecular pattern |
| PBMCs | peripheral blood mononuclear cells |
| PHA | phytohemagglutinin |
| TNF-α | tumor necrosis factor-alpha |
References
- Actor, J.K. Chapter 2—The inflammatory response. In Introductory Immunology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 21–38. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Pittman, K.; Kubes, P. Damage-associated molecular patterns control neutrophil recruitment. J. Innate Immun. 2013, 5, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Zeliger, H.I. Chapter 9—Inflammation. In Oxidative Stress; Academic Press: Cambridge, MA, USA, 2023; pp. 101–109. [Google Scholar] [CrossRef]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Muzammil, M.A.; Fariha, F.; Patel, T.; Sohail, R.; Kumar, M.; Khan, E.; Khanam, B.; Kumar, S.; Khatri, M.; Varrassi, G.; et al. Advancements in Inflammatory Bowel Disease: A Narrative Review of Diagnostics, Management, Epidemiology, Prevalence, Patient Outcomes, Quality of Life, and Clinical Presentation. Cureus 2023, 15, e41120. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Bernela, M.; Kaushal, P.; Verma, N.; Thakur, R.; Ahuja, M. Anti-Inflammatory Therapeutics: Conventional Concepts and Future with Nanotechnology. Recent. Adv. Inflamm. Allergy Drug Discov. 2023, 17, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, R.; Wiese-Szadkowska, M.; Kosmalski, T.; Frisch, D.; Ratajczak, M.; Modzelewska-Banachiewicz, B.; Studzińska, R. A Review of the Biological Activity of Amidrazone Derivatives. Pharmaceuticals 2022, 15, 1219. [Google Scholar] [CrossRef] [PubMed]
- Tullis, J.S.; VanRens, J.C.; Natchus, M.G.; Clark, M.P.; De, B.; Hsieh, L.C.; Janusz, M.J. The development of new triazole based inhibitors of tumor necrosis factor-α (TNF-α) production. Bioorganic Med. Chem. Lett. 2003, 13, 1665–1668. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.P.S.; Gonçalves, J.M.; Silva, B.F.; Pereira, E.Q.; Coutinho, P.J.G.; Pereira-Wilson, C.; Dias, A.M. Novel Imidazole-based Hydrazonoyl Cyanides and Amidrazones Containing N(N,O)-Heterocycles: Selective Synthesis, Reaction Mechanisms and Preliminary Anticancer Evaluation. Eur. J. Org. Chem. 2024, 27, e202400253. [Google Scholar] [CrossRef]
- Habashneh, A.Y.; El-Abadelah, M.M.; Bardaweel, S.K.; Taha, M.O. Synthesis and Structure-Activity Relationship; Exploration of some Potent Anti-Cancer Phenyl Amidrazone Derivatives. Med. Chem. 2018, 14, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zou, J.; Xu, W.; Hu, D.; Guo, L.-D.; Chen, J.-X.; Chen, G.-D.; So, K.-F.; Yao, X.-S.; Gao, H. Diisoprenyl-cyclohexene/ane-Type Meroterpenoids from Biscogniauxia sp. and Their Anti-inflammatory Activities. J. Org. Chem. 2021, 86, 11177–11188. [Google Scholar] [CrossRef] [PubMed]
- Gurgul, A.; Wu, Z.; Han, K.-Y.; Shetye, G.; Sydara, K.; Souliya, O.; Johnson, J.J.; Che, C.-T. Polyoxygenated cyclohexene derivatives and other constituents of Uvaria rufa stem. Phytochemistry 2023, 216, 113884. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, H.; Wu, D.; Hu, Y.; Li, J.; Yang, Y.; Li, J.; Zhou, H.; Zhang, H.; Xie, C.; et al. Anti-inflammatory polyoxygenated cyclohexene derivatives from Uvaria macclurei. Phytochemistry 2023, 214, 113797. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Cao, W.; Ma, G.; Li, W.; Li, Z.; Liu, Y.; Chen, L.; Chen, Z.; Li, X.; Cui, P.; et al. Cyclohexene oxide CA, a derivative of zeylenone, exhibits anti-cancer activity in glioblastoma by inducing G0/G1 phase arrest through interference with EZH2. Front. Pharmacol. 2024, 14, 1326245. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska-Banachiewicz, B.; Ucherek, M.; Zimecki, M.; Kutkowska, J.; Kaminska, T.; Morak-Młodawska, B.; Paprocka, R.; Szulc, M.; Lewandowski, G.; Marciniak, J.; et al. Reactions of N3-substituted amidrazones with cis-1,2-cyclohexanedicarboxylic anhydride and biological activities of the products. Arch. Pharm. 2012, 345, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Mazur, L.; Sączewski, J.; Jarzembska, K.N.; Szwarc-Karabyka, K.; Paprocka, R.; Modzelewska-Banachiewicz, B. Synthesis, structural characterization and reactivity of new trisubstituted N1-acylamidrazones: Solid state and solution studies. CrystEngComm 2018, 20, 4179–4193. [Google Scholar] [CrossRef]
- Mazur, L.; Modzelewska-Banachiewicz, B.; Paprocka, R.; Zimecki, M.; Wawrzyniak, U.E.; Kutkowska, J.; Ziółkowska, G. Synthesis, crystal structure and biological activities of a novel amidrazone derivative and its copper(II) complex—A potential antitumor drug. J. Inorg. Biochem. 2012, 114, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska, B.; Pyra, E. Synthesis of N3-substituted amidrazones. Ann. UMCS Sec. AA 1995, 50/51, 111. [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]




| Strain | MIC Values (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 2d | 2e | 2f | Amp | Flu | |
| Mycobacterium smegmatis | 64 | 512 | 64 | 512 | 512 | >512 | 16 | - |
| Staphylococcus aureus ATCC 25923 | 256 | 256 | 64 | 512 | 512 | >512 | 0.25 | - |
| Escherichia coli ATCC 25922 | 512 | 256 | 512 | 512 | >512 | 512 | 8 | - |
| Yersinia enterocolitica O3 | 512 | 64 | 512 | 512 | 512 | 128 | 16 | - |
| Klebsiella pneumoniae ATCC 700603 | 512 | 256 | 256 | 512 | 512 | >512 | >256 | - |
| Candida albicans ATCC 90028 | 512 | >512 | 512 | 512 | 512 | 256 | - | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprocka, R.; Kutkowska, J.; Paczkowska, E.; Mwaura, G.M.; Eljaszewicz, A.; Helmin-Basa, A. Synthesis, Evaluation of Biological Activity, and Structure–Activity Relationships of New Amidrazone Derivatives Containing Cyclohex-1-ene-1-Carboxylic Acid. Molecules 2025, 30, 1853. https://doi.org/10.3390/molecules30081853
Paprocka R, Kutkowska J, Paczkowska E, Mwaura GM, Eljaszewicz A, Helmin-Basa A. Synthesis, Evaluation of Biological Activity, and Structure–Activity Relationships of New Amidrazone Derivatives Containing Cyclohex-1-ene-1-Carboxylic Acid. Molecules. 2025; 30(8):1853. https://doi.org/10.3390/molecules30081853
Chicago/Turabian StylePaprocka, Renata, Jolanta Kutkowska, Ewelina Paczkowska, Godwin Munroe Mwaura, Andrzej Eljaszewicz, and Anna Helmin-Basa. 2025. "Synthesis, Evaluation of Biological Activity, and Structure–Activity Relationships of New Amidrazone Derivatives Containing Cyclohex-1-ene-1-Carboxylic Acid" Molecules 30, no. 8: 1853. https://doi.org/10.3390/molecules30081853
APA StylePaprocka, R., Kutkowska, J., Paczkowska, E., Mwaura, G. M., Eljaszewicz, A., & Helmin-Basa, A. (2025). Synthesis, Evaluation of Biological Activity, and Structure–Activity Relationships of New Amidrazone Derivatives Containing Cyclohex-1-ene-1-Carboxylic Acid. Molecules, 30(8), 1853. https://doi.org/10.3390/molecules30081853







