Media Composition Effects on Hairy Root Biomass and Tetrandrine Production in Stephania tetrandra
Abstract
1. Introduction
2. Results and Discussion
2.1. Two-Level Factorial Experimental Design
2.2. Path of Steepest Ascent Analysis for Hairy Root Biomass
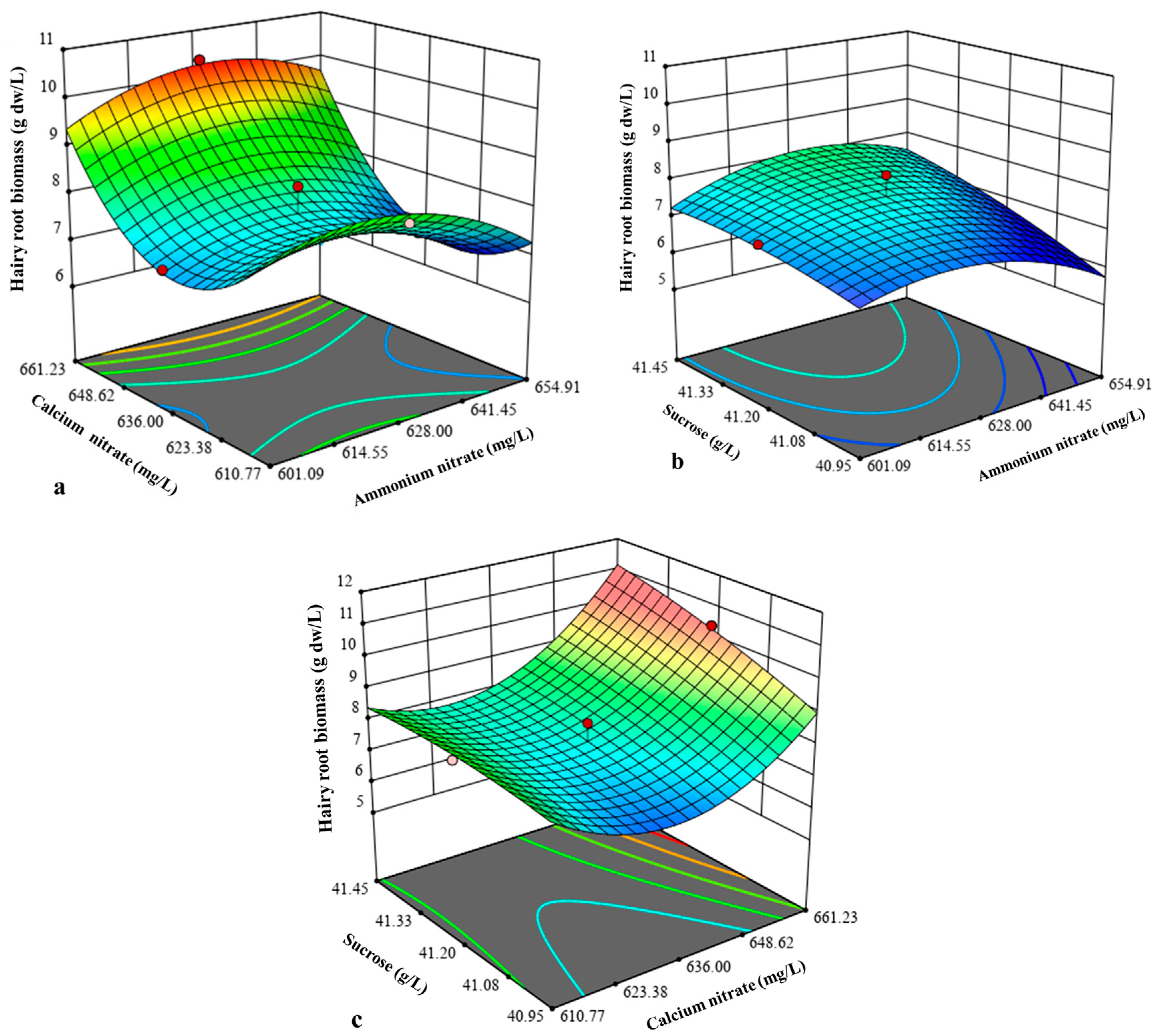
2.3. Central Composite Design Analysis for Hairy Root Biomass
0.1125X1X3 + 0.1750X2X3 − 0.2607X12 + 0.5826X22 − 0.0662X32
2.4. Path of Steepest Ascent Analysis for Tetrandrine Production
2.5. Central Composite Design Analysis for Tetrandrine Production
0.1700X1X3 + 1.42X2X3 − 0.4889X12 − 0.9857X22 − 4.38X32
3. Materials and Methods
3.1. Chemicals and Plant and Bacterial Strain
3.2. Establishment and Selection of Hairy Roots from S. tetrandra S. MOORE
3.3. Two-Level Factorial Experimental Design and Path of Steepest Ascent Design
3.4. Central Composite Experimental Design
3.5. Dry Weight Measurement of Hairy Roots
3.6. Analysis of Tetrandrine
(mg/g) × hairy root dry weight (g dw/L)
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabada-Aguirre, P.; López López, A.M.; Mendoza, K.C.O.; Garay Buenrostro, K.D.; Luna-Vital, D.A.; Mahady, G.B. Mexican traditional medicines for women’s reproductive health. Sci. Rep. 2023, 13, 2807. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Khan, M.; Ahmad, I. Chapter 1-herbal medicine: Current trends and future prospects. In New Look to Phytomedicine; Academic: Cambridge, MA, USA, 2019. [Google Scholar]
- Musa, H.H.; Musa, T.H.; Oderinde, O.; Musa, I.H.; Shonekan, O.O.; Akintunde, T.Y.; Onasanya, A.K. Traditional herbal medicine: Overview of research indexed in the scopus database. Adv. Tradit. Med. 2023, 23, 1173–1183. [Google Scholar] [CrossRef]
- Ullah, R.; Alqahtani, A.S.; Noman, O.M.; Alqahtani, A.M.; Ibenmoussa, S.; Bourhia, M. A review on ethno-medicinal plants used in traditional medicine in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Pham, B.; Le, L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-k.; Li, M.-m.; Wang, S.-y.; Hao, Z.-c.; Meng, X.; Kuang, H.-x.; Yang, B.-y.; Liu, Y. Protective effect of phenylpropionamides in the seed of Cannabis Sativa L. on Parkinson’s disease through autophagy. Fitoterapia 2024, 175, 105883. [Google Scholar] [CrossRef]
- Dai, D.-C.; Xu, X.-F.; Yan, H.; Zhang, Y. Three new indole alkaloid derivatives from Fissistigma oldhamii Levl. and their anti-inflammatory effects. Fitoterapia 2024, 175, 105910. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A compressive review about Taxol®: History and future challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Muñiz-Ramirez, A.; Perez, R.M.; Garcia, E.; Garcia, F.E. Antidiabetic activity of Aloe vera leaves. Evid.-Based Complement. Altern. Med. 2020, 2020, 6371201. [Google Scholar] [CrossRef]
- Tabuti, J.R.; Obakiro, S.B.; Nabatanzi, A.; Anywar, G.; Nambejja, C.; Mutyaba, M.R.; Omara, T.; Waako, P. Medicinal plants used for treatment of malaria by indigenous communities of Tororo District, Eastern Uganda. Trop. Med. Health 2023, 51, 34. [Google Scholar] [CrossRef]
- Rahman, M.M.; Dhar, P.S.; Anika, F.; Ahmed, L.; Islam, M.R.; Sultana, N.A.; Cavalu, S.; Pop, O.; Rauf, A. Exploring the plant-derived bioactive substances as antidiabetic agent: An extensive review. Biomed. Pharmacother. 2022, 152, 113217. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, M.; Liu, H.; Liu, S. A critical review: Traditional uses, phytochemistry, pharmacology and toxicology of Stephania tetrandra S. Moore (Fen Fang Ji). Phytochem. Rev. 2020, 19, 449–489. [Google Scholar] [CrossRef]
- Schütz, R.; Meixner, M.; Antes, I.; Bracher, F. A modular approach to the bisbenzylisoquinoline alkaloids tetrandrine and isotetrandrine. Org. Biomol. Chem. 2020, 18, 3047–3068. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, X.; Zhang, J.; Wang, C.; Xu, Q.; Hu, J.; Kai, G.; Feng, Y. Transcriptome analysis of Stephania tetrandra and characterization of norcoclaurine-6-O-methyltransferase involved in benzylisoquinoline alkaloid biosynthesis. Front. Plant Sci. 2022, 13, 874583. [Google Scholar] [CrossRef]
- He, Q.; Lei, Q.; Huang, S.; Zhou, Y.; Liu, Y.; Zhou, S.; Peng, D.; Deng, X.; Xue, J.; Li, X. Effective extraction of bioactive alkaloids from the roots of Stephania tetrandra by deep eutectic solvents-based ultrasound-assisted extraction. J. Chromatogr. A 2023, 1689, 463746. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, R.; Song, X.; Wei, M.; Xie, T.; Cao, J. Mechanochemical-assisted extraction of active alkaloids from plant with solid acids. ACS Sustain. Chem. Eng. 2018, 7, 197–207. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, D.; Gao, Y.; Liang, C.; Zhang, Y.; Ma, Z.; Liu, Y.; Peng, H.; Zhang, Y.; Qin, H. History of uses, phytochemistry, pharmacological activities, quality control and toxicity of the root of Stephania tetrandra S. Moore: A review. J. Ethnopharmacol. 2020, 260, 112995. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Bajaj, B.K. Ultrasound and surfactant assisted ionic liquid pretreatment of sugarcane bagasse for enhancing saccharification using enzymes from an ionic liquid tolerant Aspergillus assiutensis VS34. Bioresour. Technol. 2019, 285, 121319. [Google Scholar] [CrossRef]
- Fu, Y.-R.; Nargotra, P.; Kuo, C.-H.; Liu, Y.-C. Optimization of Biomass Cultivation from Tuber borchii and Effect of Additives on Triterpenoid Production. Fermentation 2023, 9, 735. [Google Scholar] [CrossRef]
- Nargotra, P.; Vaid, S.; Bajaj, B.K. Cellulase production from Bacillus subtilis SV1 and its application potential for saccharification of ionic liquid pretreated pine needle biomass under one pot consolidated bioprocess. Fermentation 2016, 2, 19. [Google Scholar] [CrossRef]
- Amdoun, R.; Khelifi, L.; Khelifi-Slaoui, M.; Amroune, S.; Asch, M.; Assaf-Ducrocq, C.; Gontier, E. Optimization of the culture medium composition to improve the production of hyoscyamine in elicited Datura stramonium L. hairy roots using the response surface methodology (RSM). Int. J. Mol. Sci. 2010, 11, 4726–4740. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Shum-Chéong-Sing, A.; Caro, Y.; Dufossé, L.; Fouillaud, M. OVAT analysis and response surface methodology based on nutrient sources for optimization of pigment production in the marine-derived fungus Talaromyces albobiverticillius 30548 submerged fermentation. Mar. Drugs 2021, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- Dwibedi, V.; Rath, S.K.; Prakash, R.; Saxena, S. Response surface statistical optimization of fermentation parameters for resveratrol production by the endophytic fungus Arcopilus aureus and its tyrosinase inhibitory activity. Biotechnol. Lett. 2021, 43, 627–644. [Google Scholar] [CrossRef]
- Liu, H.-C.; Chan, H.-S.; Nargotra, P.; Shih, H.-D.; Kuo, C.-H.; Liu, Y.-C. Development of Stephania tetrandra S. MOORE hairy root culture process for tetrandrine production. J. Biotechnol. 2024, 394, 11–23. [Google Scholar] [CrossRef]
- Vieira, R.; Severino, P.; Nalone, L.A.; Souto, S.B.; Silva, A.M.; Lucarini, M.; Durazzo, A.; Santini, A.; Souto, E.B. Sucupira oil-loaded nanostructured lipid carriers (NLC): Lipid screening, factorial design, release profile, and cytotoxicity. Molecules 2020, 25, 685. [Google Scholar] [CrossRef] [PubMed]
- Rigon, R.B.; Gonçalez, M.L.; Severino, P.; Alves, D.A.; Santana, M.H.; Souto, E.B.; Chorilli, M. Solid lipid nanoparticles optimized by 22 factorial design for skin administration: Cytotoxicity in NIH3T3 fibroblasts. Colloids Surf. B Biointerfaces 2018, 171, 501–505. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.d.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 449564. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, S.; Sanjarian, F.; Lohrasebi, T.; Karimi, M. Enhanced inulin production by hairy root cultures of Cichorium intybus in response to Pi and Fe starvation. Mol. Biol. Res. Commun. 2021, 10, 85. [Google Scholar]
- Jiao, J.; Gai, Q.-Y.; Fu, Y.-J.; Ma, W.; Yao, L.-P.; Feng, C.; Xia, X.-X. Optimization of Astragalus membranaceus hairy roots induction and culture conditions for augmentation production of astragalosides. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 120, 1117–1130. [Google Scholar] [CrossRef]
- Chen, J.; Lan, X.; Jia, R.; Hu, L.; Wang, Y. Response surface methodology (RSM) mediated optimization of medium components for mycelial growth and metabolites production of Streptomyces alfalfae XN-04. Microorganisms 2022, 10, 1854. [Google Scholar] [CrossRef]
- Feng, D.; Wang, X.; Gao, J.; Zhang, C.; Liu, H.; Liu, P.; Sun, X. Exogenous calcium: Its mechanisms and research advances involved in plant stress tolerance. Front. Plant Sci. 2023, 14, 1143963. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Cao, A.; Wang, F.; Chen, X.; Xie, S.; Shen, H.; Jin, X.; Li, H. Calcium-dependent protein kinase genes in Glycyrrhiza Uralensis appear to be involved in promoting the biosynthesis of glycyrrhizic acid and flavonoids under salt stress. Molecules 2019, 24, 1837. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium signaling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- Carr, N.F.; Boaretto, R.M.; Mattos, D., Jr. Coffee seedlings growth under varied NO3−:NH4+ ratio: Consequences for nitrogen metabolism, amino acids profile, and regulation of plasma membrane H+-ATPase. Plant Physiol. Biochem. 2020, 154, 11–20. [Google Scholar] [CrossRef]
- Bahmani, H.; Maroufi, A.; Majdi, M.; Fakheri, B.A. Thymol production in hairy root culture of Sahendian savory (Satureja sahendica Bornm). Plant Biotechnol. Rep. 2021, 15, 177–186. [Google Scholar] [CrossRef]
- Weng, X.; Li, H.; Ren, C.; Zhou, Y.; Zhu, W.; Zhang, S.; Liu, L. Calcium regulates growth and nutrient absorption in poplar seedlings. Front. Plant Sci. 2022, 13, 887098. [Google Scholar] [CrossRef] [PubMed]
- Chashmi, N.A.; Sharifi, M.; Karimi, F.; Rahnama, H. Enhanced production of tropane alkaloids by nitrate treatment in hairy root cultures of Atropa belladonna. J. Biotechnol. 2008, 136, S55. [Google Scholar] [CrossRef]
- Hoseinpanahi, B.; Bahramnejad, B.; Majdi, M.; Dastan, D.; Ashengroph, M. The effect of different elicitors on hairy root biomass and resveratrol production in wild Vitis vinifera. J. Appl. Biotechnol. Rep. 2020, 7, 25–31. [Google Scholar]
- Fazili, M.A.; Bashir, I.; Ahmad, M.; Yaqoob, U.; Geelani, S.N. In vitro strategies for the enhancement of secondary metabolite production in plants: A review. Bull. Natl. Res. Cent. 2022, 46, 35. [Google Scholar]
- Muthusamy, M.; Lee, S.I. Abiotic stress-induced secondary metabolite production in Brassica: Opportunities and challenges. Front. Plant Sci. 2024, 14, 1323085. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.-Y.; Jiao, J.; Luo, M.; Wang, W.; Gu, C.-B.; Fu, Y.-J.; Ma, W. Tremendous enhancements of isoflavonoid biosynthesis, associated gene expression and antioxidant capacity in Astragalus membranaceus hairy root cultures elicited by methyl jasmonate. Process Biochem. 2016, 51, 642–649. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Wang, X.; Zang, Y.-P.; Niu, L.-L.; Fu, Y.-J. Elicitation of Isatis tinctoria L. hairy root cultures by salicylic acid and methyl jasmonate for the enhanced production of pharmacologically active alkaloids and flavonoids. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 137, 77–86. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Sharma, S.; Bajaj, B.K. Efficacy and functional mechanisms of a novel combinatorial pretreatment approach based on deep eutectic solvent and ultrasonic waves for bioconversion of sugarcane bagasse. Renew. Energy 2021, 163, 1910–1922. [Google Scholar] [CrossRef]

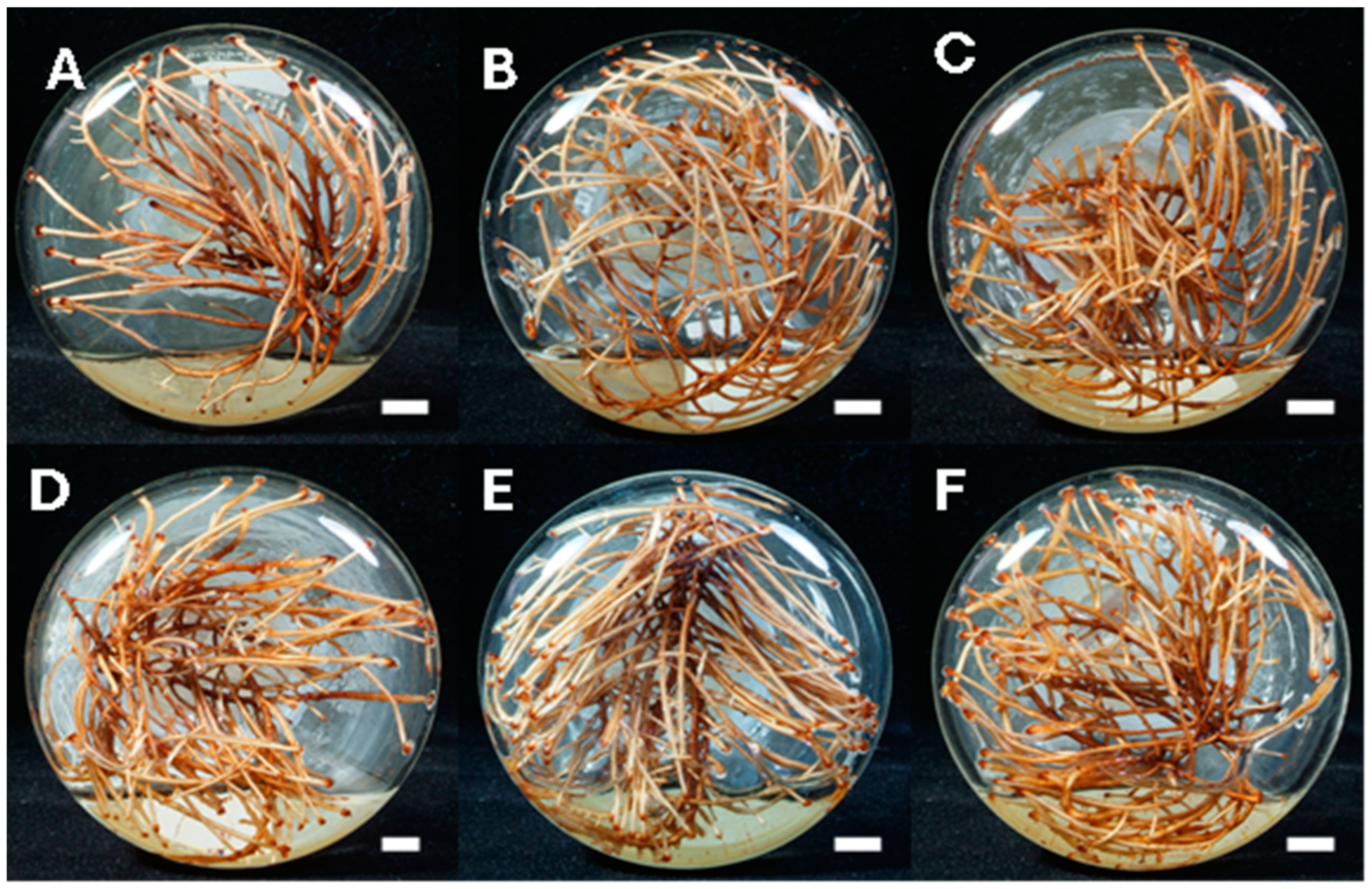


| Experiment | NH4NO3 (mg/L) | Ca(NO3)2 (mg/L) | Sucrose (g/L) | Biomass (g dw/L) | Tetrandrine Production (mg/L) |
|---|---|---|---|---|---|
| 1 | 400 | 556 | 30 | 7.31 ± 0.09 | 42.55 ± 1.06 |
| 2 | 600 | 556 | 30 | 7.96 ± 0.04 | 46.38 ± 2.13 |
| 3 | 400 | 956 | 30 | 7.35± 0.32 | 48.45 ± 0.02 |
| 4 | 600 | 956 | 30 | 7.50 ± 0.10 | 58.00 ± 4.02 |
| 5 | 400 | 556 | 50 | 7.35 ± 0.22 | 20.26 ± 1.26 |
| 6 | 600 | 556 | 50 | 8.22 ± 0.53 | 24.58 ± 1.90 |
| 7 | 400 | 956 | 50 | 6.95 ± 0.46 | 26.09 ± 2.60 |
| 8 | 600 | 956 | 50 | 7.86 ± 0.49 | 33.08 ± 0.09 |
| 9 | 500 | 756 | 40 | 8.92 ± 0.51 | 38.29 ± 3.32 |
| 10 | 500 | 756 | 40 | 8.72 ± 0.27 | 37.37 ± 2.22 |
| NH4NO3 (X1, mg/L) | Ca(NO3)2 (X2, mg/L) | Sucrose (X3, g/L) | Biomass (g dw/L) | |
|---|---|---|---|---|
| (1) Base point | 500 | 756 | 40 | |
| (2) Unit | 100 | 200 | 10 | |
| (3) Slop | 0.32 | −0.15 | 0.03 | |
| (4) New unit = (2) × (3) | 32 | −30 | 0.3 | |
| Expt. 1 | 500 | 756 | 40 | 6.89 ± 0.64 |
| Expt. 2 | 532 | 726 | 40.3 | 7.64 ± 0.46 |
| Expt. 3 | 564 | 696 | 40.6 | 7.59 ± 0.62 |
| Expt. 4 | 596 | 666 | 40.9 | 8.27 ± 0.54 |
| Expt. 5 | 628 | 636 | 41.2 | 9.33 ± 0.27 |
| Expt. 6 | 660 | 606 | 41.5 | 9.05 ± 0.85 |
| Experiment | NH4NO3 (mg/L) | Ca(NO3)2 (mg/L) | Sucrose (g/L) | Biomass (g dw/L) | |
|---|---|---|---|---|---|
| Experimental Value | Predicted Value | ||||
| 1 | 628.00 | 636.00 | 40.95 | 7.00 ± 0.02 | 6.95 |
| 2 | 644.00 | 621.00 | 41.35 | 7.62 ± 0.01 | 7.54 |
| 3 | 628.00 | 610.77 | 41.20 | 8.32 ± 0.01 | 8.50 |
| 4 | 644.00 | 651.00 | 41.35 | 9.30 ± 0.02 | 9.16 |
| 5 | 628.00 | 636.00 | 41.20 | 8.30 ± 0.02 | 7.73 |
| 6 | 601.09 | 636.00 | 41.20 | 7.23 ± 0.02 | 7.16 |
| 7 | 612.00 | 651.00 | 41.05 | 8.05 ± 0.01 | 8.08 |
| 8 | 654.91 | 636.00 | 41.20 | 6.67 ± 0.02 | 6.83 |
| 9 | 628.00 | 636.00 | 41.20 | 7.17 ± 0.01 | 7.73 |
| 10 | 628.00 | 661.23 | 41.20 | 10.35 ± 0.03 | 10.25 |
| 11 | 612.00 | 621.00 | 41.35 | 7.90 ± 0.02 | 7.74 |
| 12 | 612.00 | 651.00 | 41.35 | 8.77 ± 0.005 | 8.90 |
| 13 | 644.00 | 621.00 | 41.05 | 7.15 ± 0.02 | 6.96 |
| 14 | 644.00 | 651.00 | 41.05 | 7.78 ± 0.01 | 7.89 |
| 15 | 628.00 | 636.00 | 41.45 | 8.00 ± 0.01 | 8.13 |
| 16 | 612.00 | 621.00 | 41.05 | 7.53 ± 0.02 | 7.61 |
| NH4NO3 (X1, mg/L) | Ca(NO3)2 (X2, mg/L) | Sucrose (X3, g/L) | Tetrandrine Production (mg/L) | |
|---|---|---|---|---|
| (1) Base point | 500 | 756 | 40 | |
| (2) Unit | 100 | 200 | 10 | |
| (3) Slope | 3.09 | 3.98 | −11.42 | |
| (4) Slope/20 | 0.1545 | 0.199 | −0.571 | |
| (5) New Unit = (2) × (4) | 15.45 | 39.8 | −5.71 | |
| Expt. 1 | 500 | 756 | 40 | 44.52 ± 1.49 |
| Expt. 2 | 515.45 | 795.8 | 34.29 | 59.68 ± 3.02 |
| Expt. 3 | 530.9 | 835.6 | 28.58 | 61.98 ± 2.23 |
| Expt. 4 | 546.35 | 875.4 | 22.87 | 65.22 ± 1.42 |
| Expt. 5 | 561.8 | 915.2 | 17.16 | 50.12 ± 1.36 |
| Expt. 6 | 577.25 | 955 | 11.45 | 28.43 ± 2.90 |
| Expt. 7 | 592.7 | 994.8 | 5.74 | 9.75 ± 0.96 |
| Experiment | NH4NO3 (mg/L) | Ca(NO3)2 (mg/L) | Sucrose (g/L) | Tetrandrine Production (mg/L) | |
|---|---|---|---|---|---|
| Experimental Value | Predicted Value | ||||
| 1 | 546.35 | 841.93 | 22.87 | 67.79 ± 3.00 | 66.58 |
| 2 | 538.63 | 895.30 | 17.16 | 50.68 ± 1.77 | 51.02 |
| 3 | 554.07 | 855.50 | 28.58 | 70.02 ± 4.24 | 70.43 |
| 4 | 546.35 | 908.87 | 22.87 | 60.92 ± 2.70 | 61.07 |
| 5 | 554.07 | 895.30 | 17.16 | 53.87 ± 2.22 | 52.38 |
| 6 | 546.35 | 875.40 | 22.87 | 66.29 ± 1.73 | 66.62 |
| 7 | 546.35 | 875.40 | 32.47 | 66.44 ± 2.23 | 64.34 |
| 8 | 538.63 | 895.30 | 28.58 | 64.77 ± 2.15 | 66.21 |
| 9 | 546.35 | 875.40 | 13.27 | 43.08 ± 4.00 | 44.13 |
| 10 | 546.35 | 875.40 | 22.87 | 66.76 ± 5.18 | 66.62 |
| 11 | 559.34 | 875.40 | 22.87 | 67.32 ± 1.38 | 68.70 |
| 12 | 554.07 | 855.50 | 17.16 | 62.28 ± 4.63 | 61.59 |
| 13 | 533.36 | 875.40 | 22.87 | 64.20 ± 1.20 | 61.77 |
| 14 | 538.63 | 855.50 | 28.58 | 61.32 ± 1.67 | 63.55 |
| 15 | 538.63 | 855.50 | 17.16 | 53.34 ± 1.26 | 54.03 |
| 16 | 554.07 | 895.30 | 28.58 | 66.84 ± 5.19 | 66.90 |
| Independent Variable | Coded Level | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| NH4NO3 (mg/L) | 400 | 500 | 600 |
| Ca(NO3)2 (mg/L) | 556 | 756 | 956 |
| Sucrose (g/L) | 30 | 40 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-H.; Liu, H.-C.; Nargotra, P.; Chan, H.-S.; Shih, H.-D.; Liu, Y.-C. Media Composition Effects on Hairy Root Biomass and Tetrandrine Production in Stephania tetrandra. Molecules 2025, 30, 1859. https://doi.org/10.3390/molecules30081859
Kuo C-H, Liu H-C, Nargotra P, Chan H-S, Shih H-D, Liu Y-C. Media Composition Effects on Hairy Root Biomass and Tetrandrine Production in Stephania tetrandra. Molecules. 2025; 30(8):1859. https://doi.org/10.3390/molecules30081859
Chicago/Turabian StyleKuo, Chia-Hung, Hsuan-Chieh Liu, Parushi Nargotra, Hsiao-Sung Chan, Hsin-Der Shih, and Yung-Chuan Liu. 2025. "Media Composition Effects on Hairy Root Biomass and Tetrandrine Production in Stephania tetrandra" Molecules 30, no. 8: 1859. https://doi.org/10.3390/molecules30081859
APA StyleKuo, C.-H., Liu, H.-C., Nargotra, P., Chan, H.-S., Shih, H.-D., & Liu, Y.-C. (2025). Media Composition Effects on Hairy Root Biomass and Tetrandrine Production in Stephania tetrandra. Molecules, 30(8), 1859. https://doi.org/10.3390/molecules30081859











