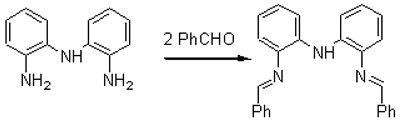
Imine and amine ligands have been widely used in the field of transition metal catalyzed olefin polymerization [1]. Here we report the synthesis of a tri-dendated imine-amine compound which we plan to use as a ligand. bis(2-Aminophenyl)amine (0.5g, 2.5mmol) [2] and benzaldehyde (0.53ml, 5.2mmol) are added into 10ml of CH2Cl2 and refluxed 4h under N2. The mixture is concentrated in vacuo, washed with cold hexane and dried in vacuo to give a red crystal product, 0.8g (yield 90%).
M. p. 195°C.
1H NMR (400 MHz, CDCl3): 8.23 (s, 1H, NH), 8.55 (s, 2H, N=CH), 6.8-8.0 (m, 18H, Ph).
13C NMR (75 MHz, CDCl3): Ar, 114.4, 119.8, 127.3, 128.6, 128.8, 131.1, 136.3, 137.7, 139.3; C=N, 157.9.
IR (KBr): 3350, 3051, 2873, 1622, 1582.
Anal. Calc. for C26H21N3 (375.50): C 83.16, H 5.65, N 11.19; Found: C 83.04, H 5.67, N 11.29.
References
- Britovsek, G. J. P.; Gibson, V. C.; Wass, D. F. Angew. Chem. Int. Ed. 1999, 38, 428–447. [CrossRef]
- Black, D. S.; Rothnie, C. N. E. Aust. J. Chem. 1983, 36, 1141–1147.
Sample Availability: available from the authors and from MDPI. |
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.
