Peptides, Antibodies, Peptide Antibodies and More
Abstract
:1. Introduction
2. Peptides
2.1. Peptide Discovery
2.2. Peptide Synthesis
2.3. Properties
2.4. Applications
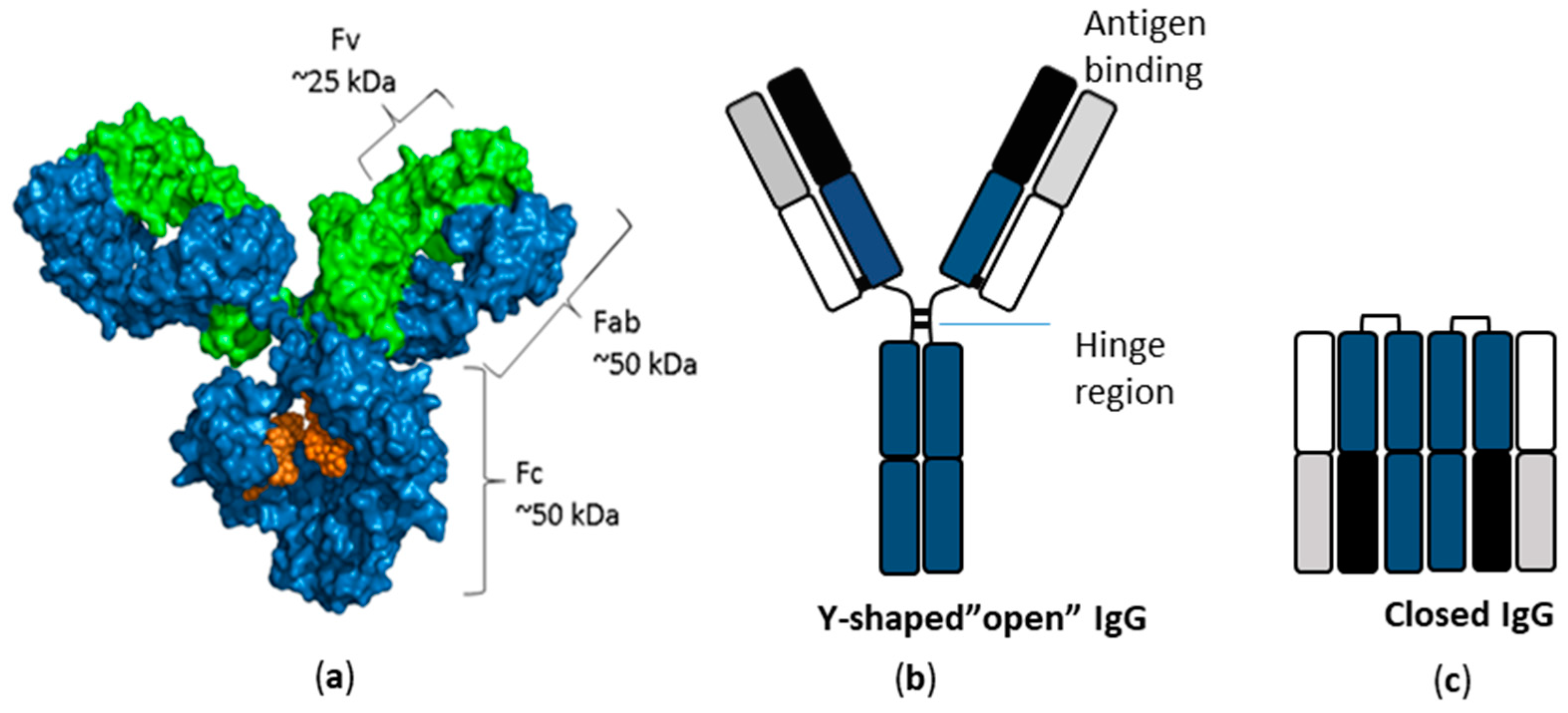
3. Antibodies
3.1. Discovery
3.2. Polyclonal Antibodies
3.3. Monoclonal Antibodies
3.4. Recombinant Antibodies
3.5. Engineered and Designed Antibodies
4. Peptide Antibodies
4.1. Discovery and Properties
4.2. Production
4.3. Applications
5. Alternative Recognition Molecules for Antigen Targeting
5.1. Peptide Libraries
5.2. Dendrimers
5.3. Aptamers
5.4. Other Recognition Molecules
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked immunosorbent assay |
| Fmoc | 9-fluorenylmethyloxycarbonyl |
| ICC | Immunocytochemistry |
| IHC | Immunohistochemistry |
| IP | Immunoprecipitation |
| FC | Flow cytometry |
| HFMV | Hand foot mouth virus |
| scFv | single chain fragment variable |
| SPPS | Solid-phase peptide synthesis |
| PTM | Posttranslational modification |
| PAb | Polyclonal antibody |
| Mab | Monoclonal antibody |
References
- Murphy, K.; Weaver, C. Janeways’s Immunobiology, 9th ed.; Garland Science: New York, NY, USA, 2016. [Google Scholar]
- Owen, J.A.; Punt, J.; Stranford, S.A. Kuby Immunology; W.H. Freeman & Co Ltd.: New York, NY, USA, 2018; Volume 8. [Google Scholar]
- Fischer, A. Ueber enige derivate des glykocolls. Ber. Dtsch. Chem. Ges. 1901, 34, 2868–2877. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, M.; Zervas, L. Uber ein allgemeines verfahren der peptide-synthese. Ber. Dtsch. Chem. Ges. 1932, 65, 1192–1201. [Google Scholar] [CrossRef]
- McKay, F.C.; Albertson, N.F. Mew amine-masking groups for peptide synthesis. J. Am. Chem. Soc. 1957, 79, 4686–4690. [Google Scholar] [CrossRef]
- Sheehan, J.C.; Hess, G.P. A method of forming peptide bonds. J. Am. Chem. Soc. 1955, 77, 1067–1068. [Google Scholar] [CrossRef]
- Vigneaud, V.D.; Ressler, C.; Swan, C.J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Edman, P. Method for determination of the amino acid sequence in peptides. Acta Chem. Scand. 1950, 4, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Edman, P.; Begg, G. A protein sequenator. Eur. J. Biochem. 1967, 1, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.; Stein, W.H. Chemical structures of pancreatic ribonuclease and deoxyribonuclease. Science 1973, 180, 458–464. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Boysen, R.I. High-Performance Liquid Chromatography of Peptides and Proteins; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Kafka, A.P.; Kleffmann, T.; Rades, T.; McDowell, A. The application of maldi tof ms in biopharmaceutical research. Int. J. Pharm. 2011, 417, 70–82. [Google Scholar] [CrossRef]
- Letzel, T. Protein and Peptide Analysis by Lc-ms: Experimentas Strategies (Rsc Chromatography Monographs); Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Hansen, P.R.; Oddo, A. Fmoc solid-phase peptide synthesis. Methods Mol. Biol. 2015, 1348, 33–50. [Google Scholar] [PubMed]
- Jad, Y.E.; El-Faham, A.; Beatriz, G.; Albericio, F. Solid-phase peptide synthesis, the state of art: Challenges and opportunities. In Peptide-Based Drug Discovery: Challenges and New Therapeutics; The Royal Society of Chemistry: London, UK, 2017; pp. 518–550. [Google Scholar]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B. Synthesis of proteins by native chemical ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Agouridas, V.; El Mahdi, O.; Diemer, V.; Cargoet, M.; Monbaliu, J.M.; Melnyk, O. Native chemical ligation and extended methods: Mechanisms, catalysis, scope, and limitations. Chem. Rev. 2019, 119, 7328–7443. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.R.; Denise, D.B.; Gregory, G.N. Peptide conformation: Stability and dynamics. In Peptide: Synthesis, Structures and Applications; Gutte, B., Ed.; Associated Press: New York, NY, USA, 1995. [Google Scholar]
- Watkins, H.A.; Rathbone, D.L.; Barwell, J.; Hay, D.L.; Poyner, D.R. Structure-activity relationships for alpha-calcitonin gene-related peptide. Br. J. Pharmacol. 2013, 170, 1308–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamysz, E.; Mickiewicz, B.; Kamysz, W.; Bielińska, S.; Rodziewicz-Motowidło, S.; Ciarkowski, J. Synthesis, biological activity and solution structure of new analogues of the antimicrobial Gramicidin S. Int. J. Pep. Sci. 2011, 17, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Lamping, M.; Gold, M.; Rottger, Y.; Brodje, D.; Dodel, R.; Frantz, R.; Mraheil, M.A.; Chakraborty, T.; Geyer, A. Synthesis of a biological active beta-hairpin peptide by addition of two structural motifs. Bioorg. Med. Chem. 2017, 25, 603–608. [Google Scholar] [CrossRef]
- Steinmetz, W.E.; Carrel, T.N.; Peprah, R.B. The conformation and assignment of the proton nmr spectrum in water of dx600, a bioactive peptide with a random coil conformation. Int. J. Spectrosc. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.L.; Grant, G.A. Synthetic Peptides. A User’s Guide, 2nd ed.; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Kaiser, E.T. Design principles in the construction of biologically active peptides. TIBS 1987, 12, 305–309. [Google Scholar] [CrossRef]
- Ranjbar, B.; Gill, P. Circular dichroism techniques: Biomolecular and nanostructural analyses—A review. Chem. Biol. Drug Des. 2009, 74, 101–120. [Google Scholar] [CrossRef]
- Bernini, A.; Termissi, P. Nuclear magnetic resonance of amino acids, peptides, and proteins. In Amino Acids, Peptides and Proteins in Organic Chemistry; Hughes, A.B., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2010; pp. 97–153. [Google Scholar]
- Gao, X.; De Maziere, A.; Iaea, D.B.; Arthur, C.P.; Klumperman, J.; Ciferri, C.; Hannoush, R.N. Visualizing the cellular route of entry of a cystine-knot peptide with xfect transfection reagent by electron microscopy. Sci. Rep. 2019, 9, 6907. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wierzbicki, M.; Du Bois, D.R.; Nowick, J.S. X-ray crystallographic structure of a teixobactin derivative reveals amyloid-like assembly. J. Am. Chem. Soc. 2018, 140, 14028–14032. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S.M.; Maecke, H.R. Chapter eight—Radiometallo-labeled peptides in tumor diagnosis and targeted radionuclide therapy. In Advances in Inorganic Chemistry; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 68, pp. 341–396. [Google Scholar]
- Trier, N.H.; Holm, B.E.; Heiden, J.; Slot, O.; Locht, H.; Lindegaard, H.; Svendsen, A.; Nielsen, C.T.; Jacobsen, S.; Theander, E.; et al. Antibodies to a strain-specific citrullinated epstein-barr virus peptide diagnoses rheumatoid arthritis. Sci. Rep. 2018, 8, 3684. [Google Scholar] [CrossRef] [PubMed]
- Opuni, K.F.M.; Al-Majdoub, M.; Yefremova, Y.; El-Kased, R.F.; Koy, C.; Glocker, M.O. Mass spectrometric epitope mapping. Mass Spectrom. Rev. 2018, 37, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Hansen, P.R.; Vedeler, C.A.; Somnier, F.E.; Houen, G. Identification of continuous epitopes of hud antibodies related to paraneoplastic diseases/small cell lung cancer. J. Neuroimmunol. 2012, 243, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Welner, S.; Trier, N.H.; Houen, G.; Hansen, P.R. Identification and mapping of a linear epitope of centromere protein f using monoclonal antibodies. J. Pept. Sci. 2013, 19, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Hansen, P.R.; Houen, G. Production and characterization of peptide antibodies. Methods 2012, 56, 136–144. [Google Scholar] [CrossRef]
- Hos, B.J.; Tondini, E.; van Kasteren, S.I.; Ossendorp, F. Approaches to improve chemically defined synthetic peptide vaccines. Front. Immunol. 2018, 9, 884. [Google Scholar] [CrossRef] [Green Version]
- Albericio, F. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef] [Green Version]
- Mathew, N.R.; Baumgartner, F.; Braun, L.; O’Sullivan, D.; Thomas, S.; Waterhouse, M.; Muller, T.A.; Hanke, K.; Taromi, S.; Apostolova, P.; et al. Erratum: Sorafenib promotes graft-versus-leukemia activity in mice and humans through il-15 production in flt3-itd-mutant leukemia cells. Nat. Med. 2018, 24, 526. [Google Scholar] [CrossRef] [Green Version]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The current state of peptide drug discovery: Back to the future? J. Med. Chem. 2017, 61, 1382–1414. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Fassi, E.M.A.; Romanato, A.; D’Annessa, I.; Odinolfi, M.T.; Brambilla, D.; Damin, F.; Chiari, M.; Gori, A.; Colombo, G.; et al. Computational analysis of dengue virus envelope protein (e) reveals an epitope with flavivirus immunodiagnostic potential in peptide microarrays. Int. J. Mol. Sci. 2019, 20, 1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, A.T.; Devkota, S.; Malvankar, S.; Bhattarai, S.; Meneely, K.M.; Williams, T.D.; Wolfe, M.S. Designed helical peptides as functional probes for gamma-secretase. Biochemistry 2019, 58, 4398–4407. [Google Scholar] [CrossRef] [PubMed]
- Maseko, S.; Padayachee, E.; Maphumulo, S.; Govender, T.; Sayed, Y.; Maguire, G.; Lin, J.; Naicker, T.; Baijnath, S.; Gerhardus, K.H. Kinetic and thermodynamic characterisation of hiv-protease inhibitors against e35d upward arrowg upward arrows mutant in the south african hiv-1 subtype c protease. J. Enzym. Inhib. Med. Chem. 2019, 34, 1451–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramil, E.; Herbi Bastian, L.; Denefle, T.; Nemati, F.; Xiao, M.; Larde, E.; Maloum, K.; Roos-Weil, D.; Chapiro, E.; Le Garff-Tavernier, M.; et al. Targeting chronic lymphocytic leukemia with n-methylated thrombospondin-1-derived peptides overcomes drug resistance. Blood Adv. 2019, 3, 2920–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routhier, C.A.; Mochel, M.C.; Lynch, K.; Dias-Santagata, D.; Louis, D.N.; Hoang, M.P. Comparison of 2 monoclonal antibodies for immunohistochemical detection of braf v600e mutation in malignant melanoma, pulmonary carcinoma, gastrointestinal carcinoma, thyroid carcinoma, and gliomas. Hum. Pathol. 2013, 44, 2563–2570. [Google Scholar] [CrossRef]
- Zarei, O.; Irajian, G.R.; Zarnani, A.H.; Chamani-Tabriz, L.; Emami, S.; Jeddi-Tehrani, M.; Rabbani, H. Peptide-based polyclonal antibody production against p110 protein of mycoplasma genitalium. Avicenna J. Med. Biotechnol. 2011, 3, 79–85. [Google Scholar]
- Amrutkar, S.D.; Trier, N.H.; Hansen, P.R.; Houen, G. Fine mapping of a monoclonal antibody to the n-methyl d-aspartate receptor reveals a short linear epitope. Biopolymers 2012, 98, 567–575. [Google Scholar] [CrossRef]
- Valdarnini, N.; Holm, B.; Hansen, P.; Rovero, P.; Houen, G.; Trier, N. Fine mapping of glutamate decarboxylase 65 epitopes reveals dependency on hydrophobic amino acids for specific interactions. Int. J. Mol. Sci. 2019, 20, 2909. [Google Scholar] [CrossRef] [Green Version]
- Trier, N.H.; Holm, B.E.; Heiden, J.; Slot, O.; Locht, H.; Jensen, B.; Lindegaard, H.; Svendsen, A.; Nielsen, C.T.; Jacobsen, S.; et al. The use of synthetic peptides for detection of anti-citrullinated protein antibodies in rheumatoid arthritis. J. Immunol. Methods 2018, 454, 6–14. [Google Scholar] [CrossRef]
- Trier, N.H.; Holm, B.E.; Slot, O.; Locht, H.; Lindegaard, H.; Svendsen, A.; Nielsen, C.T.; Jacobsen, S.; Theander, E.; Houen, G. Application of synthetic peptides for detection of anti-citrullinated peptide antibodies. Peptides 2016, 76, 87–95. [Google Scholar] [CrossRef]
- Prince, H.E. Evaluation of the inova diagnostics enzyme-linked immunosorbent assay kits for measuring serum immunoglobulin g (igg) and iga to deamidated gliadin peptides. Clin. Vaccine Immunol. 2006, 13, 150–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.C.; Chen, M.J.; Ren, Z.Q.; Hou, J.Y.; Lin, G.F.; Wu, Y.S. Development of an improved time-resolved fluoroimmunoassay for simultaneous quantification of c-peptide and insulin in human serum. Clin. Biochem. 2014, 47, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.; Misicka, A.; Olma, A.; Bankowski, K.; Stoev, S.; Chini, B.; Durroux, T.; Mouillac, B.; Corbani, M.; Guillon, G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J. Neuroendocrinol. 2012, 24, 609–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, A.C.; Meethal, S.V.; Bowen, R.L.; Atwood, C.S. Leuprolide acetate: A drug of diverse clinical applications. Expert Opin. Investig. Drugs 2007, 16, 1851–1863. [Google Scholar] [CrossRef]
- Davoodi, S.; Bolhassani, A.; Sadat, S.M.; Irani, S. Design and in vitro delivery of hiv-1 multi-epitope DNA and peptide constructs using novel cell-penetrating peptides. Biotechnol. Lett. 2019, 41, 1283–1298. [Google Scholar] [CrossRef]
- Hey, A. History and practice: Antibodies in infectious diseases. Microbiol. Spectr. 2015, 3, AID-0026-2014. [Google Scholar] [CrossRef] [Green Version]
- Fahey, J.L. Antibodies and immunoglobulins. I. Structure and function. JAMA 1965, 194, 71–74. [Google Scholar] [CrossRef]
- Alzari, P.M.; Lascombe, M.B.; Poljak, R.J. Three-dimensional structure of antibodies. Annu. Rev. Immunol. 1988, 6, 555–580. [Google Scholar] [CrossRef]
- Harris, L.J.; Larson, S.B.; Hasel, K.W.; Day, J.; Greenwood, A.; McPherson, A. The three-dimensional structure of an intact monoclonal antibody for canine lymphoma. Nature 1992, 360, 369–372. [Google Scholar] [CrossRef]
- Saphire, E.O.; Stanfield, R.L.; Crispin, M.D.; Morris, G.; Zwick, M.B.; Pantophlet, R.A.; Parren, P.W.; Rudd, P.M.; Dwek, R.A.; Burton, D.R.; et al. Crystal structure of an intact human igg: Antibody asymmetry, flexibility, and a guide for hiv-1 vaccine design. Adv. Exp. Med. Biol. 2003, 535, 55–66. [Google Scholar]
- Blech, M.; Horer, S.; Kuhn, A.B.; Kube, S.; Goddeke, H.; Kiefer, H.; Zang, Y.; Alber, Y.; Kast, S.M.; Westermann, M.; et al. Structure of a therapeutic full-length anti-npra igg4 antibody: Dissecting conformational diversity. Biophys. J. 2019, 116, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.J.; Skaletsky, E.; McPherson, A. Crystallographic structure of an intact igg1 monoclonal antibody. J. Mol. Biol. 1998, 275, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maibom-Thomsen, S.L.; Trier, N.H.; Holm, B.E.; Hansen, K.B.; Rasmussen, M.I.; Chailyan, A.; Marcatili, P.; Hojrup, P.; Houen, G. Immunoglobulin g structure and rheumatoid factor epitopes. PLoS ONE 2019, 14, e0217624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, L.A.; Majidi, J.; Baradaran, B.; Abdolalizadeh, J.; Akbari, A.M. Production and characterization of murine monoclonal antibody against synthetic peptide of cd34. Hum. Antibodies 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Maleki, L.A.; Shanehbandi, D.; Majidi, J.; Yusefi, M.; Abdolalizadeh, J.; Orangi, M.; Baradaran, B. Production of anti-cd14 monoclonal antibody using synthetic peptide of human cd14 as immunizing antigen. Hum. Antibodies 2013, 22, 67–71. [Google Scholar] [CrossRef]
- Sepehr, K.S.; Baradaran, B.; Majidi, J.; Abdolalizadeh, J.; Aghebati, L.; Shahneh, F.Z. Development and characterization of monoclonal antibodies against human cd20 in balb/c mice. Hum. Antibodies 2012, 21, 57–64. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, C.; Deng, Y.; Wang, M.; Luo, P.; Wu, C.; Yue, J.; Fang, N.; Wang, M.; Wei, S. Immunohistochemistry as a quick screening method for clinical detection of braf(v600e) mutation in melanoma patients. Tumour Biol. 2014, 35, 5727–5733. [Google Scholar] [CrossRef]
- Cooper, W.A.; Yu, B.; Yip, P.Y.; Ng, C.C.; Lum, T.; Farzin, M.; Trent, R.J.; Mercorella, B.; Clarkson, A.; Kohonen-Corish, M.R.; et al. Egfr mutant-specific immunohistochemistry has high specificity and sensitivity for detecting targeted activating egfr mutations in lung adenocarcinoma. J. Clin. Pathol. 2013, 66, 744–748. [Google Scholar] [CrossRef]
- Loussouarn, D.; Le Loupp, A.G.; Frenel, J.S.; Leclair, F.; Von Deimling, A.; Aumont, M.; Martin, S.; Campone, M.; Denis, M.G. Comparison of immunohistochemistry, DNA sequencing and allele-specific pcr for the detection of idh1 mutations in gliomas. Int. J. Oncol. 2012, 40, 2058–2062. [Google Scholar]
- Costumbrado, J.; Mansour, T.; Ghassemzadeh, S. Rh incompatibility; Statpearls: Treasure Island, FL, USA, 2019; Available online: https://www.ncbi.nlm.nih.gov/books/NBK459353/ (accessed on 12 December 2019).
- Habermann, E.; Bernath, S. Preparation, measurement and possible use of human antitoxin against cl. Botulinum a, b, and e toxins. Med. Microbiol. Immunol. 1975, 161, 203–210. [Google Scholar] [CrossRef]
- Torgeman, A.; Ozeri, E.; Ben David, A.; Diamant, E.; Rosen, O.; Schwartz, A.; Barnea, A.; Makovitzki, A.; Mimran, A.; Zichel, R. Role of homologous fc fragment in the potency and efficacy of anti-botulinum antibody preparations. Toxins 2017, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Bigaut, K.; De Seze, J.; Collongues, N. Ocrelizumab for the treatment of multiple sclerosis. Expert Rev. Neurother. 2019, 19, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Molina, A. A decade of rituximab: Improving survival outcomes in non-hodgkin’s lymphoma. Annu. Rev. Med. 2008, 59, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Bondeson, J.; Maini, R.N. Tumour necrosis factor as a therapeutic target in rheumatoid arthritis and other chronic inflammatory diseases: The clinical experience with infliximab (remicade). Int. J. Clin. Pract. 2001, 55, 211–216. [Google Scholar] [PubMed]
- Koppolu, V.; Rekha Vasigala, V.K. Checkpoint immunotherapy by nivolumab for treatment of metastatic melanoma. J. Cancer Res. Ther. 2018, 14, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, F.; Dadduzio, V.; Bergamo, F.; Manai, C.; Schirripa, M.; Lonardi, S.; Zagonel, V.; Loupakis, F. Anti-egfr monoclonal antibody panitumumab for the treatment of patients with metastatic colorectal cancer: An overview of current practice and future perspectives. Expert Opin. Biol. Ther. 2017, 17, 1297–1308. [Google Scholar] [CrossRef]
- Saghafi, H.; Rahbar, K.; Nobakht Haghighi, A.; Qoreishi, M.; Safdari, F. Efficacy of anti-interleukin-2 receptor antibody (daclizumab) in reducing the incidence of acute rejection after renal transplantation. Nephrourol. Mon. 2012, 4, 475–477. [Google Scholar] [CrossRef] [Green Version]
- MacGregor, P.; Gonzalez-Munoz, A.L.; Jobe, F.; Taylor, M.C.; Rust, S.; Sandercock, A.M.; Macleod, O.J.S.; Van Bocxlaer, K.; Francisco, A.F.; D’Hooge, F.; et al. A single dose of antibody-drug conjugate cures a stage 1 model of african trypanosomiasis. PLoS Negl. Trop. Dis. 2019, 13, e0007373. [Google Scholar] [CrossRef] [Green Version]
- Bankamp, B.; Hickman, C.; Icenogle, J.P.; Rota, P.A. Successes and challenges for preventing measles, mumps and rubella by vaccination. Curr. Opin. Virol. 2019, 34, 110–116. [Google Scholar] [CrossRef]
- Reynolds, D.L.; Vidor, E. Fully liquid dtap-ipv-hib pediatric combination vaccine (pediacel): A review of 18 years of clinical experience. Expert Rev. Vaccines 2014, 13, 943–968. [Google Scholar] [CrossRef]
- Skibinski, D.A.; Baudner, B.C.; Singh, M.; O’Hagan, D.T. Combination vaccines. J. Glob. Infect. Dis. 2011, 3, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Dtap-ipv-hepb-hib vaccine (hexyon((r))): An updated review of its use in primary and booster vaccination. Paediatr. Drugs 2019, 21, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018, 5, CD009069. [Google Scholar] [CrossRef] [PubMed]
- Roden, R.B.S.; Stern, P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Mukumoto, M.; Miyatake, K. Production and purification of polyclonal antibodies. Methods Mol. Biol. 2010, 657, 63–74. [Google Scholar] [PubMed]
- Nakazawa, M.; Mukumoto, M.; Miyatake, K. Production and Purification of Polyclonal Antibodies; Humana Press: New York, NY, USA, 2016; Volume 1474, pp. 49–59. [Google Scholar]
- Pihl, T.H.; Illigen, K.E.; Houen, G. Polyclonal peptide antisera. Methods Mol. Biol. 2015, 1348, 103–107. [Google Scholar] [PubMed]
- Guven, E.; Duus, K.; Laursen, I.; Hojrup, P.; Houen, G. Aluminum hydroxide adjuvant differentially activates the three complement pathways with major involvement of the alternative pathway. PLoS ONE 2013, 8, e74445. [Google Scholar] [CrossRef]
- Trier, N.H.; Guven, E.; Skogstrand, K.; Ciplys, E.; Slibinskas, R.; Houen, G. Comparison of immunological adjuvants. APMIS 2019, 127, 635–641. [Google Scholar] [CrossRef]
- Islam, T.; Naik, A.D.; Hashimoto, Y.; Menegatti, S.; Carbonell, R.G. Optimization of sequence, display, and mode of operation of igg-binding peptide ligands to develop robust, high-capacity affinity adsorbents that afford high igg product quality. Int. J. Mol. Sci. 2019, 20, 161. [Google Scholar] [CrossRef] [Green Version]
- Lundstrom, S.L.; Heyder, T.; Wiklundh, E.; Zhang, B.; Eklund, A.; Grunewald, J.; Zubarev, R.A. Spotlight proteomics-a igg-enrichment phenotype profiling approach with clinical implications. Int. J. Mol. Sci. 2019, 20, 2157. [Google Scholar] [CrossRef] [Green Version]
- Kohler, G.; Howe, S.C.; Milstein, C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur. J. Immunol. 1976, 6, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. 1975. J. Immunol. 2005, 174, 2453–2455. [Google Scholar] [PubMed]
- Trier, N.H.; Mortensen, A.; Schiolborg, A.; Friis, T. Production and screening of monoclonal peptide antibodies. Methods Mol. Biol. 2015, 1348, 109–126. [Google Scholar] [PubMed]
- Agca, S.; Houen, G.; Trier, N.H. Characterization of continuous b-cell epitopes in the n-terminus of glutamate decarboxylase67 using monoclonal antibodies. J. Pept. Sci. 2014, 20, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Dam, C.E.; Houen, G.; Hansen, P.R.; Trier, N.H. Identification and fine mapping of a linear b cell epitope of human vimentin. Scand. J. Clin. Lab. Investig. 2014, 74, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tronstrom, J.; Draborg, A.H.; Hansen, P.R.; Houen, G.; Trier, N.H. Identification of a linear epitope recognized by a monoclonal antibody directed to the heterogeneous nucleoriboprotein a2. Protein Pept. Lett. 2014, 21, 25–31. [Google Scholar] [CrossRef]
- Harlow, E.L.D. Antibodies—A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1988. [Google Scholar]
- Copestake, D.E.; Indyk, H.E.; Otter, D.E. Affinity liquid chromatography method for the quantification of immunoglobulin g in bovine colostrum powders. J. AOAC Int. 2006, 89, 1249–1256. [Google Scholar]
- Gall-Debreceni, A.; Lazar, J.; Kadas, J.; Balogh, A.; Ferenczi, A.; Sos, E.; Takacs, L.; Kurucz, I. Specific detection and quantitation of bovine igg in bioreactor derived mouse mab preparations. J. Immunol. Methods 2016, 438, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Todd, P.A.; Brogden, R.N. Muromonab cd3. A review of its pharmacology and therapeutic potential. Drugs 1989, 37, 871–899. [Google Scholar] [CrossRef]
- Neuberger, M.S.; Williams, G.T.; Mitchell, E.B.; Jouhal, S.S.; Flanagan, J.G.; Rabbitts, T.H. A hapten-specific chimaeric ige antibody with human physiological effector function. Nature 1985, 314, 268–270. [Google Scholar] [CrossRef]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M.; Rosensweig, C.J.; Faden, L.B.; Dewitz, M.C. Monoclonal antibody successes in the clinic. Nat. Biotechnol. 2005, 23, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Basu, K.; Green, E.M.; Cheng, Y.; Craik, C.S. Why recombinant antibodies—Benefits and applications. Curr. Opin. Biotechnol. 2019, 60, 153–158. [Google Scholar] [CrossRef]
- Ma, H.; O’Kennedy, R. The structure of natural and recombinant antibodies. Methods Mol. Biol. 2015, 1348, 7–11. [Google Scholar] [PubMed]
- Tomszak, F.; Weber, S.; Zantow, J.; Schirrmann, T.; Hust, M.; Frenzel, A. Selection of recombinant human antibodies. Adv. Exp. Med. Biol. 2016, 917, 23–54. [Google Scholar]
- Baird, C.L.; Fischer, C.J.; Pefaur, N.B.; Miller, K.D.; Kagan, J.; Srivastava, S.; Rodland, K.D. Developing recombinant antibodies for biomarker detection. Cancer Biomark. 2010, 6, 271–279. [Google Scholar] [CrossRef]
- Dubel, S. Recombinant therapeutic antibodies. Appl. Microbiol. Biotechnol. 2007, 74, 723–729. [Google Scholar] [CrossRef]
- Koch, J.; Tesar, M. Recombinant antibodies to arm cytotoxic lymphocytes in cancer immunotherapy. Transfus. Med. Hemother. 2017, 44, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Shen, Z.; Mernaugh, R. Recombinant antibodies and their use in biosensors. Anal. Bioanal. Chem. 2012, 402, 3027–3038. [Google Scholar] [CrossRef] [Green Version]
- Casan, J.M.L.; Wong, J.; Northcott, M.J.; Opat, S. Anti-cd20 monoclonal antibodies: Reviewing a revolution. Hum. Vaccin Immunother. 2018, 14, 2820–2841. [Google Scholar] [CrossRef]
- Dangi, A.K.; Sinha, R.; Dwivedi, S.; Gupta, S.K.; Shukla, P. Cell line techniques and gene editing tools for antibody production: A review. Front. Pharmacol. 2018, 9, 630. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.C.; Choong, Y.S.; Lim, T.S. Cognizance of molecular methods for the generation of mutagenic phage display antibody libraries for affinity maturation. Int. J. Mol. Sci. 2019, 20, 1861. [Google Scholar] [CrossRef] [Green Version]
- Ahamadi-Fesharaki, R.; Fateh, A.; Vaziri, F.; Solgi, G.; Siadat, S.D.; Mahboudi, F.; Rahimi-Jamnani, F. Single-chain variable fragment-based bispecific antibodies: Hitting two targets with one sophisticated arrow. Mol. Ther. Oncolytics 2019, 14, 38–56. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, A.; Jimeno, A. Bispecific antibodies for cancer therapy: A review. Pharm. Ther. 2018, 185, 122–134. [Google Scholar] [CrossRef]
- Runcie, K.; Budman, D.R.; John, V.; Seetharamu, N. Bi-specific and tri-specific antibodies- the next big thing in solid tumor therapeutics. Mol. Med. 2018, 24, 50. [Google Scholar] [CrossRef]
- Walsh, Z.; Yang, Y.; Kohler, M.E. Immunobiology of chimeric antigen receptor t cells and novel designs. Immunol. Rev. 2019, 290, 100–113. [Google Scholar] [CrossRef]
- Borrebaeck, C.A.; Carlsson, R. Human therapeutic antibodies. Curr. Opin. Pharmacol. 2001, 1, 404–408. [Google Scholar] [CrossRef]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Stahl, S.; Uhlen, M.; Nygren, P.A. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef]
- Pluckthun, A. Designed ankyrin repeat proteins (darpins): Binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef]
- Richter, A.; Eggenstein, E.; Skerra, A. Anticalins: Exploiting a non-ig scaffold with hypervariable loops for the engineering of binding proteins. FEBS Lett. 2014, 588, 213–218. [Google Scholar] [CrossRef]
- Simeon, R.; Chen, Z. In vitro-engineered non-antibody protein therapeutics. Protein Cell 2018, 9, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowlands, D.J.; Clarke, B.E.; Carroll, A.R.; Brown, F.; Nicholson, B.H.; Bittle, J.L.; Houghten, R.A.; Lerner, R.A. Chemical basis of antigenic variation in foot-and-mouth disease virus. Nature 1983, 306, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, M.H. Antigenicity and immunogenicity of synthetic peptides. Biologicals 2001, 29, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Houen, G. Peptide Antibodies: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2016. [Google Scholar]
- Kao, D.J.; Hodges, R.S. Advantages of a synthetic peptide immunogen over a protein immunogen in the development of an anti-pilus vaccine for pseudomonas aeruginosa. Chem. Biol. Drug Des. 2009, 74, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trier, N.H.; Houen, G. Peptide antibodies in clinical laboratory diagnostics. Adv. Clin. Chem. 2017, 81, 43–96. [Google Scholar]
- Grant, G.A. Synthetic peptides for production of antibodies that recognize intact proteins. Chapter 11. Curr. Protoc. Mol. Biol. 2002, 59, 11–16. [Google Scholar] [CrossRef]
- Barnard, G.; Hopkins, L.; Moorthie, S.; Seilly, D.; Tonks, P.; Dabaghian, R.; Clewley, J.; Coward, J.; McConnell, I. Direct detection of disease associated prions in brain and lymphoid tissue using antibodies recognizing the extreme n terminus of prpc. Prion 2007, 1, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Bhullar, S.S.; Chandak, N.H.; Baheti, N.N.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F.; Kashyap, R.S. Diagnosis of herpes simplex encephalitis by elisa using antipeptide antibodies against type-common epitopes of glycoprotein b of herpes simplex viruses. J. Immunoass. Immunochem. 2016, 37, 217–227. [Google Scholar] [CrossRef]
- Field, S.; Uyttenhove, C.; Stroobant, V.; Cheou, P.; Donckers, D.; Coutelier, J.P.; Simpson, P.T.; Cummings, M.C.; Saunus, J.M.; Reid, L.E.; et al. Novel highly specific anti-periostin antibodies uncover the functional importance of the fascilin 1-1 domain and highlight preferential expression of periostin in aggressive breast cancer. Int. J. Cancer 2016, 138, 1959–1970. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, J.; Criado, R.; Citti, R.; Martin, M.; Herranz, C.; Fernandez, M.F.; Cintas, L.M.; Hernandez, P.E. Performance and applications of polyclonal antipeptide antibodies specific for the enterococcal bacteriocin enterocin p. J. Agric. Food Chem. 2004, 52, 2247–2255. [Google Scholar] [CrossRef]
- Skovbjerg, H.; Koch, C.; Anthonsen, D.; Sjostrom, H. Deamidation and cross-linking of gliadin peptides by transglutaminases and the relation to celiac disease. Biochim. Biophys. Acta 2004, 1690, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, P.Y.; Fasman, G.D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. Relat. Areas Mol. Biol. 1978, 47, 45–148. [Google Scholar] [PubMed]
- Garnier, J.; Osguthorpe, D.J.; Robson, B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J. Mol. Biol. 1978, 120, 97–120. [Google Scholar] [CrossRef]
- Lee, B.S.; Huang, J.S.; Jayathilaka, G.D.; Lateef, S.S.; Gupta, S. Production of antipeptide antibodies. Methods Mol. Biol. 2010, 657, 93–108. [Google Scholar]
- Rubinstein, N.D.; Mayrose, I.; Halperin, D.; Yekutieli, D.; Gershoni, J.M.; Pupko, T. Computational characterization of b-cell epitopes. Mol. Immunol. 2008, 45, 3477–3489. [Google Scholar] [CrossRef] [Green Version]
- Houen, G.; Jakobsen, M.H.; Svaerke, C.; Koch, C.; Barkholt, V. Conjugation to preadsorbed preactivated proteins and efficient generation of anti peptide antibodies. J. Immunol. Methods 1997, 206, 125–134. [Google Scholar] [CrossRef]
- Houen, G.; Jensen, O.M. Conjugation to preactivated proteins using divinylsulfone and iodoacetic acid. J. Immunol. Methods 1995, 181, 187–200. [Google Scholar] [CrossRef]
- Hurdayal, R.; Achilonu, I.; Choveaux, D.; Coetzer, T.H.; Dean Goldring, J.P. Anti-peptide antibodies differentiate between plasmodial lactate dehydrogenases. Peptides 2010, 31, 525–532. [Google Scholar] [CrossRef]
- Hancock, D.C.; O’Reilly, N.J. Synthetic peptides as antigens for antibody production. Methods Mol. Biol. 2005, 295, 13–26. [Google Scholar]
- Berghoff, A.S.; Lassmann, H.; Preusser, M.; Hoftberger, R. Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin. Exp. Metastasis 2013, 30, 69–81. [Google Scholar] [CrossRef]
- Anasir, M.I.; Poh, C.L. Advances in antigenic peptide-based vaccine and neutralizing antibodies against viruses causing hand, foot, and mouth disease. Int. J. Mol. Sci. 2019, 20, 1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favoino, E.; Prete, M.; Catacchio, G.; Conteduca, G.; Perosa, F. Cd20-mimotope peptides: A model to define the molecular basis of epitope spreading. Int. J. Mol. Sci. 2019, 20, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddalena, A.; Papassotiropoulos, A.; Muller-Tillmanns, B.; Jung, H.H.; Hegi, T.; Nitsch, R.M.; Hock, C. Biochemical diagnosis of alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide42. Arch. Neurol. 2003, 60, 1202–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapiola, T.; Alafuzoff, I.; Herukka, S.K.; Parkkinen, L.; Hartikainen, P.; Soininen, H.; Pirttila, T. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of alzheimer-type pathologic changes in the brain. Arch. Neurol. 2009, 66, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Kalia, L.V.; Kalia, S.K.; McLean, P.J.; Lozano, A.M.; Lang, A.E. Alpha-synuclein oligomers and clinical implications for parkinson disease. Ann. Neurol. 2013, 73, 155–169. [Google Scholar] [CrossRef]
- Shen, N.; Song, G.; Yang, H.; Lin, X.; Brown, B.; Hong, Y.; Cai, J.; Cao, C. Identifying the pathological domain of alpha- synuclein as a therapeutic for parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 2338. [Google Scholar] [CrossRef] [Green Version]
- Cohn, M. Degeneracy, mimicry and crossreactivity in immune recognition. Mol. Immunol. 2005, 42, 651–655. [Google Scholar] [CrossRef]
- Cunto-Amesty, G.; Luo, P.; Monzavi-Karbassi, B.; Kieber-Emmons, T. Exploiting molecular mimicry: Defining rules of the game. Int. Rev. Immunol. 2001, 20, 157–180. [Google Scholar] [CrossRef]
- Fournel, S.; Muller, S. Synthetic peptides in the diagnosis of systemic autoimmune diseases. Curr. Protein Pept. Sci. 2003, 4, 261–274. [Google Scholar] [CrossRef]
- Jain, D.; Salunke, D.M. Antibody specificity and promiscuity. Biochem. J. 2019, 476, 433–447. [Google Scholar] [CrossRef]
- Rose, N.R.; Mackay, I.R. Molecular mimicry: A critical look at exemplary instances in human diseases. Cell. Mol. Life Sci. 2000, 57, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Marasco, D.; Perretta, G.; Sabatella, M.; Ruvo, M. Past and future perspectives of synthetic peptide libraries. Curr. Protein Pept. Sci. 2008, 9, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Sandomenico, A.; Caporale, A.; Doti, N.; Cross, S.; Cruciani, G.; Chambery, A.; De Falco, S.; Ruvo, M. Synthetic peptide libraries. From random mixtures to in vivo testing. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Marcaurelle, L.A.; Seeberger, P.H. Combinatorial carbohydrate chemistry. Curr. Opin. Chem. Biol. 2002, 6, 289–296. [Google Scholar] [CrossRef]
- Sofia, M.J. Carbohydrate-based combinatorial libraries. Mol. Divers 1997, 3, 75–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [Green Version]
- Reymond, J.L.; Darbre, T. Peptide and glycopeptide dendrimer apple trees as enzyme models and for biomedical applications. Org. Biomol. Chem. 2012, 10, 1483–1492. [Google Scholar] [CrossRef]
- Sebestik, J.; Niederhafner, P.; Jezek, J. Peptide and glycopeptide dendrimers and analogous dendrimeric structures and their biomedical applications. Amino Acids 2011, 40, 301–370. [Google Scholar] [CrossRef]
- Haupt, K.; Linares, A.V.; Bompart, M.; Bui, B.T. Molecularly imprinted polymers. Top. Curr. Chem. 2012, 325, 1–28. [Google Scholar]
- Takeuchi, T.; Sunayama, H. Beyond natural antibodies—A new generation of synthetic antibodies created by post-imprinting modification of molecularly imprinted polymers. Chem. Commun. 2018, 54, 6243–6251. [Google Scholar] [CrossRef]
- Batra, D.; Shea, K.J. Combinatorial methods in molecular imprinting. Curr. Opin. Chem. Biol. 2003, 7, 434–442. [Google Scholar] [CrossRef]
- Ye, L.; Haupt, K. Molecularly imprinted polymers as antibody and receptor mimics for assays, sensors and drug discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S. Pegaptanib sodium for the treatment of age-related macular degeneration. Expert Opin. Pharmacother. 2008, 9, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Babin, B.M.; Kasperkiewicz, P.; Janiszewski, T.; Yoo, E.; Dra, G.M.; Bogyo, M. Leveraging peptide substrate libraries to design inhibitors of bacterial lon protease. ACS Chem. Biol. 2019, 14, 2453–2462. [Google Scholar] [CrossRef]
- Dooley, C.T.; Ferrer, T.; Pagan, H.; O’Corry-Crowe, G.M. Bridging immunogenetics and immunoproteomics: Model positional scanning library analysis for major histocompatibility complex class ii dq in tursiops truncatus. PLoS ONE 2018, 13, e0201299. [Google Scholar] [CrossRef]
- Nino-Vasquez, J.J.; Allicotti, G.; Borras, E.; Wilson, D.B.; Valmori, D.; Simon, R.; Martin, R.; Pinilla, C. A powerful combination: The use of positional scanning libraries and biometrical analysis to identify cross-reactive t cell epitopes. Mol. Immunol. 2004, 40, 1063–1074. [Google Scholar] [CrossRef]
- Pinilla, C.; Appel, J.R.; Judkowski, V.; Houghten, R.A. Identification of b cell and t cell epitopes using synthetic peptide combinatorial libraries. Chapter 9. Curr. Protoc. Immunol. 2012, 99. [Google Scholar] [CrossRef] [Green Version]
- Pulido, D.; Prats-Ejarque, G.; Villalba, C.; Albacar, M.; Moussaoui, M.; Andreu, D.; Volkmer, R.; Torrent, M.; Boix, E. Positional scanning library applied to the human eosinophil cationic protein/rnase3 n-terminus reveals novel and potent anti-biofilm peptides. Eur. J. Med. Chem. 2018, 152, 590–599. [Google Scholar] [CrossRef]
- Rut, W.; Kasperkiewicz, P.; Byzia, A.; Poreba, M.; Groborz, K.; Drag, M. Recent advances and concepts in substrate specificity determination of proteases using tailored libraries of fluorogenic substrates with unnatural amino acids. Biol. Chem. 2015, 396, 329–337. [Google Scholar] [CrossRef]
- Diafa, S.; Hollenstein, M. Generation of aptamers with an expanded chemical repertoire. Molecules 2015, 20, 16643–16671. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Zheng, X.; Jiao, B.; Wang, L. Post-selex optimization of aptamers. Anal. Bioanal. Chem. 2016, 408, 4567–4573. [Google Scholar] [CrossRef]
- Jijakli, K.; Khraiwesh, B.; Fu, W.; Luo, L.; Alzahmi, A.; Koussa, J.; Chaiboonchoe, A.; Kirmizialtin, S.; Yen, L.; Salehi-Ashtiani, K. The in vitro selection world. Methods 2016, 106, 3–13. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Rosenthal, M.; Siegl, J.; Ewers, J.; Mayer, G. Customised nucleic acid libraries for enhanced aptamer selection and performance. Curr. Opin. Biotechnol. 2017, 48, 111–118. [Google Scholar] [CrossRef]
- Pinheiro, V.B.; Taylor, A.I.; Cozens, C.; Abramov, M.; Renders, M.; Zhang, S.; Chaput, J.C.; Wengel, J.; Peak-Chew, S.Y.; McLaughlin, S.H.; et al. Synthetic genetic polymers capable of heredity and evolution. Science 2012, 336, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.Y.; Acquah, C.; Sidhu, A.; Ongkudon, C.M.; Yon, L.S.; Danquah, M.K. Selex modifications and bioanalytical techniques for aptamer-target binding characterization. Crit. Rev. Anal. Chem. 2016, 46, 521–537. [Google Scholar] [CrossRef]
- Beckman, R.A.; Weiner, L.M.; Davis, H.M. Antibody constructs in cancer therapy: Protein engineering strategies to improve exposure in solid tumors. Cancer 2007, 109, 170–179. [Google Scholar] [CrossRef]


| Name | Sequence | Biological Activity | Secondary Structure | References |
|---|---|---|---|---|
| Calcitonin- Gene Related Peptide | ACDTATCVTHRLAGLLSRSGGVVKNNFVPTNVGSKAF | Vasodilator, Migrane | α-helix | [20] |
| Gramicidin S | cyclo[D-Phe-Pro-Val-Orn-Leu]2 | Antimicrobial | Antiparallel β-sheet, cyclic | [21] |
| Peptide 2 | KHQCHWECT-Cit-GRCRLVCGRSGS | Reacts with rheumatoid autoantibodies | β-hairpin, disulfide bonds (C4–C17, C8–C13) | [22] |
| DX600 | GDYSHCSPLRYYPWWKCTYPDPEGGG | Inhibitor of angiotensin converting enzyme 2 | Random coil, disulfide bond (C6–C17) | [23] |
| Area | Examples | References |
|---|---|---|
| Research | ||
| Protease substrates | γ-secretase TM peptide substrates/AD | [41] |
| Protease inhibitors | HIV-protease inhibitors/HIV subtype C | [42] |
| Cell adhesion | N-methylated TSP-1 peptides/CLL | [43] |
| Peptide antibody production | P110/ mycoplasma genitalium, b-raf/ malignant melanoma | [44,45] |
| Epitope identification | GAD/ diabetes, CENPF/ cancer, NMDAR/ encephalitis | [34,46,47] |
| Diagnostics | ||
| Antibody detection | ACPA/RA, Gliadin/CD, DVEP/Dengue fever | [31,40,48,49,50] |
| Peptide quantification | Insulin/diabetes, C peptide/diabetes | [51] |
| Therapeutics | ||
| Peptide drugs | Leuprolide/cancer, desmopressin/diabetes | [52,53] |
| Vaccine development | Cell-penetrating peptides/DNA vaccine delivery | [54] |
| Antibodies | Examples/Uses | Antibody Type | References |
|---|---|---|---|
| Research | Target Recognition | ||
| CD14, CD20, CD34/ELISA, ICC, WB, FC | Peptide Ab (MAb) | [63,64,65] | |
| Diagnostics | Target Quantification | ||
| Isocitrate dehydrogenase, B-Raf, Epidermal growth factor receptor/IHC | Peptide Ab (MAb) | [66,67,68] | |
| melanoma Glycoprotein B, Herpes simplex encephalitis/IHC, ELISA | Peptide Ab (MAb) | ||
| Therapeutics | Target Neutralization | ||
| Rhesus-D Ig/rhesus syndrome prophylaxis | PAb (Sp IVIG) | [69] | |
| Anti-toxins/botulism | PAb (Sp IVIG) | [70,71] | |
| Rituximab/lymphoma, Ocrelizumab/multiple sclerosis | MAb (recombinant), chimeric | [72,73] | |
| Infliximab/rheumatoid arthritis | MAb (recombinant), chimeric | [74] | |
| Nivolumab/malignant melanoma | MAb (recombinant), human | [75] | |
| Panitumumab/EGFR metastatic colorectal carcinoma | MAb (recombinant), human | [76] | |
| Daclizumab/allograft rejection | Mab (recombinant), humanized | [77] | |
| HpHbR Ab-PBD conjugate/African trypanosomiasis treatment (mouse model) | MAb-drug conjugate | [78] | |
| Vaccines | |||
| MMR live attenuated viruses vaccine/measles, mumps, rubella prophylaxis | PAb in vivo | [79] | |
| DiTePePolHiB SU vaccine/diphtheria-tetanus-pertussis-polio-hemophilus prophylaxis | PAb in vivo | [80,81,82] | |
| HPV SU vaccine/cervix cancer prophylaxis | PAb in vivo | [83,84] |
| Selecting Factors | Examples | References |
|---|---|---|
| Amino acid composition | Hydrophilic aas, charged aas, Pro, Gly (represented in loops) | [33,138] |
| Peptide length | 8–25 aa | [46,130,132,133,134] |
| Peptide structure | Linear, flexible, cyclic, loops, turns, helices | [25,86,87,93,94,95] |
| Protein target | Accessible epitope, areas of high conservation, areas of hypervariability, N/C-termini, post-translational modifications | [128,129,138] |
| Area/Molecule Types | Examples/Uses | References |
|---|---|---|
| Research | Target Recognition/Identification | |
| Peptide libraries | Multiple (Proteases, inhibitors, B-cell and T-cell epitopes) | [155,156] |
| Carbohydrate libraries | Few | [157,158] |
| Aptamers | Multiple | [159] |
| Dendrimers | Few | [160,161] |
| Molecular imprints | Few | [162,163] |
| Diagnostics | Target Quantification | |
| Aptamers | Multiple (research/development stage) | [164,165] |
| Therapeutics | Target Neutralization | |
| Aptamers | Pegaptanib/ocular neo-vascularization | [166] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trier, N.; Hansen, P.; Houen, G. Peptides, Antibodies, Peptide Antibodies and More. Int. J. Mol. Sci. 2019, 20, 6289. https://doi.org/10.3390/ijms20246289
Trier N, Hansen P, Houen G. Peptides, Antibodies, Peptide Antibodies and More. International Journal of Molecular Sciences. 2019; 20(24):6289. https://doi.org/10.3390/ijms20246289
Chicago/Turabian StyleTrier, Nicole, Paul Hansen, and Gunnar Houen. 2019. "Peptides, Antibodies, Peptide Antibodies and More" International Journal of Molecular Sciences 20, no. 24: 6289. https://doi.org/10.3390/ijms20246289
APA StyleTrier, N., Hansen, P., & Houen, G. (2019). Peptides, Antibodies, Peptide Antibodies and More. International Journal of Molecular Sciences, 20(24), 6289. https://doi.org/10.3390/ijms20246289





