Abstract
Preterm birth (PTB) refers to the birth of infants before 37 weeks of gestation and is a challenging issue worldwide. Evidence reveals that PTB is a multifactorial dysregulation mediated by a complex molecular mechanism. Thus, a better understanding of the complex molecular mechanisms underlying PTB is a prerequisite to explore effective therapeutic approaches. During early pregnancy, various physiological and metabolic changes occur as a result of endocrine and immune metabolism. The microbiota controls the physiological and metabolic mechanism of the host homeostasis, and dysbiosis of maternal microbial homeostasis dysregulates the mechanistic of fetal developmental processes and directly affects the birth outcome. Accumulating evidence indicates that metabolic dysregulation in the maternal or fetal membranes stimulates the inflammatory cytokines, which may positively progress the PTB. Although labour is regarded as an inflammatory process, it is still unclear how microbial dysbiosis could regulate the molecular mechanism of PTB. In this review based on recent research, we focused on both the pathological and therapeutic contribution of microbiota-generated metabolites to PTB and the possible molecular mechanisms.
1. Introduction
Preterm birth (PTB) refers to the birth of infants before 37 weeks of gestation by World Health Organization [1]. PTB is a challenging issue worldwide with a prevalence of 5% to 18%, which increases the risk of morbidity and mortality or long-term complications to neonatal life [2,3]. During early pregnancy, various physiological and metabolic changes occur as a result of endocrine and immune metabolism [4]. Environmental and clinical factors such as toxicant particulate matter (PM 2.5–10, bisphenol, etc.), consumption of a high-fat diet, family PTB history, low education, low socioeconomic status, ethnicity (PTB is higher in non-Hispanic black women), previous PTB history, multiple pregnancies, short pregnancy interval, early (<16 years) or late (>36 years) pregnancy, tobacco or alcohol consumption, high stress, hypertension, obesity, low body mass index, infection, short cervix, uterine anomaly, and miscarriage can affect fetal developmental plasticity, gestational age, or birth outcome [5,6,7,8,9].
Based on clinical observations, PTB is classified as spontaneous PTB (sPTB) and iatrogenic; sPTB is due to preterm labour (PTL) or preterm premature rupture of the membranes (P-PROM) [10,11]. Several lines of evidence indicate that sPTB is commonly associated with intrauterine infection/inflammation [12,13]. Increased inflammatory molecules in different maternal bio-fluids indicate the onset of PTB [13,14,15]. It has been noted that inflammatory molecules (IL1, TNF, and IL6) are associated with the initiation of PTB and as predictive markers of PTB in symptomatic women [14]. Moreover, polymorphisms or hyper-methylation in genes or RNA transcript expression mediates inflammation and are associated with PTB [16,17,18].
Accumulating evidence indicates that the host microbiota regulates the maternal and fetal immune interaction as well as the birth outcome [19,20,21]. In addition, current lines of evidence also indicate that the host microbiota-generated metabolites control various metabolic mechanisms and inflammatory processes including PTB (Figure 1) [22,23]. Although labour is regarded as an inflammatory process, it is still unclear which microbiota and metabolites control PTB. In this review, we focused on the recent research-based evidence to elucidate the probable molecular basis of the involvement of microbial metabolites in PTB, emphasizing both pathogenic and therapeutic insights.
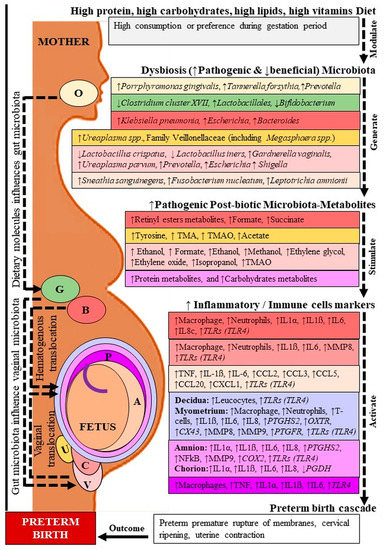
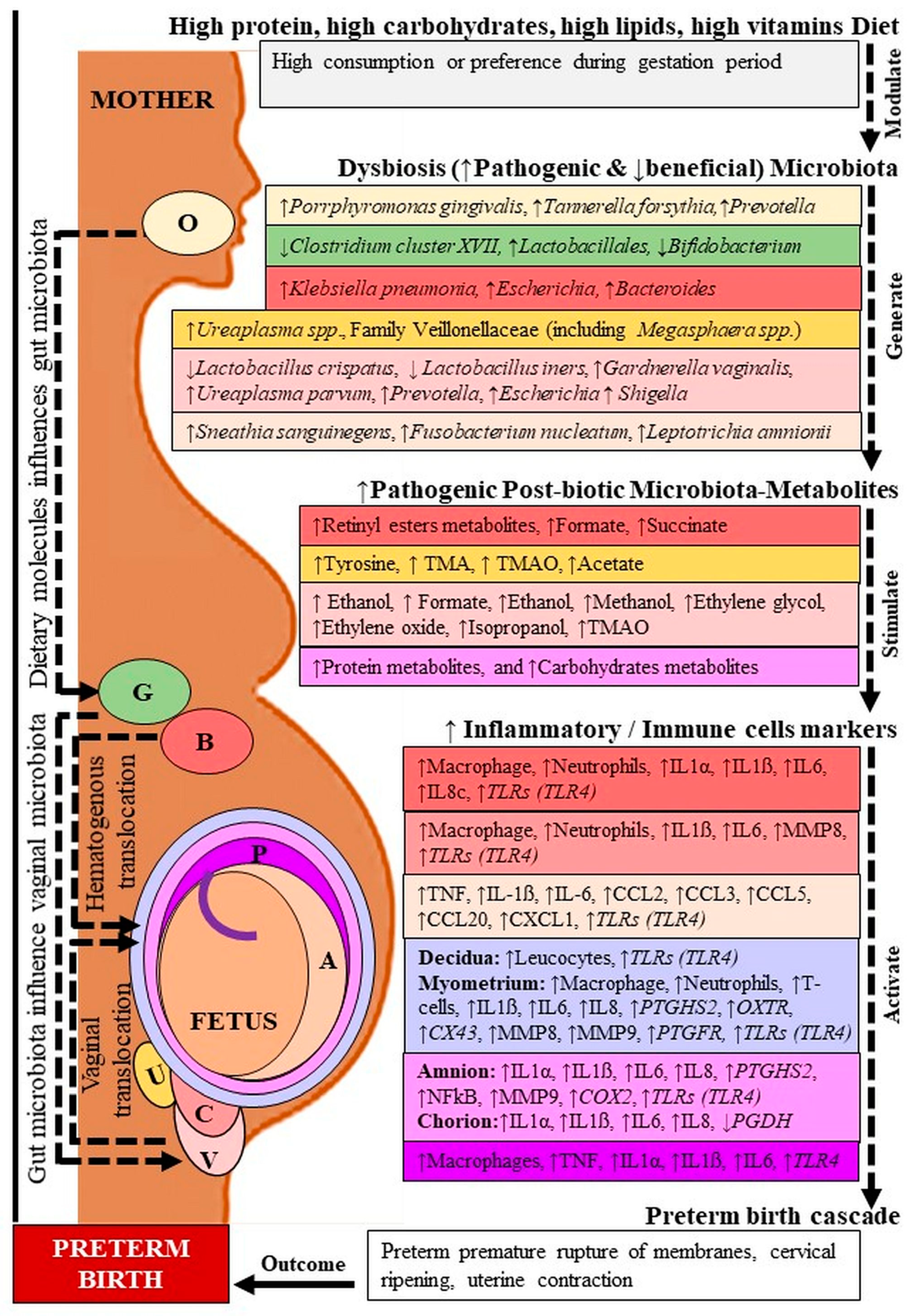
Figure 1.
Microbiota-metabolites and inflammatory markers in preterm birth. O: Oral; G: Gut; B: Blood; U: Urine; V: Vagina; C: Cervix; P: Placenta; A: Amniotic fluid. TMA: Trimethylamine, TMAO: Trimethylamine N-oxide; IL: Interleukin; TNF: Tumor necrosis factor; MMP: Matrix metalloproteinase; CCL: C-C motif chemokine ligand; CXCL: C-X-C motif chemokine; PTGDS2: Prostaglandin D2 synthase; OXTR: Oxytocin receptor; CX: Connexin; NFkB: Nuclear factor-kappa B; COX: Cyclooxygenase; PGDH: Prostaglandin dehydrogenase. Dashed lines indicate several steps, ↓ decreased, ↑ increased level. Location followed by color code.
2. Microbiota Pathological Insight in PTB
In early pregnancy, various physiological processes change dynamically, including hormonal and immunity for placentation and implementation [24,25]. During the course of normal pregnancy, healthy microbiota colonization can be a prerequisite for immune maturation as well as metabolic and hormonal homeostasis [26,27,28]. However, during pregnancy, the microbiota remains relatively stable or fluctuates dramatically in different trimesters at different regions [29,30,31,32]. Concerning healthy pregnancies, the intrauterine cavity microbiota colonization originates exclusively from ascending route through the urogenital tract (urinary, cervical, and vaginal) and the hematogenous route through the placenta after translocation from the digestive tract (oral and gut) [33]. The oral and gut microbiota stability is affected by extrinsic factors, especially diets, which influence the cervicovaginal microbiota dynamics [34,35]. The vaginal microbiota fluctuates almost daily because of its unstable environment in pregnant women [36]. Normally, the dominance of Lactobacillus spp. in the vaginal tract reflects healthy microbial colonization as members of this bacterial spp. promote the maintenance of vaginal homeostasis and prohibit the colonization and growth of adverse microorganisms, including those contributing to sexually transmitted infections. The protective role of Lactobacillus spp. is exerted through several mechanisms, such as the creation of an acidic environment by reducing vaginal pH at around 4.0, the production of bioactive compounds, competition for nutrients and adhesion sites, and modulation of host immunity [37,38,39,40]. Instead of the defensive function of dominant Lactobacillus spp. and low level of host-derived antimicrobial peptide, immune modulator β-Defensin 2 in cervicovaginal fluid is associated with increased risk of PTB in African American women [41].
A previous study analyzing microbial species composition in 396 samples from a population of asymptomatic North American women representing four ethnic groups (white, black, Hispanic, and Asian) by pyrosequencing of barcoded 16S rRNA genes revealed the clustering of vaginal microbial taxonomic communities into five groups called community state types (CST) [42]. Among them, four were dominated by Lactobacillus spp. as follows: CST I (Lactobacillus crispatus), CST II (Lactobacillus gasseri), CST III (Lactobacillus iners), and CST V (Lactobacillus jensenii), while CST IV was represented by a lower proportion of Lactobacillus and an array of strict and facultative anaerobes including Prevotella, Dialister, Atopobium, Gardnerella, Megasphaera, Peptoniphilus, Sneathia, Eggerthella, Aerococcus, Finegoldia, and Mobiluncus. Additionally, communities in CST I have the lowest median pH (4.0 ± 0.3), whereas communities belonging to CST IV had the highest median pH (5.3 ± 0.6) [42,43]. It was found that women with both CST IV and short cervixes are at a higher risk for sPTB than women with either factor alone [44]. In contrast, in another study, the association between term birth and PTB with Lactobacillus community composition classified vaginal microbiota into three categories: normal (>90% Lactobacillus spp.), intermediate (30–90% Lactobacillus spp.), and dysbiotic (<30% Lactobacillus spp.) [30,45], Gardnerella vaginalis is commonly isolated from patients with BV, while for characterization by 16S rRNA gene of V2 region, PCR products are an indicator of BV [46,47].
It has been found that in early pregnancy, higher microbial richness and diversity in different bio-fluids (blood, urine, cervicovaginal fluid, amniotic fluid) are similar to non-pregnant women before the second trimester. The microbial dysbiosis occurring during the very crucial second trimester, along with racial disparity, directly affects the normal developmental physiology and birth outcome [30,35]. Broadly speaking, dysbiosis in Gram-negative bacteria acts as an inducer of PTB [48,49]. In early pregnancy, an increase in pathogenic microbiota (Gardnerella, Ureaplasma, Closteridiam, Provetella, Mycoplasma) provides permissible colonization and metabolic signatures of bacterial vaginosis (BV), which double the risk of PTB [50,51,52,53]. Additionally, African American women have a higher rate of BV-related microbiota than Caucasian women [54]. The microbial translocation is not yet clear, but the ascending and hematogenous route pathways are common, as mentioned earlier [33]. The abundance of Lactobacillus spp., particularly the Lactobacillus crispatus-dominated vaginal environment (CST I), maintains gestational health and results in term birth. While the Lactobacillus iners dominated vaginal milieu (CST III), and there was an abundance of Clostridiales, Bacteroidales, and Actinomycetales, which are known to lead to vaginal dysbiosis associated with PTB [31,43,55,56]. In addition, the relatively lower abundance of vaginal Lactobacillus crispatus and relatively higher abundance of Anaerococcus vaginalis and Prevotella timonensis were observed in obese women and are significantly related to BV and PTB [8,30].
The abundance of Bacteroides and Escherichia-Shigella were observed in the blood, while the dominance of BV-associated Leptotrichia/Sneathia, Mobiluncus spp., and Mycoplasma spp. was reported in the vagina of PTB women [35,51,57]. A high level of Lactobacillales was observed in the feces and an abundance of Weissella and Rickettsiales were observed in the blood of women who reached full term [35,58]. The abundance of Ureaplasma spp. and the family Veillonellaceae (including Megasphaera spp.) was observed in the urine of PTB women [59]. The BV strains Sneathia sanguinegens and Fusobacterium nucleatum were identified in amniotic fluid samples at mid-trimester of women with PTB [60]. The low levels of Clostridium subcluster (XVIII, XIVa) and Bacteroides and a high level of Lactobacillales were observed in the feces of the PTB [58]. In pregnancy, the oral microbiota generally exhibits a relatively stable bacterial population to the vaginal microbiome. However, one study has revealed that high levels of common periodontal pathogens Porphyromonas gingivalis, Tannerella forsythia, Prevotella intermedia, and Prevotella nigrescens are associated with an increased risk of PTB [61]. Lactobacillus crispatus and Lactobacillus iners have a protective role against pathogenic microbiota such as Gardnerella, Ureaplasma, Closteridiam, and Provetella through beneficial metabolites to prevent virginal dysbiosis, maintain vaginal pH, and protect mucus layer integrity [62,63]. Furthermore, subjects with high concentrations of Lactobacillus crispatus at follow-up had high concentrations of metabolites negatively associated with BV, which affected their pregnancies (Figure 2, Table 1) [38,52,56,64].
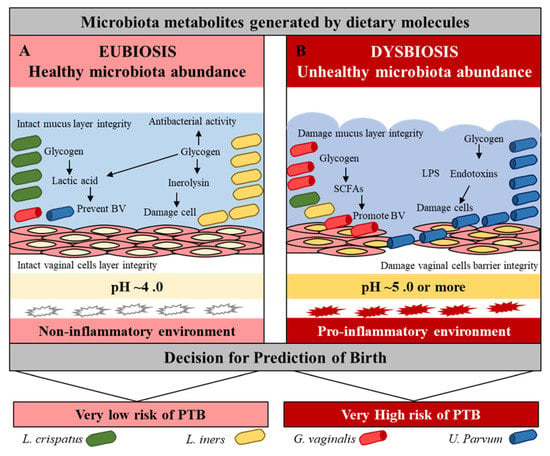
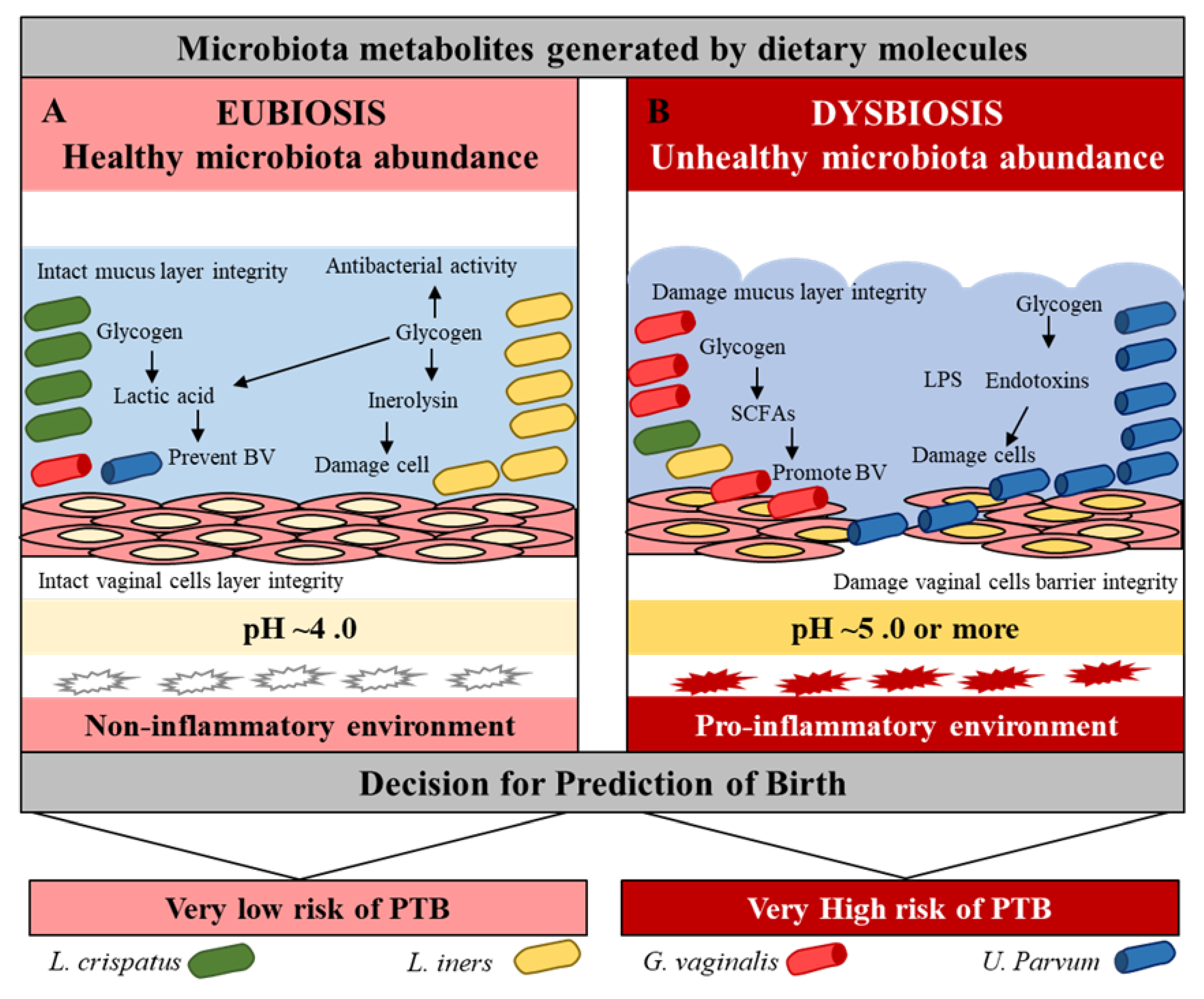
Figure 2.
Vaginal microbiota eubiosis and dysbiosis. Effect of beneficial and pathogenic microbiota in the vaginal environment. Pink = normal, while red = inflammation conditions.

Table 1.
Microbial richness in preterm birth.
3. Microbiota Metabolites Pathological Insight in PTB
Microbiota affects the metabolic process directly or indirectly by their generated metabolites, also termed as ‘post-biotic metabolites’ (PBM), which produce similar or much better effects compared to their own live parents [22,68]. In general, bacterial metabolites may impact human cell function, inflammation, and disease susceptibility. Small molecule metabolites (the metabolome) represent the enzymatic pathways and complex metabolic networks that execute microbial transformation of host-derived products. Various external environmental factors, especially diet containing high carbohydrates, high protein, and high fat affect the gut microbiota dysbiosis and metabolic dysregulation [69,70]. Disturbed metabolic dysregulation due to high consumption of carbohydrate and fat diet increases the chances of obesity and relative abundance of pathogenic microbiota [71,72]. Consumption of high carbohydrates, high-protein, high-fat, and/or high-vitamin diets influences maternal extra and intrauterine factors by their pathogenic microbiota and metabolites, thereby increasing the risk of PTB [23,73]. In connection, microbiota dysbiosis directly affects the production of microbiota-metabolites, and the presence of metabolites at higher or lower levels impacts metabolic function including PTB [74,75]. These PBM are represented by several active compounds including short-chain fatty acids, polyamines, polyphosphates, and peptides, which exert a significant effect on several metabolic activities [76]. Principally, there are two basic phenomena involved in the generation of microbial PBM: first, the bio-production of short-chain fatty acids (SCFAs) or alcohol from fermenting sugars or fibers, and second, the bio-conversion of derivative molecules [77,78]. Microbial metabolites generated from pathogenic bacteria such as peptidoglycan (PGN), lipopolysaccharides (LPS), and lipoteichoic acid (LTA) represent pathogen-associated molecular patterns (PAMPs), while damage-associated molecular patterns (DAMPs) are derived from dietary factors. These PAMPs and DAMPs are generated in response to infection and inflammation [79]. These two microbe-specific molecular signatures are recognized by the innate immune system via germline-encoded pattern-recognition receptors (PRRs). In the mammalian system, among the major members of PRR families, Toll-like receptors (TLRs) were the first to be identified, and are the best-characterized molecules. Following PAMPs and DAMPs recognition, TLRs recruit Toll/IL1 receptor (IL1R) domain-containing adaptor proteins such as MyD88 and TRIF, which induce signal transduction pathways that ultimately lead to the activation of transcription factors NFκB and IRFs or MAP kinases to regulate the expression of pro-inflammatory cytokines, chemokines, and type I IFNs. Such events dictate the outcome of innate immune responses that protect the host from microbial infections [79].
The SCFAs (formate, acetate, succinate, and lactate) are produced from indigestible carbohydrates and dietary fibers in the presence of microbiota. Formate is produced by Lactobacillus pentosus, acetate is produced by Lactobacillus acidophilus CRL 1014, while various strains of Clostridium spp., Ruminococcus spp. produce butyrate, propionate, and succinate. Alcoholic metabolites (methanol, ethanol, formate, and isopropanol) are generated through fermentation-mediated production of methanol and ethylene by ammonia-oxidizing bacteria such as Nitrosomonas europaea and Nitrosococcus oceani [80,81], while acetone is produced mostly by Clostridium acetobutylicum, Clostridium beijerinckii, and Clostridium saccharobutylicum. Ethylene glycol is produced by Corynebacterium glutamicum, glycolate by Corynebacterium glutamicum, isopropanol by Clostridium acetobutylicum ATCC 824, and Escherichia coli, and ethanol by Lactobacillus fermentum. Several bacterial pathogens (LPS) depend on polyamines for their survival and virulence within the host, including Helicobacter pylori, Salmonella enterica subsp. enterica serovar Typhimurium, Shigella spp., Staphylococcus aureus, Streptococcus pneumonia, and Vibrio cholera [82]. Finally, the derived metabolite ‘TMAO’ is a bioconverted derivative of TMA that is generated from choline, betaine, and carnitine by the action of eight distinct bacterial strains, including Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Edwardsiella tarda, Escherichia fergusonii, Proteus penneri, and Providencia rettgeri [83]. Lactobacillus iners produces a pore-forming toxin (Inerolysin) similar to the one produced by Gardenella vaginalis, which is capable of lysing erythrocytes and increase the pH facilitate PTB (<4.5; Figure 3, Table 2) [84].
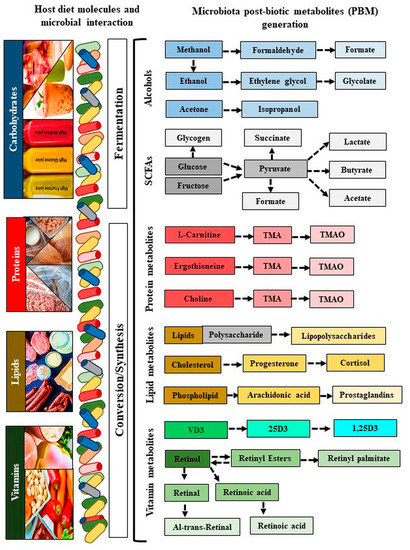
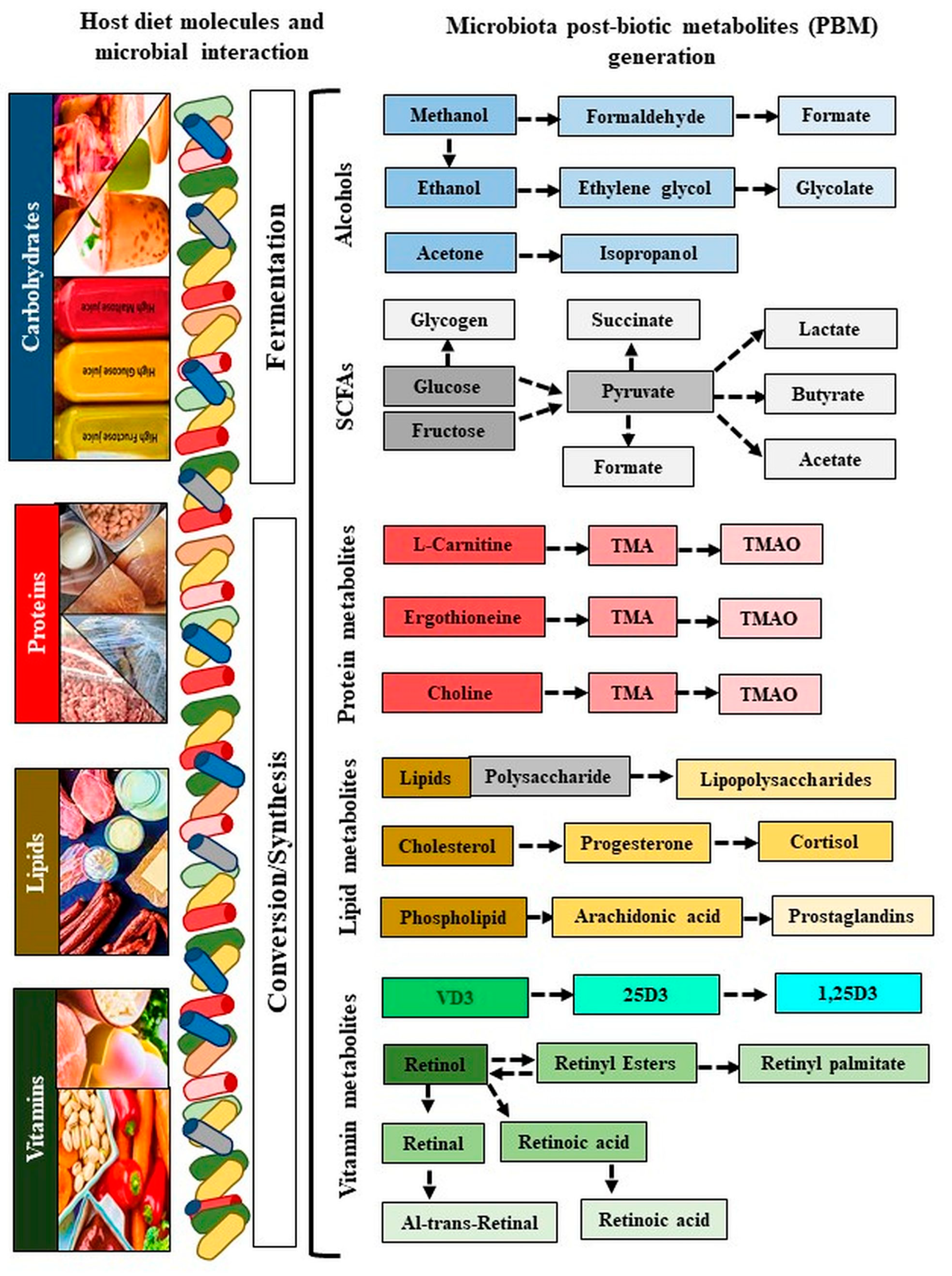
Figure 3.
Generation of microbiota metabolites. The blue color refers to carbohydrates, the red color refers to proteins, the yellow color refers to lipids, and the green color refers to vitamins. Colors indicate metabolites generated by their specific diatery molecules and microbiota. Colors from dark to light color shed indicate derivatives of metabolites conversion. VD: Vitamin D; 25D: 25-hydroxy vitamin D; 1,25(OH)2D3: 1α,25-dihydroxy vitamin D.

Table 2.
Metabolites generate by microbiota.
The generation or availability of microbial metabolites is directly influenced by the dysbiosis of the microbial population, which in turn is affected by environmental factors, especially diet [36]. During pregnancy, microbiota-generated post-biotic metabolites concentration depends upon microbial richness and diversity in different bio-fluids (blood, urine, cervicovaginal fluid, amniotic fluid), which are associated with PTB (Table 3). It has been reported that the levels of polyamines that are associated with mothers’ dietary intake are higher in the preterm women’s breast milk [107]. Alcoholic and acetone microbial metabolites are harmful to the birth outcome. Exposure to a low level of methanol can shorten the pregnancy and promote labour complications, while ethylene metabolite, i.e., ethylene oxide may increase the risk of spontaneous abortion, PTB, and post-term birth. It has been found that the consumption of natural highly sugar-sweetened (e.g., fructose) or artificially sweetened (e.g., aspartame) beverages may be likened to an increased risk of PTB. Aspartame breaks down into methanol and other substances in the body, which in turn can be converted into toxic metabolites such as formaldehyde and formate that adversely affect PTB [23]. LPS is a microbiota endotoxin, which acts on TLRs and induces PTB [108]. The high protein content of the amniotic fluid observed in the second-trimester is also considered as one of the contributing factors for PTB [109] since the high level of protein metabolites TMA/TMAO in the second-trimester are known to be associated with PTB [23].

Table 3.
Microbiota metabolites generated in preterm birth.
4. Molecular Mechanism of Microbiota Metabolites in PTB
Although labour is considered an inflammatory process, accumulating evidence indicates that the molecular mechanisms underlying PTB are multifactorial, involving many biological pathways [11]. More specifically, the estrogen metabolism pathway, intrauterine infection, extracellular matrix degradation, fetal stress, and fetal anomalies are the most reported pathways associated with PTB [118]. However, the most common mechanism of PTB is found to be linked to the inflammatory signaling pathways [12,118]. Several reports indicate the activation of inflammatory reactions in the gestational tissues and secretion of inflammatory cytokines as an immune response to the ascending infection of the genital tract and pathogenic microbial composition [10,74]. More specifically, microbe-induced inflammatory signals arising from urinary tract infection, sexually transmitted infections, including trichomoniasis, or BV are the major factors contributing to PTB [119,120]. The abundance of certain Lactobacilli in the vagina has been shown to trigger a distinct inflammatory cascade that largely contributes to CST-specific response. It was revealed that the vaginal presence of Lactobacillus iners in CST III and CST IV were associated with a higher baseline in pro-inflammatory factors including macrophage migration inhibitory factor (MIF), IL1α, IL18, and TNF, which are known to induce the activation of inflammatory responses [43,121]. In agreement, a previous clinical study performing longitudinal analyses of 16S rRNA, metagenomic, metatranscriptomic, and nine cytokine profiles from forty-five preterm and ninety term birth controls demonstrated higher vaginal levels of eotaxin, IL1β, IL6, and macrophage inflammatory protein (MIP)1β in PTB compared to TB samples [122]. The study also found a strong negative correlation between Lactobacillus crispatus and several taxa associated with dysbiosis and PTB (for instance, Gardnerella. vaginalis, Prevotella cluster 2, S. amnii, and, to a lesser extent, TM7-H1), as well as with vaginal cytokines, further supporting the benefits of Lactobacillus crispatus on vaginal health [121]. Additionally, the examined cytokines, which were largely pro-inflammatory, showed a loose correlation both with each other and with taxa associated with dysbiosis and PTB. While the proinflammatory chemokine IP10/CXCL10 was positively correlated with Lactobacillus iners. In contrast, in women with PTB, the proinflammatory cytokines and dysbiotic taxa (for instance, Atopobium vaginae, Gardnerella vaginalis, and Megasphaera type 1) exhibited a tighter cluster, signifying a stronger positive correlation [122]. Using 16S rRNA and GC-MS/LC–MS a correlation between microbiota (Gardnerella vaginalis), and metabolites (2-hydroxyisovalerate and γ-hydroxybutyrate) was observed and identified the biomarkers for clinical BV [123]. Additionally, shortgun sequencing of vaginal microbiota is a powerful molecular technique that reveals the community profiles, as well as functional potential regarding PTB [124].
In recent years, evidence on maternal interactions with microbial metabolites and associated immune responses contributing to the adverse pregnancy outcomes, including PTB, has emerged [125,126,127]. It has been found that dysbiosis of the vaginal, gut, or placental microbiota and subsequent alterations to secondary metabolite biosynthesis are vital for the onset and progression of infection, inflammation, and pathogenesis of PTL [74,125,127,128]. A previous clinical study revealed significant alterations in lipid metabolism in BV as reflected by the higher levels of 12-hydroxyeicosatetraenoic acid (12-HETE), a signaling eicosanoid mediating inflammatory response pathways, and lower levels of its precursor arachidonate, suggesting bioconversion of arachidonate to 12-HETE by BV-associated microbes [52]. Chorioamnionitis, an inflammatory condition of the fetal membranes (amnion and chorion) usually caused by bacterial infection, is known to contribute to PTB. It has been found that chorioamnionitis membranes are often positive for vaginal organisms, particularly those involved in BV [129]. Alterations in the biosynthesis of secondary metabolites (e.g., phenylpropanoid, stilbenoid, diarylheptanoid, and gingerol) and lipid (glycerolipid, glycerophospholipid, arachidonic acid, and unsaturated fatty acids) metabolism accompanied by a higher abundance of oral commensal bacteria-Streptococcus thermophilus and Fusobacterium sp. are seen in women with chorioamnionitis [130].
Microbial metabolites functionally play an important role in the proliferation, differentiation, and development of epithelial cells, as well as in the maintenance of homeostasis of the immune system [131]. Reports of untargeted metabolites of microbiota generated in the vaginal fluid (formate, methanol, acetone, and TMAO), blood (retinyl palmitate, At-Retinal, 13-cis-Retinoic acid), and targeted metabolites (folate) in the blood reveal a significant association with inflammation, which facilitate PTB cascades [23,73,132]. PTB has also been found to be associated with T-cell activation, which is involved in adaptive immune response [133]. It has been found that glucose and glucose-derived metabolites regulate T-cell activation and signaling through the modulation of particular pathways. For instance, succinate and fumarate, two important metabolites in both the host and microbial processes, are the potent allosteric inhibitors of the 2OG-dependent dioxygenases, which are the members of histone demethylases [134]. Accumulating evidence indicates that epigenetic events, such as histone modifications including methylations are often associated with T-cell activation, differentiation, and commitment [135]. Therefore, it is conceivable that the production and consumption of these metabolites and their transport from the mitochondria to the cytosol facilitating histone methylation dynamics in the nucleus may contribute to the PTB [134].
As aforementioned, several lines of evidence indicate that BV is associated with PTB [136]. BV is manifested by an alteration in the proportion of a particular bacterial population affecting the profile of metabolites in vaginal fluid accompanied by increased cell-shedding from the cervicovaginal epithelium [137]. More specifically, BV is represented by a shift in the vaginal microbial population from the normally Lactobacillus-dominated to a highly complex polymicrobial community characterized by the presence of anaerobic bacteria, such as Gardnerella vaginalis, Atopobium spp., Prevotella spp., and high levels of several biogenic amines (putrescine, cadaverine, and trimethylamine), short-chain fatty acids (especially acetate and succinate), and low concentrations of certain amino acids (tyrosine and glutamate) [138,139,140]. These findings are also in agreement with a previous clinical study that used mass spectrometry to link specific metabolites with particular bacteria detected in the human vagina by broad-range PCR [52]. The report demonstrated dramatic differences in metabolite compositions and concentrations associated with BV by addressing a global metabolomics approach. More specifically, a total of 279 named biochemicals were detected; among them, the levels of 62% of metabolites in women with BV were significantly different from those in women without BV. BV was particularly associated with strong metabolic signatures across multiple pathways influencing amino acid, carbohydrate, and lipid metabolism. Furthermore, unsupervised clustering of metabolites separated subjects with BV from participants without BV. More specifically, women with BV had metabolite profiles characterized by lower concentrations of amino acids and dipeptides, accompanied by higher levels of amino acid catabolites. Such events indicate augmented utilization of amino acids and increased catabolism in BV, supporting the notion that BV-associated bacteria may use amino acids as a source of carbon and nitrogen. This is in contrast to lactobacilli, which are known to metabolize sugars, such as glycogen. Furthermore, in agreement with previous reports described above, this study also detected well-known amines putrescine, cadaverine, and tyramine in women with BV. N-acetylputrescine (a degradation product of putrescine), cadaverine, and tyramine were associated with elevated pH. Such BV-specific signatures were found to be associated with the presence and concentrations of particular vaginal bacteria. The study also revealed that BV-associated bacterial levels were positively correlated with succinate, while lactobacilli were negatively associated [52].
In early pregnancy, permissible colonization by BV-associated pathogens induces secretion of pro-inflammatory cytokines in vaginal epithelial cells, and BV doubles the risk of PTB [51]. Studies have revealed that microbial compositions of the cervicovaginal fluid (CVF) are associated with metabolic profiles in healthy pregnancy [30,38] Reduce lactate is associated with BV, while succinate acts as an immunomodulatory molecule [141]. Lactobacillus abundance has a strong positive association with lactate and, to a lesser extent, with levels of several amino acids, such as isoleucine, leucine, tryptophan, phenylalanine, and aspartate [142]. Lactic acid generated by Lactobacillus species, L. crispatus in particular, acidifies the vaginal environment and thereby strongly prevents the growth of potentially harmful microorganisms [143]. In addition, vitamins play a significant role in the composition of various microbiota, including vitamin A metabolite (retinoic acid) as a key player in embryogenesis, and vitamin D shows an immunomodulatory through TLRs pathway and effect pregnancy [144,145]. Deficiency or efficiency of vitamins (A, or D, etc.), significantly reflect the microbiota dysbiosis, which might directly affect the production of their metabolites and immunomodulation [146,147]. During pregnancy, deficiency of vitamin D (25-hydroxy (OH) and 1, 25-dihydroxy (OH) 2), associated with PTB, while increased concentrations of vitamin A metabolites (retinyl palmitate, At-Retinal, 13-cis-Retinoic acid), also contribute to PTB [75,111].
As mentioned before, TLRs (TLR2, TLR3, TLR4, TLR5, TLR6) play an important role in the inflammatory activation processes by binding to the PAMPs or DAMPs [79]. These molecules serve as upstream mediators of the synthesis of inflammatory cytokines and chemokines in infections/inflammation-induced PTB [148]. The metabolites that belong to PAMPs (endotoxins and exotoxins) activate PRRs such as TLRs and nod-like receptors that are expressed by amnion epithelial cells, intermediate trophoblasts in the chorion, decidual cells, macrophages, and neutrophils. LPS, an endotoxin and an essential component of the outer membrane of Gram-negative bacteria, acts on TLRs, manifesting a strong response to immune systems and an induction of PTB [108,149]. The PRRs induce the pleiotropic NFκB signal transduction pathway which regulates the expression of proinflammatory chemokines (e.g., IL8 and C-C motif ligand 2 (CCL2)), cytokines (e.g., IL1β, IL6, TNFα, IFNγ), prostaglandins, and proteases, leading to activation of the common pathway of parturition [118,125] (Figure 4).
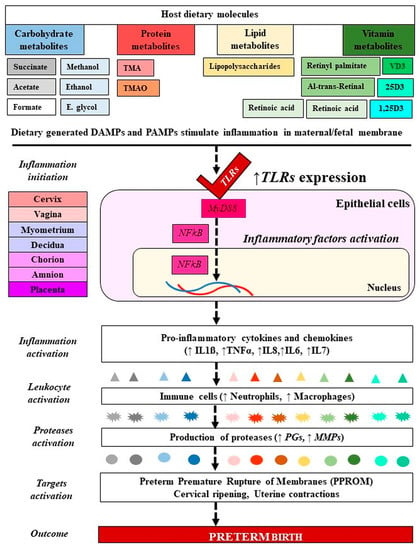
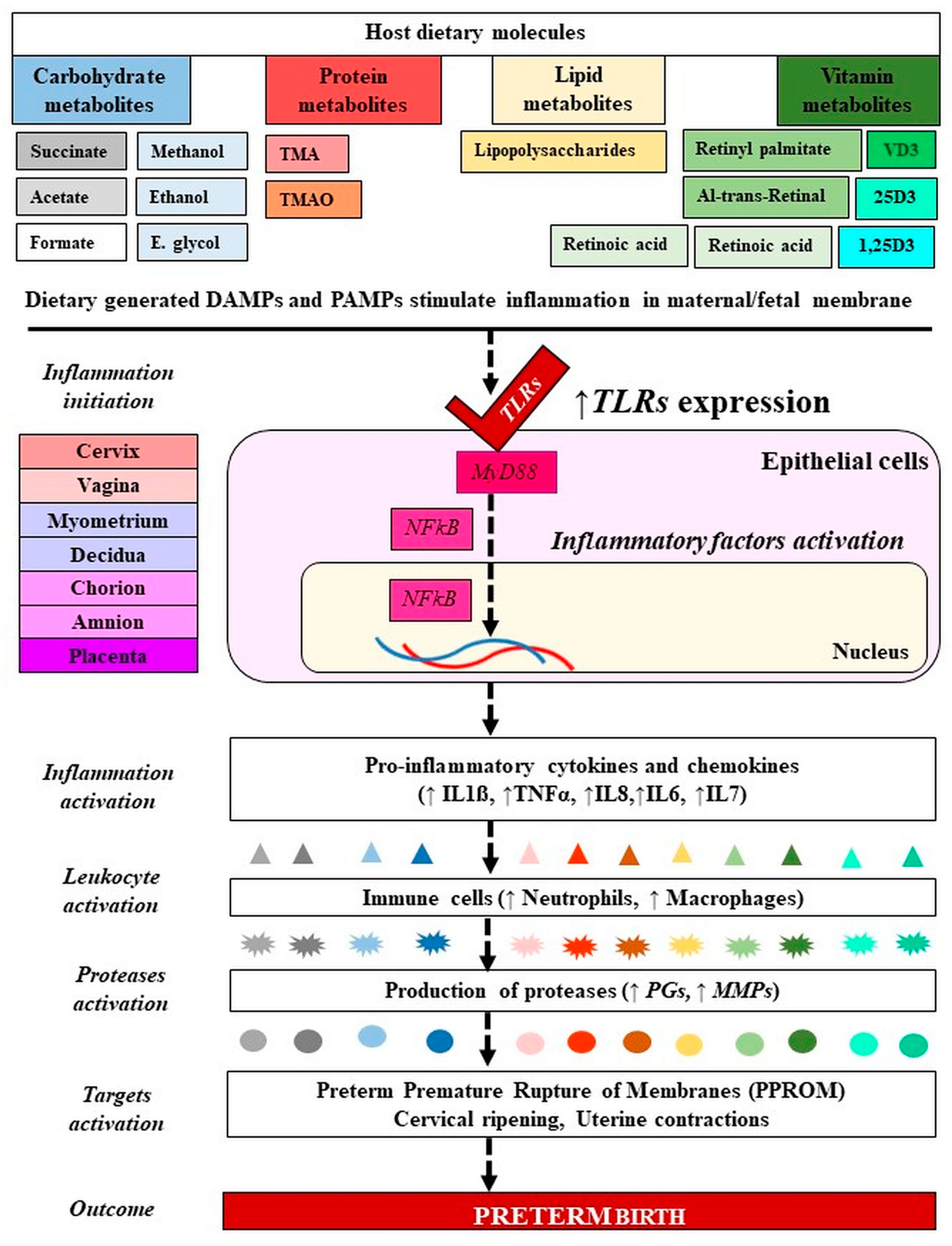
Figure 4.
Molecular mechanism of microbiota-metabolites in preterm birth. Signaling of PAMPs and DAMPs derived from gut microbiota and dietary molecules have activated TLRs in PTB. The blue and gray colors refer to carbohydrates, the red color refers to proteins, the yellow color refers to lipids, and the green color refers to vitamins. From dark color to light color indicate metabolites forms and their generated inflammatory markers. PAMPs: Ppathogen-associated molecular patterns, DAMPs: Damage-associated molecular patterns; VD: Vitamin D; 25D: 25-hydroxy vitamin D; 1,25(OH)2D3: 1α,25-dihydroxy vitamin D. TMA: Trimethylamine, TMAO: Trimethylamine N-oxide; IL: Interleukin; TNF: Tumor necrosis factor; MMP: Matrix metalloproteinase; PGs: Prostaglandins. TLRs: Toll like receptors. Dashed lines indicate several steps, ↓ decreased, ↑ increased level.
5. Microbiota Metabolites Therapeutic Insight in PTB
Specifically, a wide variety of drugs designed to inhibit the contraction of myometrial smooth muscle cells are commonly used to prevent PTB. However, therapies are largely ineffective in delaying the delivery and reducing neonatal mortality substantially [12]. As an alternative, many studies have evaluated the potential of probiotics to restrain PTB as they are known to displace and kill pathogens and modulate the immune response by interfering with the inflammatory cascade that leads to PTL and PTB [150]. Although live lactic acid bacteria (LAB) are commonly used as probiotics to treat a wide range of diseases, they are shown to potentiate PTB [151]. However, novel trends in probiotics supplementation are oriented towards the replacement of live microbes with non-viable microbial extracts and metabolic by-products, the PBM [76]. This new approach reduces health risks associated with the consumption of live bacteria, especially concerning their high immune-stimulating potential [22]. Recent data showed that Lactobacillus postbiotic metabolites can modulate inflammatory pathways and have potential cytoprotective effects against hepatotoxicity [152]. Healthy microbiota is associated with maintaining a low pH (4–4.5) of vaginal fluid by lactate-produced Lactobacilli and maternal physiological factors. Lactobacillus crispatus was shown to dominate, and they have been shown to inhibit the growth of Escherichia coli and biofilm formation by Gardnerella vaginalis [56]. Antimicrobial and immune-modulatory effects of lactic acid and SCFAs produced by vaginal microbiota associated with microbiota eubiosis and BV [153]. LPS increases fetal membrane expression of GPR43, which was significantly higher in women delivering preterm. GPR43-SCFA interactions may represent novel pathways that regulate inflammatory processes involved in labour. In addition, Lactobacilli produce hydrogen peroxide and secrete various factors such as bacteriocins and anti-adhesive molecules that suppress the growth of Gardnerella vaginalis and compete for anaerobic species [63]. Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel have been used to treat symptomatic BV [154]. It has been found that the lactic acid generated by Lactobacillus species, Lactobacillus crispatus in particular, acidifies the vaginal environment and thereby strongly prevents the growth of potentially harmful microorganisms [155]
A healthy dietary pattern containing fibrous food consumption during pregnancy decreases the risk of PTB by increasing beneficial SCFA metabolites and lowering pH, compared with a diet consisting of the consumption of Western-style junk foods [156,157]. Prebiotic and probiotics consumption created a barrier effect and protected against pathogens and metabolites production during the gestational period and birth control [158,159]. In early-life exposure to SCFAs during a critical window, protection against pathogenic microbiota through immunopathologies was revealed [160]. SCFA consists of anti-inflammatory role labour, though modulating inflammatory pathways in fetal membranes through GPR43 and GPR41 RAR-related orphan receptor gamma t–positive (RORγt +) [160]. Suppression of inflammatory pathways by SCFA may be therapeutically beneficial for pregnant women at risk of pathogen-induced PTB [161]. Ritodrine is a phenethylamine (amine) derivative by certain bacteria (Lactobacillus, Clostridium, Pseudomonas, and the family Enterobacteriaceae) and acts as a potent antimicrobial against certain pathogenic strains of Escherichia coli [162]. Phenethylamine derivatives isolated from the strain of Arenibacter nanhaiticus sp. nov. NH36AT consist of antimicrobial activity against Staphylococcus aureus and Bacillus subtilis, Escherichia coli [163]. Lactobacillus iners also produce a moderate level of lactic acid and prevent BV [164]. These microbiota metabolites consist of therapeutic effects on PTB. Consumption of controlled carbohydrate and protein-rich diets decreases the production of toxic microbial metabolites and reduces the risk of PTB. Identification of microbiota metabolites of pathogenic bacteria could be used as a non-invasive, quick, and cost-effective proxy marker for the characterization of the prevailing microbial community and the attendant inflammatory mechanisms of inflammation-induced PTB, as well as uncover potential novel therapies. More specifically, the replacement of live microbes with beneficial post-biotic metabolites might account for a promising therapeutic option to reduce the risks of PTB. Additionally, adequate amounts of vitamins (A, D) supplements reduced the chances of their pathogenic metabolites production and risk of PTB [75,111,165].
6. Conclusions
The interaction of microbial metabolites in the gestational stage is involved in both maternal and neonatal health, and pathogenic metabolites increase the risk of PTB. To understand the molecular mechanisms underlying such events, there is a need to elucidate the role of the microbiota and their metabolites in pregnant women. Comparative analyses of omics markers in maternal and fetal bio-fluid (plasma, cervicovaginal, and amniotic fluid) at different trimesters in a well-defined population could reveal the accurate cellular and molecular mechanisms, predictive biomarkers, and biotics-mediated therapeutic approaches for PTB. The advancement in omics research opens a new horizon to elucidate the precise cellular and molecular events in the mechanistic pathway of PTB.
Author Contributions
Conceptualization, A.A., Y.Y., S.P. and Y.K. validation, S.B., Y.Y., S.P; resources, A.A.; writing—original draft preparation, A.A.; writing—review and editing, S.B., Y.Y., S.P.; supervision, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by BK21 FOUR (Fostering Outstanding Universities for Research) funded by the Ministry of Education (MOE, Korea) and the National Research Foundation of Korea (NRF-2020R1A2C3011850).
Acknowledgments
Thank you to author’s contributions and funding agencies support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beck, S.; Wojdyla, D.; Say, L.; Betran, A.P.; Merialdi, M.; Requejo, J.H.; Rubens, C.; Menon, R.; Van Look, P.F. The worldwide incidence of preterm birth: A systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010, 88, 31–38. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Sohn, S.; Hong, K.; Kim, J.; Kim, R.; Lee, S.; Youn, H.; Kim, Y.J. Maternal, infant, and perinatal mortality statistics and trends in Korea between 2009 and 2017. Obstet. Gynecol. Sci. 2020, 63, 623–630. [Google Scholar] [CrossRef]
- Duthie, L.; Reynolds, R.M. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology 2013, 98, 106–115. [Google Scholar] [CrossRef]
- Bateson, P.; Barker, D.; Clutton-Brock, T.; Deb, D.; D’Udine, B.; Foley, R.A.; Gluckman, P.; Godfrey, K.; Kirkwood, T.; Lahr, M.M.; et al. Developmental plasticity and human health. Nature 2004, 430, 419–421. [Google Scholar] [CrossRef]
- Patel, C.J.; Yang, T.; Hu, Z.; Wen, Q.; Sung, J.; El-Sayed, Y.Y.; Cohen, H.; Gould, J.; Stevenson, D.K.; Shaw, G.M.; et al. Investigation of maternal environmental exposures in association with self-reported preterm birth. Reprod. Toxicol. 2014, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Keiser, A.M.; Salinas, Y.D.; DeWan, A.T.; Hawley, N.L.; Donohue, P.K.; Strobino, D.M. Risks of preterm birth among non-Hispanic black and non-Hispanic white women: Effect modification by maternal age. Paediatr. Perinat. Epidemiol. 2019, 33, 346–356. [Google Scholar] [CrossRef]
- Raglan, O.; MacIntyre, D.A.; Mitra, A.; Lee, Y.S.; Smith, A.; Assi, N.; Nautiyal, J.; Purkayastha, S.; Gunter, M.J.; Gabra, H.; et al. The association between obesity and weight loss after bariatric surgery on the vaginal microbiota. Microbiome 2021, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.S.; Park, S.; You, Y.A.; Jee, Y.; Ansari, A.; Kim, S.M.; Lee, G.; Kim, Y.J. Prenatal Exposure to Alcohol, Tobacco and Coffee: Associated Congenital Complications and Adverse Birth Outcomes. Int. J. Environ. Res. Public Health 2021, 18, 3140. [Google Scholar] [CrossRef]
- Chan, R.L. Biochemical markers of spontaneous preterm birth in asymptomatic women. Bio. Med. Res. Int. 2014, 2014, 164081. [Google Scholar] [CrossRef]
- Bollopragada, S.; Youssef, R.; Jordan, F.; Greer, I.; Norman, J.; Nelson, S. Term labour is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am. J. Obstet. Gynecol. 2009, 200, 104.e1–104.e11. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.K.; Rinaldi, S.F.; Norman, J.E.; Stock, S.J. Preterm birth: Inflammation, fetal injury and treatment strategies. J. Reprod. Immunol. 2017, 1, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, M.; Bella, S.D.; Ferrazzi, E.; Mavilio, D.; Divanovic, S. Inflammation and preterm birth. J. Leukoc. Biol. 2016, 99, 67–78. [Google Scholar] [CrossRef]
- Park, S.; You, Y.A.; Yun, H.; Choi, S.J.; Hwang, H.S.; Choi, S.K.; Lee, S.M.; Kim, Y.J. Cervicovaginal fluid cytokines as predictive markers of preterm birth in symptomatic women. Obstet. Gynecol. Sci. 2020, 63, 455–463. [Google Scholar] [CrossRef]
- Son, G.H.; You, Y.A.; Kwon, E.J.; Lee, K.Y.; Kim, Y.J. Comparative analysis of midtrimester amniotic fluid cytokine levels to predict spontaneous very pre-term birth in patients with cervical insufficiency. Am. J. Reprod. Immunol. 2016, 75, 155–161. [Google Scholar] [CrossRef]
- You, Y.A.; Kwon, E.J.; Hwang, H.S.; Choi, S.J.; Choi, S.K.; Kim, Y.J. Hypermethylation of the VTRNA2-1 Promoter in Maternal Blood is Associated with Preterm Birth. 24 July 2020. Available online: https://www.researchsquare.com/article/rs-20052/v2 (accessed on 31 May 2021).
- Baek, M.Y.; Hwang, H.S.; Park, J.Y.; Chung, J.E.; Lee, K.E.; Lee, G.Y.; Seong, J.W.; Yee, J.; Kim, Y.J.; Gwak, H.S. Association between CACNA1C gene polymorphisms and ritodrine-induced adverse events in preterm labour patients. Eur. J. Clin. Pharmacol. 2017, 73, 837–842. [Google Scholar] [CrossRef]
- Chim, S.S.; Lee, W.S.; Ting, Y.H.; Chan, O.K.; Lee, S.W.; Leung, T.Y. Systematic identification of spontaneous preterm birth-associated RNA transcripts in maternal plasma. PLoS ONE 2012, 7, e34328. [Google Scholar] [CrossRef] [PubMed]
- Nyangahu, D.D.; Jaspan, H.B. Influence of maternal microbiota during pregnancy on infant immunity. Clin. Exp. Immunol. 2019, 198, 47–56. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Claesson, M.J. Gut microbiota: Changes throughout the lifespan from infancy to elderly. Int. Dairy J. 2010, 20, 281–291. [Google Scholar] [CrossRef]
- Bayar, E.; Bennett, P.R.; Chan, D.; Sykes, L.; MacIntyre, D.A. The pregnancy microbiome and preterm birth. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2020; Volume 42, pp. 487–499. [Google Scholar]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef]
- Ansari, A.; Lee, H.; You, Y.A.; Jung, Y.; Park, S.; Kim, S.M.; Hwang, G.S.; Kim, Y.J. Identification of Potential Biomarkers in the Cervicovaginal Fluid by Metabolic Profiling for Preterm Birth. Metabolites 2020, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Costa, S.D.; Zenclussen, A.C. Endocrine factors modulating immune responses in pregnancy. Front. Immunol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; O’Callaghan, L.; McCarthy, J.; Shilling, D.; Scully, P.; Sibartie, S.; Kavanagh, E.; Kirwan, W.O.; Redmond, H.P.; Collins, J.K.; et al. Differential cytokine response from dendritic cells to commensal and pathogenic bacteria in different lymphoid compartments in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, 839–845. [Google Scholar] [CrossRef]
- Perez-Muñoz, M.E.; Arrieta, M.C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 1–19. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The influence of the gut microbiome on host metabolism through the regulation of gut hormone release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyell, D.J.; Robaczewska, A.; Sun, C.L.; Goltsman, D.S.; Wong, R.J.; Shaw, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Nat. Acad. Sci. USA 2015, 112, 11060–11065. [Google Scholar] [CrossRef]
- You, Y.A.; Kwon, E.J.; Choi, S.J.; Hwang, H.S.; Choi, S.K.; Lee, S.M.; Kim, Y.J. Vaginal microbiome profiles of pregnant women in Korea using a 16S metagenomics approach. Am. J. Reprod. Immunol. 2019, 82, e13124. [Google Scholar] [CrossRef]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Van den Veyver, I.; Milosavljevic, A.; et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef]
- Laghi, L.; Zagonari, S.; Patuelli, G.; Zhu, C.; Foschi, C.; Morselli, S.; Pedna, M.F.; Sambri, V.; Marangoni, A. Vaginal metabolic profiles during pregnancy: Changes between first and second trimester. PLoS ONE 2021, 16, e0249925. [Google Scholar] [CrossRef] [PubMed]
- Neu, J. The microbiome during pregnancy and early postnatal life. In Seminars in Fetal and Neonatal Medicine; W.B. Saunders, Ltd., Elsevier: Philadelphia, PA, USA, 2016; Volume 21, pp. 373–379. [Google Scholar]
- Chilton, S.N.; Enos, M.K.; Burton, J.P.; Reid, G. The effects of diet and the microbiome on reproduction and longevity: A comparative review across 5 continents. Nutr. Food Sci. 2015, 5, e364. [Google Scholar] [CrossRef]
- You, Y.A.; Yoo, J.Y.; Kwon, E.J.; Kim, Y.J. Blood microbial communities during pregnancy are associated with preterm birth. Front. Microbiol. 2019, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Song, S.D.; Acharya, K.D.; Zhu, J.E.; Deveney, C.M.; Walther-Antonio, M.R.; Tetel, M.J.; Chia, N. Daily Vaginal Microbiota Fluctuations Associated with Natural Hormonal Cycle, Contraceptives, Diet, and Exercise. Msphere 2020, 5, e00593-20. [Google Scholar] [CrossRef] [PubMed]
- Boris, S.; Barbés, C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000, 2, 543–546. [Google Scholar] [CrossRef]
- Oliver, A.; LaMere, B.; Weihe, C.; Wandro, S.; Lindsay, K.L.; Wadhwa, P.D.; Mills, D.A.; Pride, D.T.; Fiehn, O.; Northen, T.; et al. Cervicovaginal microbiome composition is associated with metabolic profiles in healthy pregnancy. Mbio 2020, 11, e01851-20. [Google Scholar] [CrossRef]
- Calonghi, N.; Parolin, C.; Sartor, G.; Verardi, L.; Giordani, B.; Frisco, G.; Marangoni, A.; Vitali, B. Interaction of vaginal Lactobacillus strains with HeLa cells plasma membrane. Benef. Microbes 2017, 8, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Frisco, G.; Foschi, C.; Giordani, B.; Salvo, M.; Vitali, B.; Marangoni, A.; Calonghi, N. Lactobacillus crispatus BC5 interferes with Chlamydia trachomatis infectivity through integrin modulation in cervical cells. Front. Microbiol. 2018, 9, 2630. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Gajer, P.; Riis, V.; Brown, A.G.; Humphrys, M.S.; Holm, J.B.; Ravel, J. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun. 2019, 10, 1305. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Nat. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Gerson, K.D.; McCarthy, C.; Elovitz, M.A.; Ravel, J.; Sammel, M.D.; Burris, H.H. Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am. J. Obstet. Gynecol. 2020, 222, 491.e1–491.e8. [Google Scholar] [CrossRef]
- Kindinger, L.M.; Bennett, P.R.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; MacIntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef]
- Nath, K.; Sarosy, J.W.; Stylianou, S.P.J.B. Suitability of a unique 16S rRNA gene PCR product as an indicator of Gardnerella vaginalis. Biotechniques 2000, 28, 222–226. [Google Scholar] [CrossRef]
- Nath, K.; Chen, X.; Ahn, K.-S.; Chen, S. Characterization of the 16S rRNA gene V2 region and the rrn operonsof Gardnerella vaginalis. Res. Microbiol. 2000, 151, 747–754. [Google Scholar] [CrossRef]
- Fei, N.; Bruneau, A.; Zhang, X.; Wang, R.; Wang, J.; Rabot, S.; Gérard, P.; Zhao, L. Endotoxin producers overgrowing in human gut microbiota as the causative agents for nonalcoholic fatty liver disease. MBio 2020, 11, e03263-19. [Google Scholar] [CrossRef]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.M.; O’loughlin, J.A.; Jolley, P.; Vigneswaran, R.; McDonald, P.J. Vaginal infection and preterm labour. Int. J. Obstet. Gynaecol. 1991, 98, 427–435. [Google Scholar] [CrossRef]
- Nelson, D.B.; Hanlon, A.; Nachamkin, I.; Haggerty, C.; Mastrogiannis, D.S.; Liu, C.; Fredricks, D.N. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr. Perinat. Epidemiol. 2014, 28, 88–96. [Google Scholar] [CrossRef]
- Srinivasan, S.; Morgan, M.T.; Fiedler, T.L.; Djukovic, D.; Hoffman, N.G.; Raftery, D.; Marrazzo, J.M.; Fredricks, D.N. Metabolic signatures of bacterial vaginosis. MBio 2015, 6, e00204-15. [Google Scholar] [CrossRef]
- Srinivasan, S.; Fredricks, D.N. The human vaginal bacterial biota and bacterial vaginosis. Interdiscip. Perspect. Infect. Dis. 2008, 2008, 750479. [Google Scholar] [CrossRef] [PubMed]
- Koumans, E.H.; Sternberg, M.; Bruce, C.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef]
- Jakobsson, T.; Forsum, U. Lactobacillus iners: A marker of changes in the vaginal flora? J. Clin. Microbiol. 2007, 45, 3145. [Google Scholar] [CrossRef]
- Verstraelen, H.; Verhelst, R.; Claeys, G.; De Backer, E.; Temmerman, M.; Vaneechoutte, M. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol. 2009, 9, 116. [Google Scholar] [CrossRef]
- Wen, A.; Srinivasan, U.; Goldberg, D.; Owen, J.; Marrs, C.F.; Misra, D.; Wing, D.A.; Ponnaluri, S.; Miles-Jay, A.; Bucholz, B.; et al. Selected vaginal bacteria and risk of preterm birth: An ecological perspective. J. Infect. Dis. 2014, 209, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, A.; Yoneda, S.; Yoneda, N.; Yonezawa, R.; Matsubayashi, T.; Seo, G.; Saito, S. Intestinal microbiota is different in women with preterm birth: Results from terminal restriction fragment length polymorphism analysis. PLoS ONE 2014, 9, e111374. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Rho, M.; You, Y.A.; Kwon, E.J.; Kim, M.H.; Kym, S.; Jee, Y.K.; Kim, Y.K.; Kim, Y.J. 16S rRNA gene-based metagenomic analysis reveals differences in bacteria-derived extracellular vesicles in the urine of pregnant and non-pregnant women. Exp. Mol. Med. 2016, 48, e208. [Google Scholar] [CrossRef]
- You, Y.A.; Son, G.H.; Kwon, E.J.; Park, M.H.; Lee, K.Y.; Kim, Y.J. New insight into the analysis of amniotic fluid microflora using 16S rRNA gene sequencing. JMM Case Rep. 2016, 3, e005021. [Google Scholar] [CrossRef][Green Version]
- Lin, D.; Moss, K.; Beck, J.D.; Hefti, A.; Offenbacher, S. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J. Periodontol. 2007, 78, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Oh, D.; Heo, H.; Lee, G.; Kim, S.M.; Ansari, A.; You, Y.A.; Jung, Y.J.; Kim, Y.H.; Lee, M.; et al. Prediction of preterm birth based on machine learning using bacterial risk score in cervicovaginal fluid. Am. J. Reprod. Immunol. 2021, 2021, e13435. [Google Scholar]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.M.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of vaginal bacteria and D-and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: Implications for protection against upper genital tract infections. MBio 2013, 4, e00460-13. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O. The vaginal microenvironment: The physiologic role of lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Son, K.A.; Kim, M.; Kim, Y.M.; Kim, S.H.; Choi, S.J.; Oh, S.Y.; Roh, C.R.; Kim, J.H. Prevalence of vaginal microorganisms among pregnant women according to trimester and association with preterm birth. Obstet. Gynecol. Sci. 2018, 61, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; DiGiulio, D.B.; Goltsman, D.S.; Sun, C.L.; Costello, E.K.; Jeganathan, P.; Biggio, J.R.; Wong, R.J.; Druzin, M.L.; Shaw, G.M.; et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Nat. Acad. Sci. USA 2017, 114, 9966–9971. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 1, 1–19. [Google Scholar]
- Ansari, A.; Bose, S.; Patra, J.K.; Shin, N.R.; Lim, D.W.; Kim, K.W.; Wang, J.H.; Kim, Y.M.; Chin, Y.W.; Kim, H. A Controlled Fermented Samjunghwan Herbal Formula Ameliorates Non-alcoholic Hepatosteatosis in HepG2 Cells and OLETF Rats. Front. Pharmacol. 2018, 9, 596. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You are what you eat—The relationship between diet, microbiota, and metabolic disorders—A review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- He, C.; Cheng, D.; Peng, C.; Li, Y.; Zhu, Y.; Lu, N. High-fat diet induces dysbiosis of gastric microbiota prior to gut microbiota in association with metabolic disorders in mice. Front. Microbiol. 2018, 9, 639. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Lominchar, M.G.; San Juan, C.; Larrosa, M. Microbiota features associated with a high-fat/low-fiber diet in healthy adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
- You, Y.A.; Hwang, S.Y.; Kim, S.M.; Park, S.; Lee, G.I.; Park, S.; Ansari, A.; Lee, J.; Kwon, Y.; Kim, Y.J. Identification of Indicators for Preterm Birth Using Retinoid Metabolites. Metabolites 2021, 11, 443. [Google Scholar] [CrossRef]
- Stafford, G.P.; Parker, J.L.; Amabebe, E.; Kistler, J.; Reynolds, S.; Stern, V.; Paley, M.; Anumba, D.O. Spontaneous preterm birth is associated with differential expression of vaginal metabolites by lactobacilli-dominated microflora. Front. Physiol. 2017, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Denning, P.W. Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: What is the current evidence? Clin. Perinatol. 2013, 40, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Martinez, K.B.; Leone, V.; Chang, E.B. Microbial metabolites in health and disease: Navigating the unknown in search of function. J. Biol. Chem. 2017, 292, 8553–8559. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Voysey, P.A.; Wood, P.M. Methanol and formaldehyde oxidation by an autotrophic nitrifying bacterium. Microbiology 1987, 133, 283–290. [Google Scholar] [CrossRef][Green Version]
- Hyman, M.R.; Wood, P.M. Ethylene oxidation by Nitrosomonas europaea. Arch. Microbiol. 1984, 137, 155–158. [Google Scholar] [CrossRef]
- Di Martino, M.L.; Campilongo, R.; Casalino, M.; Micheli, G.; Colonna, B.; Prosseda, G. Polyamines: Emerging players in bacteria–host interactions. Int. J. Med. Microbiol. 2013, 303, 484–491. [Google Scholar] [CrossRef]
- Bergeron, N.; Williams, P.T.; Lamendella, R.; Faghihnia, N.; Grube, A.; Li, X.; Wang, Z.; Knight, R.; Jansson, J.K.; Hazen, S.L.; et al. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br. J. Nutr. 2016, 116, 2020–2029. [Google Scholar] [CrossRef]
- Rampersaud, R.; Planet, P.J.; Randis, T.M.; Kulkarni, R.; Aguilar, J.L.; Lehrer, R.I.; Ratner, A.J. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. Res. 2011, 193, 1034–1041. [Google Scholar] [CrossRef]
- Cselovszky, J.; Wolf, G.; Hammes, W.P. Production of formate, acetate, and succinate by anaerobic fermentation of Lactobacillus pentosus in the presence of citrate. Appl. Microbiol. Biotechnol. 1992, 37, 94–97. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Taylor, T.D.; Ohno, H.; Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 2012, 3, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Salazar, N.; Binetti, A.; Gueimonde, M.; Alonso, A.; Garrido, P.; Del Rey, C.G.; González, C.; Ruas-Madiedo, P.; Clara, G. Safety and intestinal microbiota modulation by the exopolysaccharide-producing strains Bifidobacterium animalis IPLA R1 and Bifidobacterium longum IPLA E44 orally administered to Wistar rats. Int. J. Food Microbiol. 2011, 144, 342–351. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; Leitch, C.M.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Abdin, A.A.; Saeid, E.M. An experimental study on ulcerative colitis as a potential target for probiotic therapy by Lactobacillus acidophilus with or without “olsalazine”. J. Crohn Colitis 2008, 2, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio 2014, 5, e00889-14. [Google Scholar] [CrossRef]
- Serpa, J.; Caiado, F.; Carvalho, T.; Torre, C.; Gonçalves, L.G.; Casalou, C.; Lamosa, P.; Rodrigues, M.; Zhu, Z.; Lam, E.W.; et al. Butyrate-rich colonic microenvironment is a relevant selection factor for metabolically adapted tumor cells. J. Biol. Chem. 2010, 285, 39211–39223. [Google Scholar] [CrossRef]
- Mackie, R.I.; Gilchrist, F.M. Changes in lactate-producing and lactate-utilizing bacteria in relation to pH in the rumen of sheep during stepwise adaptation to a high-concentrate diet. Appl. Environ. Microbiol. 1979, 38, 422–430. [Google Scholar] [CrossRef]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab. 2016, 24, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Ceperuelo-Mallafré, V.; Keiran, N.; Queipo-Ortuño, M.I.; Bernal, R.; Gomez-Huelgas, R.; Urpi-Sarda, M.; Sabater, M.; Pérez-Brocal, V.; Andrés-Lacueva, C.; et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018, 12, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.; Bockelmann, W.; Meske, D.; de Vrese, M.; Walte, H.G.; Schrezenmeir, J.; Heller, K.J. Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front. Microbiol. 2016, 7, 47. [Google Scholar] [CrossRef]
- Lee, J.; Jang, Y.S.; Choi, S.J.; Im, J.A.; Song, H.; Cho, J.H.; Papoutsakis, E.T.; Bennett, G.N.; Lee, S.Y. Metabolic engineering of Clostridium acetobutylicum ATCC 824 for isopropanol-butanol-ethanol fermentation. Appl. Environ. Microbiol. 2012, 78, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Atsumi, S.; Liao, J.C. Engineered synthetic pathway for isopropanol production in Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 7814–7818. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Choi, J.I.; Woo, H.M. Bioconversion of xylose to ethylene glycol and glycolate in engineered Corynebacterium glutamicum. ACS Omega 2019, 4, 21279–21287. [Google Scholar] [CrossRef]
- Buakhiaw, B.; Sanguanchaipaiwong, V. Effect of media on acetone-butanol-ethanol fermentation by isolated Clostridium spp. Energy Procedia 2017, 138, 864–869. [Google Scholar] [CrossRef]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 2015, 6, e02481-14. [Google Scholar] [CrossRef] [PubMed]
- Monack, D.M. Helicobacter and salmonella persistent infection strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010348. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Atiya Ali, A.M.; Strandvik, B.; Sabel, K.G.; Kilander, C.P.; Strömberg, R.; Yngve, A. Polyamine levels in breast milk are associated with mothers’ dietary intake and are higher in preterm than full-term human milk and formulas. J. Hum. Nutr. Diet. 2014, 27, 459–467. [Google Scholar] [CrossRef]
- Elovitz, M.A.; Wang, Z.; Chien, E.K.; Rychlik, D.F.; Phillippe, M. A new model for inflammation-induced preterm birth: The role of platelet-activating factor and Toll-like receptor-4. Am. J. Pathol. 2003, 163, 2103–2111. [Google Scholar] [CrossRef]
- Power, K.M.; Sanchez-Galan, J.E.; Luskey, G.W.; Koski, K.G.; Burns, D.H. Use of near-infrared spectroscopic analysis of second trimester amniotic fluid to assess preterm births. J. Pregnancy. 2011, 2011, 980985. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; McCulloch, K.M.; Worrell, L.; Vidyasagar, D. Vitamin A deficiency and severe bronchopulmonary dysplasia in very low birth weight infants. Am. J. Perinatol. 1996, 13, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Thota, C.; Menon, R.; Fortunato, S.J.; Brou, L.; Lee, J.-E.; Al-Hendy, A.J.R.S. 1, 25-Dihydroxyvitamin D deficiency is associated with preterm birth in African American and Caucasian women. Reprod Sci. 2014, 21, 244–250. [Google Scholar] [CrossRef]
- Maitre, L.; Fthenou, E.; Athersuch, T.; Coen, M.; Toledano, M.B.; Holmes, E.; Kogevinas, M.; Chatzi, L.; Keun, H.C. Urinary metabolic profiles in early pregnancy are associated with preterm birth and fetal growth restriction in the Rhea mother–Child cohort study. BMC Med. 2014, 12, 110. [Google Scholar] [CrossRef]
- Wolrath, H.; Ståhlbom, B.; Hallén, A.; Forsum, U. Trimethylamine and trimethylamine oxide levels in normal women and women with bacterial vaginosis reflect a local metabolism in vaginal secretion as compared to urine. Apmis 2005, 113, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Hu, H.; Cantonwine, D.E.; Lamadrid-Figueroa, H.; Calafat, A.M.; Ettinger, A.S.; Hernandez-Avila, M.; Loch-Caruso, R.; Téllez-Rojo, M.M. Urinary phthalate metabolites in relation to preterm birth in Mexico City. Environ. Health Perspect. 2009, 117, 1587–1592. [Google Scholar] [CrossRef]
- Diaz, S.O.; Pinto, J.; Graça, G.; Duarte, I.F.; Barros, A.S.; Galhano, E.; Pita, C.; Almeida, M.D.; Goodfellow, B.J.; Carreira, I.M.; et al. Metabolic biomarkers of prenatal disorders: An exploratory NMR metabonomics study of second trimester maternal urine and blood plasma. J. Proteome Res. 2011, 10, 3732–3742. [Google Scholar] [CrossRef]
- Gershuni, V.; Li, Y.; Elovitz, M.; Li, H.; Wu, G.D.; Compher, C.W. Maternal gut microbiota reflecting poor diet quality is associated with spontaneous preterm birth in a prospective cohort study. Am. J. Clin. Nutr. 2021, 113, 602–611. [Google Scholar] [CrossRef]
- Alcon-Giner, C.; Dalby, M.J.; Caim, S.; Ketskemety, J.; Shaw, A.; Sim, K.; Lawson, M.A.; Kiu, R.; Leclaire, C.; Chalklen, L.; et al. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: An observational study. Cell Rep. Med. 2020, 1, 100077. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labour: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Donders, G.G.; Van Calsteren, K.; Bellen, G.; Reybrouck, R.; Van den Bosch, T.; Riphagen, I.; Van Lierde, S. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. Int. J. Obstet. Gynaecol. 2009, 116, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Campisciano, G.; Zanotta, N.; Ricci, G.; Comar, M. The vaginal community state types microbiome-immune network as key factor for bacterial vaginosis and aerobic vaginitis. Front. Microbiol. 2019, 10, 2451. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.; Rulisa, S.; Sumarah, M.; Macklaim, J.M.; Renaud, J.; Bisanz, J.E.; Gloor, G.B.; Reid, G. A multi-platform metabolomics approach identifies highly specific biomarkers of bacterial diversity in the vagina of pregnant and non-pregnant women. Sci. Rep. 2015, 5, 14174. [Google Scholar] [CrossRef]
- Feehily, C.; Crosby, D.; Walsh, C.J.; Lawton, E.M.; Higgins, S.; McAuliffe, F.M.; Cotter, P.D. Shotgun sequencing of the vaginal microbiome reveals both a species and functional potential signature of preterm birth. NPJ Biofilms Microbiomes 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Parris, K.M.; Amabebe, E.; Cohen, M.C.; Anumba, D.O. Placental microbial–metabolite profiles and inflammatory mechanisms associated with preterm birth. J. Clin. Pathol. 2021, 74, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Flaviani, F.; Hezelgrave, N.L.; Kanno, T.; Prosdocimi, E.M.; Chin-Smith, E.; Ridout, A.E.; von Maydell, D.K.; Mistry, V.; Wade, W.G.; Shennan, A.H.; et al. Cervicovaginal microbiota and metabolome predict preterm birth risk in an ethnically diverse cohort. JCI Insight 2021, 2021, 1–28. [Google Scholar]
- Amabebe, E.; Reynolds, S.; Stern, V.; Stafford, G.; Paley, M.; Anumba, D.O. Cervicovaginal fluid acetate: A metabolite marker of preterm birth in symptomatic pregnant women. Front. Med. 2016, 3, 48. [Google Scholar] [CrossRef]
- Klebanoff, M.A.; Brotman, R.M. Treatment of bacterial vaginosis to prevent preterm birth. Lancet 2018, 392, 2141–2142. [Google Scholar] [CrossRef]
- Antony, K.M.; Ma, J.; Mitchell, K.B.; Racusin, D.A.; Versalovic, J.; Aagaard, K. The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am. J. Obstet. Gynecol. 2015, 212, 653-e1. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Himes, K.P.; Venkataramanan, R.; Chen, J.Y.; Evans, R.W.; Meyer, J.L.; Simhan, H.N. Maternal serum folate species in early pregnancy and risk of preterm birth. Am. J. Clin. Nutr. 2010, 92, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Shin, Y.; Kim, S.M.; You, Y.A.; Kim, D.; Hwang, D.; Kim, Y.J. Integrative analysis of transcriptomic data for identification of T-cell activation-related mRNA signatures indicative of preterm birth. Sci. Rep. 2021, 11, 2392. [Google Scholar] [CrossRef]
- Shyer, J.A.; Flavell, R.A.; Bailis, W. Metabolic signaling in T cells. Cell Res. 2020, 30, 649–659. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, Q. Epigenetic alterations in cellular immunity: New insights into autoimmune diseases. Cell. Physiol. Biochem. 2017, 41, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L.; Nugent, R.P.; Eschenbach, D.A.; Krohn, M.A.; Gibbs, R.S.; Martin, D.H.; Cotch, M.F.; Edelman, R.; Pastorek, J.G.; Rao, A.V.; et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N. Engl. J. Med. 1995, 333, 1737–1742. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Gajer, P.; Brotman, R.M.; Ravel, J. Asymptomatic Bacterial Vaginosis is Associated with Depletion of Mature Superficial Cells Shed from the Vaginal Epithelium. Front. Cell. Infect. Microbiol. 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Vitali, B.; Cruciani, F.; Picone, G.; Parolin, C.; Donders, G.; Laghi, L. Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2367–2376. [Google Scholar] [CrossRef]
- Watson, E.; Reid, G. Metabolomics as a clinical testing method for the diagnosis of vaginal dysbiosis. Am. J. Reprod. Immunol. 2018, 80, e12979. [Google Scholar] [CrossRef] [PubMed]
- Parolin, C.; Foschi, C.; Laghi, L.; Zhu, C.; Banzola, N.; Gaspari, V.; D’Antuono, A.; Giordani, B.; Severgnini, M.; Consolandi, C.; et al. Insights into vaginal bacterial communities and metabolic profiles of Chlamydia trachomatis infection: Positioning between eubiosis and dysbiosis. Front. Microbiol. 2018, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Al-Mushrif, S.; Eley, A.; Jones, B.M. Inhibition of chemotaxis by organic acids from anaerobes may prevent a purulent response in bacterial vaginosis. J. Med. Microbiol. 2000, 49, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Ceccarani, C.; Foschi, C.; Parolin, C.; D’Antuono, A.; Gaspari, V.; Consolandi, C.; Laghi, L.; Camboni, T.; Vitali, B.; Severgnini, M.; et al. Diversity of vaginal microbiome and metabolome during genital infections. Sci. Rep. 2019, 9, 14095. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Cyprian, F.; Lefkou, E.; Varoudi, K.; Girardi, G. Immunomodulatory effects of vitamin D in pregnancy and beyond. Front. Immunol. 2019, 10, 2739. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Silva, H.; Araújo-Silva, H.; Correia-Pinto, J.; Moura, R.S. Retinoic acid: A key regulator of lung development. Biomolecules 2020, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.; Ferreira, S.R.G.J.M. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism. 2017, 69, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Grizotte-Lake, M.; Zhong, G.; Duncan, K.; Kirkwood, J.; Iyer, N.; Smolenski, I.; Isoherranen, N.; Vaishnava, S.J.I. Commensals suppress intestinal epithelial cell retinoic acid synthesis to regulate interleukin-22 activity and prevent microbial dysbiosis. Immunity 2018, 49, 1103–1115.e6. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Severson, E.M.; Verstraeten, B.S.; Ng, J.W.; McCreary, J.K.; Metz, G.A. Allostatic load and preterm birth. Int. J. Mol. Sci. 2015, 16, 29856–29874. [Google Scholar] [CrossRef]
- Salminen, A.; Paananen, R.; Vuolteenaho, R.; Metsola, J.; Ojaniemi, M.; Autio-Harmainen, H.; Hallman, M. Maternal endotoxin-induced preterm birth in mice: Fetal responses in toll-like receptors, collectins, and cytokines. Pediatri. Res. 2008, 63, 280–286. [Google Scholar] [CrossRef]
- Piso, B.; Zechmeister-Koss, I.; Winkler, R. Antenatal interventions to reduce preterm birth: An overview of Cochrane Systematic Reviews. BMC Res. Notes 2014, 7, 1–10. [Google Scholar] [CrossRef]
- Ljungh, A.; Wadstrom, T. Lactic acid bacteria as probiotics. Curr. Issues Intest. Microbiol. 2006, 7, 73–90. [Google Scholar]
- Kanmani, P.; Ansari, A.; Villena, J.; Kim, H. Immunobiotics beneficially modulate TLR4 signaling triggered by lipopolysaccharide and reduce hepatic steatosis in vitro. J. Immunol. Res. 2019, 2019, 3876896. [Google Scholar] [CrossRef]
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef]
- Anukam, K.; Osazuwa, E.; Ahonkhai, I.; Ngwu, M.; Osemene, G.; Bruce, A.W.; Reid, G. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: Randomized, double-blind, placebo controlled trial. Microbes Infect. 2006, 8, 1450–1454. [Google Scholar] [CrossRef]
- Hallén, A.; Jarstrand, C.O.; Påhlson, C. Treatment of bacterial vaginosis with lactobacilli. Sex. Transm. Dis. 1992, 19, 146–148. [Google Scholar]
- Martin, C.L.; Sotres-Alvarez, D.; Siega-Riz, A.M. Maternal dietary patterns during the second trimester are associated with preterm birth. Nutr. J. 2015, 145, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.S.; He, J.R.; Chen, Q.; Lu, J.; Wei, X.; Zhou, Q.; Chan, F.; Zhang, L.; Chen, N.; Qiu, L.; et al. Maternal dietary patterns during pregnancy and preterm delivery: A large prospective cohort study in China. Nutr. J. 2018, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Alfirevic, Z.; Neilson, J.P. Probiotics for preventing preterm labour. Cochrane Database Syst. Rev. 2007, 1, CD005941. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.; Underwood, M.A. Prenatal and postnatal administration of prebiotics and probiotics. In Seminars in Fetal and Neonatal Medicine; WB Saunders Ltd., Elsevier: Philadelphia, PA, USA, 2017; Volume 22, pp. 284–289. [Google Scholar]
- Jetten, A.M.; Takeda, Y.; Slominski, A.; Kang, H.S. Retinoic acid-related orphan receptor γ (RORγ): Connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr. Oppn. Toxicol. 2018, 8, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Voltolini, C.; Battersby, S.; Etherington, S.L.; Petraglia, F.; Norman, J.E.; Jabbour, H.N. A novel antiinflammatory role for the short-chain fatty acids in human labour. Endocrinology 2012, 153, 395–403. [Google Scholar] [CrossRef]
- Lynnes, T.; Horne, S.M.; Prüß, B.M. β-phenylethylamine as a novel nutrient treatment to reduce bacterial contamination due to Escherichia coli O157: H7 on beef meat. Meat Sci. 2014, 96, 165–171. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Tang, X.; Wang, C.; Lian, Y.; Shao, Z.; Yao, X.; Gao, H. New phenethylamine derivatives from Arenibacter nanhaiticus sp. nov. NH36A T and their antimicrobial activity. J. Antibiot. 2013, 66, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or foe? Trends Microbiol. 2017, 25, 182–191. [Google Scholar] [CrossRef]
- Zhou, S.S.; Tao, Y.H.; Huang, K.; Zhu, B.B.; Tao, F.B. Vitamin D and risk of preterm birth: Up-to-date meta-analysis of randomized controlled trials and observational studies. J. Obstet. Gynaecol. Res. 2017, 43, 247–256. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).