Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches
Abstract
:1. Introduction
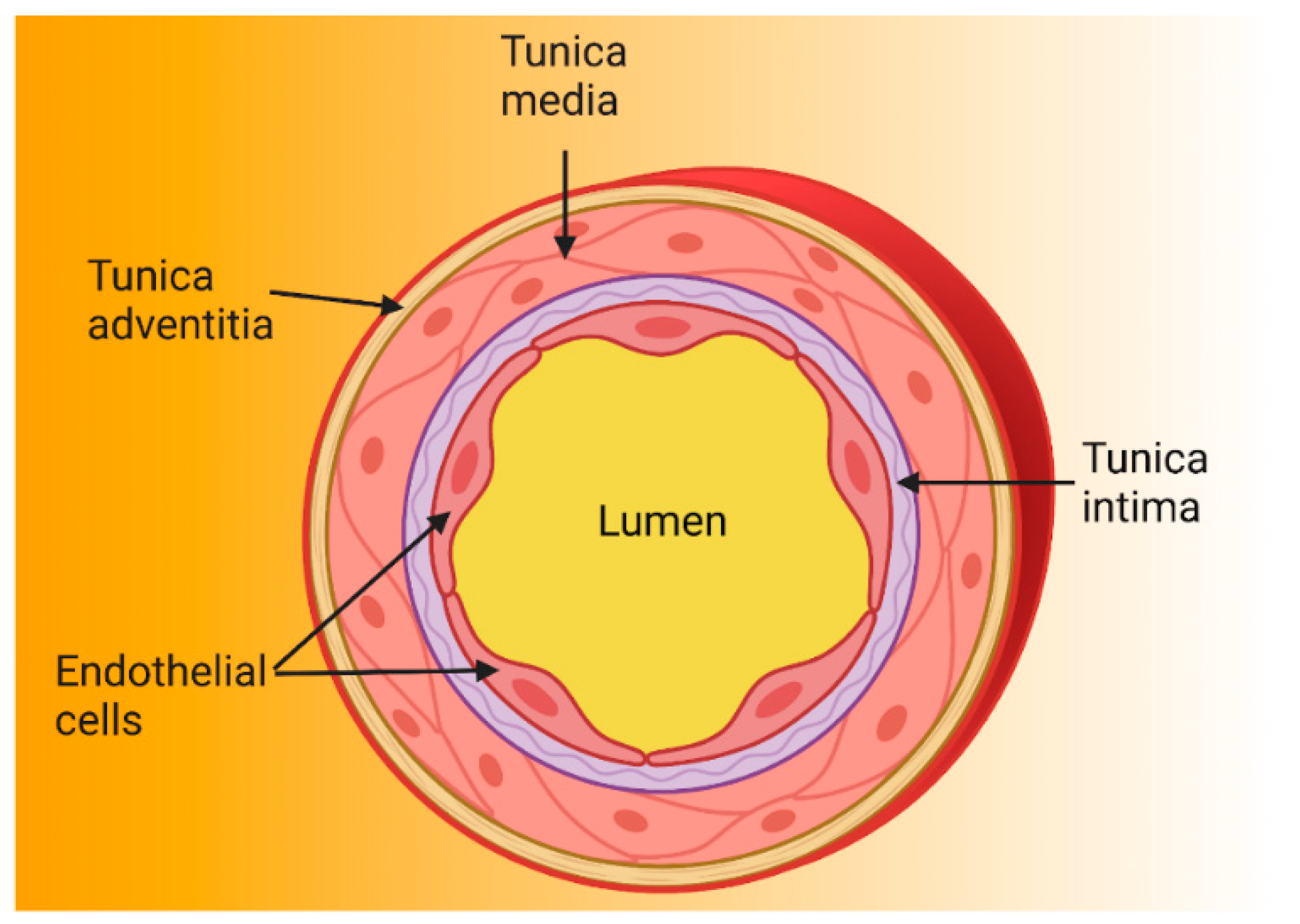
2. The Endothelium
2.1. Function as a Selective Barrier
2.2. Regulation of Haemostasis and Thrombosis
2.3. Regulation of Vascular Tone
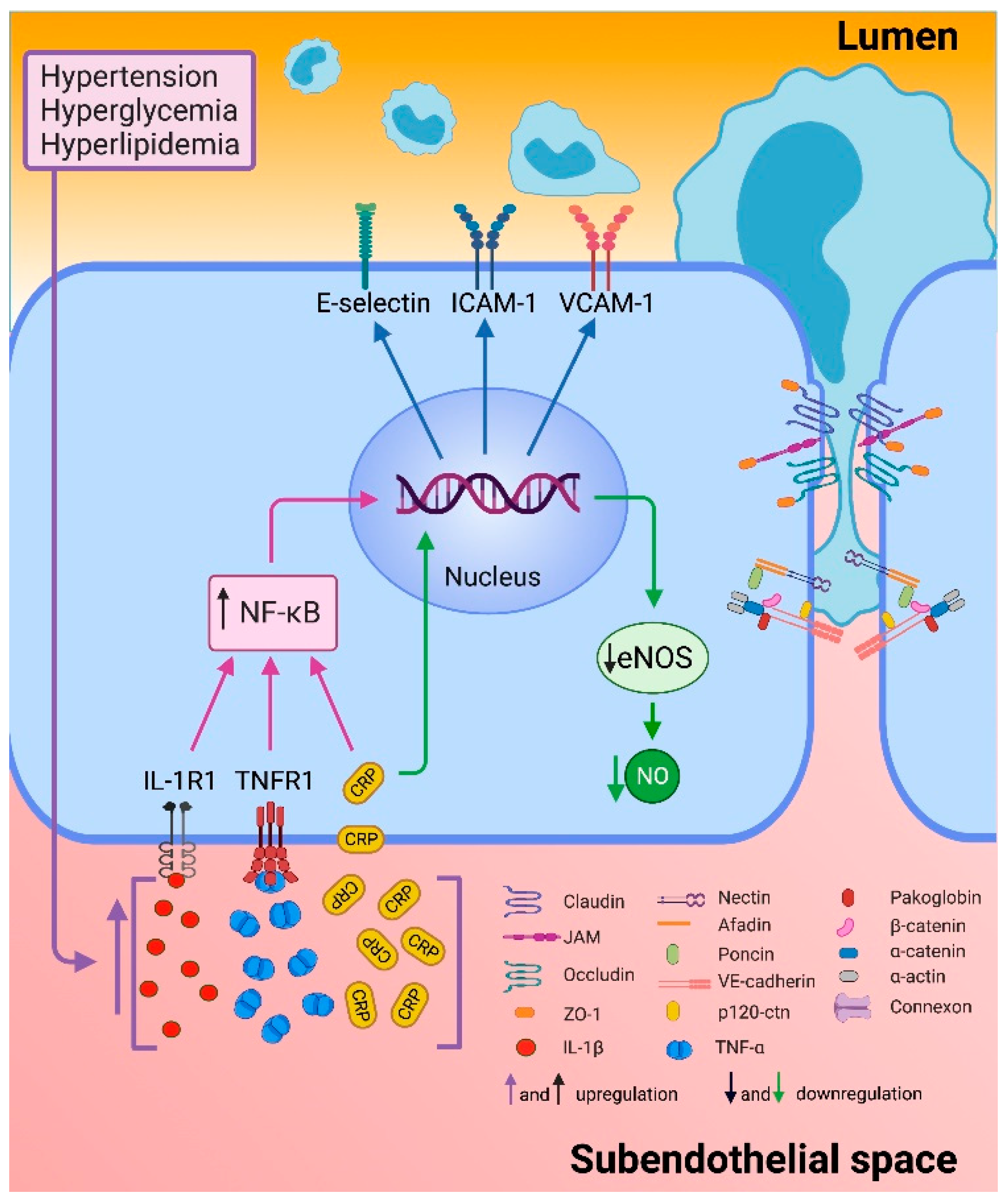
2.4. Endothelial Dysfunction
3. Biomarkers
3.1. Traditional Biomarkers
3.1.1. Acute-Phase Proteins
3.1.2. Cytokines
3.1.3. Cell Adhesion Molecules (CAM)
3.1.4. Cellular Markers
3.1.5. Others
3.2. Novel Potential Biomarkers
3.3. Therapeutic Strategies by Prevent Endothelial Dysfunction
3.3.1. Pharmacological Therapy
3.3.2. Non-Pharmacological Therapies
Lifestyle Modifications
Antioxidant Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Las Principales Causas de Defunción OMS. 2018. Available online: https://www.who.int/es/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 16 February 2019).
- Gao, W.; Liu, H.; Yuan, J.; Wu, C.; Huang, D.; Ma, Y.; Zhu, J.; Ma, L.; Guo, J.; Shi, H.; et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappaB pathway. J. Cell. Mol. Med. 2016, 20, 2318–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero-Fernandez, B.; Gomez-Bris, R.; Somovilla-Crespo, B.; Gonzalez-Granado, J.M. Immunobiology of Atherosclerosis: A Complex Net of Interactions. Int. J. Mol. Sci. 2019, 20, 5293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Chiu, J.-J. Atherosclerosis and flow: Roles of epigenetic modulation in vascular endothelium. J. Biomed. Sci. 2019, 26, 56. [Google Scholar] [CrossRef] [PubMed]
- Strohbach, A.; Pennewitz, M.; Glaubitz, M.; Palankar, R.; Gross, S.; Lorenz, F.; Materzok, I.; Rong, A.; Busch, M.C.; Felix, S.B.; et al. The apelin receptor influences biomechanical and morphological properties of endothelial cells. J. Cell. Physiol. 2018, 233, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Boen, J.R.A.; Gevaert, A.B.; De Keulenaer, G.W.; Van Craenenbroeck, E.M.; Segers, V.F.M. The role of endothelial miRNAs in myocardial biology and disease. J. Mol. Cell. Cardiol. 2020, 138, 75–87. [Google Scholar] [CrossRef]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of endothelial dysfunction in cardiovascular diseases: The link between inflammation and hydrogen sulfide. Front. Pharmacol. 2019, 10, 1568. [Google Scholar] [CrossRef] [Green Version]
- Medina-Leyte, D.J.; Domínguez-Pérez, M.; Mercado, I.; Villarreal-Molina, M.T.; Jacobo-Albavera, L. Use of Human Umbilical Vein Endothelial Cells (HUVEC) as a Model to Study Cardiovascular Disease: A Review. Appl. Sci. 2020, 10, 938. [Google Scholar] [CrossRef] [Green Version]
- Florey, L. The endothelial cell. Br. Med. J. 1966, 2, 487–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Triggle, C.R.; Samuel, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012, 90, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Der ateromatose prozess der arterien. Wien. Med. Wochenschr. 1856, 6, 825–827. [Google Scholar]
- Gimbrone, M.A., Jr.; Alexander, R.W. Angiotensin II stimulation of prostaglandin production in cultured human vascular endothelium. Science 1975, 189, 219–220. [Google Scholar] [CrossRef]
- Weksler, B.B.; Marcus, A.J.; Jaffe, E.A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc. Natl. Acad. Sci. USA 1977, 74, 3922–3926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncada, S.; Herman, A.G.; Higgs, E.A.; Vane, J.R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb. Res. 1977, 11, 323–344. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ordovas, J.M.; Auger, K.R.; Robbins, A.H.; Birinyi, L.K.; Dinarello, C.A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am. J. Pathol. 1986, 124, 179–185. [Google Scholar] [PubMed]
- Vane, J.R. The Croonian Lecture, 1993. The endothelium: Maestro of the blood circulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 343, 225–246. [Google Scholar] [PubMed] [Green Version]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Fuchgott, R.F. Studies on relaxation of rabbit aorta by sodium nitrite: The basis for the proposal that the acid-activatable inhibitory factor from bovine retractor penis is inorganic nitrite and the endothelium-derived relaxing factor is nitric oxide. In Vasodilatation Vascular Smooth Muscle, Peptides Autonomic Nerves, and Endothelium; Vanhoutte, P., Ed.; Raven Press: New York City, NY, USA, 1988; pp. 401–414. [Google Scholar]
- Komori, K.; Vanhoutte, P.M. Endothelium-derived hyperpolarizing factor. Blood Vessel. 1990, 27, 238–245. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Botelho, L.H. Endothelins. FASEB J. 1991, 5, 2713–2720. [Google Scholar] [CrossRef]
- De Graaf, J.; Banga, J.; Moncada, S.; Palmer, R.; de Groot, P.; Sixma, J. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation 1992, 85, 2284–2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnittler, H.J. Structural and functional aspects of intercellular junctions in vascular endothelium. Basic Res. Cardiol. 1998, 93 (Suppl. 3), 30–39. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.L.; Thurston, G. Mechanics of endothelial cell architecture and vascular permeability. Crit. Rev. Biomed. Eng. 2001, 29, 247–278. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial dysfunction and hypertension. In Hypertension: From Basic Research to Clinical Practice; Springer: Berlin/Heidelberg, Germany, 2016; pp. 511–540. [Google Scholar]
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef]
- Lampugnani, M.G. Endothelial cell-to-cell junctions: Adhesion and signaling in physiology and pathology. Cold Spring Harb. Perspect. Med. 2012, 2, a006528. [Google Scholar] [CrossRef] [Green Version]
- Pries, A.R.; Secomb, T.W.; Gaehtgens, P. The endothelial surface layer. Pflug. Arch. 2000, 440, 653–666. [Google Scholar] [CrossRef]
- van den Berg, B.M.; Nieuwdorp, M.; Stroes, E.S.; Vink, H. Glycocalyx and endothelial (dys) function: From mice to men. Pharmacol. Rep. 2006, 58, 75–80. [Google Scholar] [PubMed]
- Buonassisi, V. Sulfated mucopolysaccharide synthesis and secretion in endothelial cell cultures. Exp. Cell Res. 1973, 76, 363–368. [Google Scholar] [CrossRef]
- Gerrity, R.G.; Richardson, M.; Somer, J.B.; Bell, F.P.; Schwartz, C.J. Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. Am. J. Pathol. 1977, 89, 313–334. [Google Scholar]
- Baldwin, A.L.; Winlove, C.P. Effects of perfusate composition on binding of ruthenium red and gold colloid to glycocalyx of rabbit aortic endothelium. J. Histochem. Cytochem. 1984, 32, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Simionescu, M.; Simionescu, N.; Palade, G.E. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J. Cell Biol. 1975, 67, 863–885. [Google Scholar] [CrossRef] [Green Version]
- Dejana, E.; Corada, M.; Lampugnani, M.G. Endothelial cell-to-cell junctions. FASEB J. 1995, 9, 910–918. [Google Scholar] [CrossRef]
- Simionescu, M.; Antohe, F. Functional ultrastructure of the vascular endothelium: Changes in various pathologies. In The Vascular Endothelium I; Springer: Berlin/Heidelberg, Germany, 2006; pp. 41–69. [Google Scholar]
- Nieuwdorp, M.; Meuwese, M.C.; Vink, H.; Hoekstra, J.B.; Kastelein, J.J.; Stroes, E.S. The endothelial glycocalyx: A potential barrier between health and vascular disease. Curr. Opin. Lipidol. 2005, 16, 507–511. [Google Scholar] [CrossRef]
- Zhang, X.; Sessa, W.C.; Fernandez-Hernando, C. Endothelial Transcytosis of Lipoproteins in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Rahimi, N. Defenders and Challengers of Endothelial Barrier Function. Front. Immunol. 2017, 8, 1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, K.Y.Y.; Fairn, G.D.; Lee, W.L. Transcellular vesicular transport in epithelial and endothelial cells: Challenges and opportunities. Traffic 2018, 19, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, S.M.; Sugiyama, M.G.; Fung, K.Y.; Gao, Y.; Wang, C.; Levy, A.S.; Azizi, P.; Roufaiel, M.; Zhu, S.N.; Neculai, D.; et al. A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis. Cardiovasc. Res. 2015, 108, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, L.; Cavelier, C.; Fuchs, S.; Schlüter, M.A.; Völker, W.; von Eckardstein, A. Binding, internalization and transport of apolipoprotein AI by vascular endothelial cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2006, 1761, 186–194. [Google Scholar] [CrossRef]
- Cavelier, C.; Rohrer, L.; Von Eckardstein, A. ATP-Binding cassette transporter A1 modulates apolipoprotein AI transcytosis through aortic endothelial cells. Circ. Res. 2006, 99, 1060–1066. [Google Scholar] [CrossRef] [Green Version]
- Vaisman, B.L.; Demosky, S.J.; Stonik, J.A.; Ghias, M.; Knapper, C.L.; Sampson, M.L.; Dai, C.; Levine, S.J.; Remaley, A.T. Endothelial expression of human ABCA1 in mice increases plasma HDL cholesterol and reduces diet-induced atherosclerosis. J. Lipid Res. 2012, 53, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerterp, M.; Tsuchiya, K.; Tattersall, I.W.; Fotakis, P.; Bochem, A.E.; Molusky, M.M.; Ntonga, V.; Abramowicz, S.; Parks, J.S.; Welch, C.L.; et al. Deficiency of ATP-Binding Cassette Transporters A1 and G1 in Endothelial Cells Accelerates Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1328–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatikos, A.; Dronadula, N.; Ng, P.; Palmer, D.; Knight, E.; Wacker, B.K.; Tang, C.; Kim, F.; Dichek, D.A. ABCA1 Overexpression in Endothelial Cells In Vitro Enhances ApoAI-Mediated Cholesterol Efflux and Decreases Inflammation. Hum. Gene Ther. 2019, 30, 236–248. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Hinsbergh, V.W. Endothelium—Role in Regulation of Coagulation and Inflammation. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 93–106. [Google Scholar]
- Kirsch, J.; Schneider, H.; Pagel, J.-I.; Rehberg, M.; Singer, M.; Hellfritsch, J.; Chillo, O.; Schubert, K.M.; Qiu, J.; Pogoda, K. Endothelial dysfunction, and a prothrombotic, proinflammatory phenotype is caused by loss of mitochondrial thioredoxin reductase in endothelium. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1891–1899. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; He, S.; Sun, X.; Franck, G.; Deng, Y.; Yang, D.; Haemmig, S.; Wara, A.K.; Icli, B.; Li, D.; et al. MicroRNA-181b inhibits thrombin-mediated endothelial activation and arterial thrombosis by targeting caspase recruitment domain family member 10. FASEB J. 2016, 30, 3216–3226. [Google Scholar] [CrossRef] [Green Version]
- Yau, J.W.; Singh, K.K.; Hou, Y.; Lei, X.; Ramadan, A.; Quan, A.; Teoh, H.; Kuebler, W.M.; Al-Omran, M.; Yanagawa, B.; et al. Endothelial-specific deletion of autophagy-related 7 (ATG7) attenuates arterial thrombosis in mice. J. Thorac. Cardiovasc. Surg. 2017, 154, 978–988.e1. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Hu, Y.; Jiang, M.; Wang, F.; Gong, G. Effect of Autophagy Regulated by Sirt1/FoxO1 Pathway on the Release of Factors Promoting Thrombosis from Vascular Endothelial Cells. Int. J. Mol. Sci. 2019, 20, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, S.S. Regulation of blood flow in the microcirculation. Microcirculation 2005, 12, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Khaddaj Mallat, R.; Mathew John, C.; Kendrick, D.J.; Braun, A.P. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit. Rev. Clin. Lab. Sci. 2017, 54, 458–470. [Google Scholar] [CrossRef]
- Heathcote, H.R.; Lee, M.D.; Zhang, X.; Saunter, C.D.; Wilson, C.; McCarron, J.G. Endothelial TRPV4 channels modulate vascular tone by Ca(2+) -induced Ca(2+) release at inositol 1,4,5-trisphosphate receptors. Br. J. Pharmacol. 2019, 176, 3297–3317. [Google Scholar] [CrossRef] [Green Version]
- Iring, A.; Jin, Y.J.; Albarran-Juarez, J.; Siragusa, M.; Wang, S.; Dancs, P.T.; Nakayama, A.; Tonack, S.; Chen, M.; Kunne, C.; et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Investig. 2019, 129, 2775–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacol. Ther. 2018, 192, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.V.G.; de Figueiredo, R.C.; Rios, D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3458. [Google Scholar] [CrossRef] [Green Version]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Osman, R.; L’Allier, P.L.; Elgharib, N.; Tardif, J.C. Critical appraisal of C-reactive protein throughout the spectrum of cardiovascular disease. Vasc. Health Risk Manag. 2006, 2, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodriguez Guzman, R.; Centofanti, F.; Doldo, E.; Cespedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 483–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Victor, V.M.; Rocha, M.; Sola, E.; Banuls, C.; Garcia-Malpartida, K.; Hernandez-Mijares, A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr. Pharm. Des. 2009, 15, 2988–3002. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon Dos Santos, J.; Quadros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and biomarkers of endothelium: When something is rotten in the state. Oxid. Med. Cell Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Maruhashi, T.; Noma, K.; Kihara, Y. Oxidative stress and endothelial dysfunction: Clinical evidence and therapeutic implications. Trends Cardiovasc. Med. 2014, 24, 165–169. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: Oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Jenny, N.S.; Olson, N.C.; Allison, M.A.; Rifkin, D.E.; Daniels, L.B.; de Boer, I.H.; Wassel, C.L.; Tracy, R.P. Biomarkers of Key Biological Pathways in CVD. Glob. Heart 2016, 11, 327–336 e3. [Google Scholar] [CrossRef] [Green Version]
- Kura, B.; Szeiffova Bacova, B.; Kalocayova, B.; Sykora, M.; Slezak, J. Oxidative Stress-Responsive MicroRNAs in Heart Injury. Int. J. Mol. Sci. 2020, 21, 358. [Google Scholar] [CrossRef] [Green Version]
- Lyngbakken, M.N.; Myhre, P.L.; Rosjo, H.; Omland, T. Novel biomarkers of cardiovascular disease: Applications in clinical practice. Crit. Rev. Clin. Lab. Sci. 2019, 56, 33–60. [Google Scholar] [CrossRef]
- Ruparelia, N.; Choudhury, R. Inflammation and atherosclerosis: What is on the horizon? Heart 2020, 106, 80–85. [Google Scholar] [CrossRef]
- Antoniades, C.; Demosthenous, M.; Tousoulis, D.; Antonopoulos, A.S.; Vlachopoulos, C.; Toutouza, M.; Marinou, K.; Bakogiannis, C.; Mavragani, K.; Lazaros, G.; et al. Role of asymmetrical dimethylarginine in inflammation-induced endothelial dysfunction in human atherosclerosis. Hypertension 2011, 58, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Kharbanda, R.K.; Walton, B.; Allen, M.; Klein, N.; Hingorani, A.D.; MacAllister, R.J.; Vallance, P. Prevention of inflammation-induced endothelial dysfunction: A novel vasculo-protective action of aspirin. Circulation 2002, 105, 2600–2604. [Google Scholar] [CrossRef] [Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Wang, C.H.; Li, S.H.; Dumont, A.S.; Fedak, P.W.; Badiwala, M.V.; Dhillon, B.; Weisel, R.D.; Li, R.K.; Mickle, D.A.; et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation 2002, 106, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Pasceri, V.; Willerson, J.T.; Yeh, E.T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation 2000, 102, 2165–2168. [Google Scholar] [CrossRef] [PubMed]
- Bisoendial, R.J.; Boekholdt, S.M.; Vergeer, M.; Stroes, E.S.; Kastelein, J.J. C-reactive protein is a mediator of cardiovascular disease. Eur. Heart J. 2010, 31, 2087–2091. [Google Scholar] [CrossRef]
- Sara, J.D.S.; Prasad, M.; Zhang, M.; Lennon, R.J.; Herrmann, J.; Lerman, L.O.; Lerman, A. High-sensitivity C-reactive protein is an independent marker of abnormal coronary vasoreactivity in patients with non-obstructive coronary artery disease. Am. Heart J. 2017, 190, 1–11. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Bradwin, G.; Hasan, A.A.; Rifai, N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: Secondary analyses from the Cardiovascular Inflammation Reduction Trial. Eur. Heart J. 2020, 41, 2952–2961. [Google Scholar] [CrossRef] [Green Version]
- Luyendyk, J.P.; Schoenecker, J.G.; Flick, M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019, 133, 511–520. [Google Scholar] [CrossRef]
- Danik, J.S.; Pare, G.; Chasman, D.I.; Zee, R.Y.; Kwiatkowski, D.J.; Parker, A.; Miletich, J.P.; Ridker, P.M. Novel loci, including those related to Crohn disease, psoriasis, and inflammation, identified in a genome-wide association study of fibrinogen in 17 686 women: The Women’s Genome Health Study. Circ. Cardiovasc. Genet. 2009, 2, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarnell, J.; McCrum, E.; Rumley, A.; Patterson, C.; Salomaa, V.; Lowe, G.; Evans, A. Association of European population levels of thrombotic and inflammatory factors with risk of coronary heart disease: The Monica Optional Haemostasis Study. Eur. Heart J. 2005, 26, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Reinhart, W.H. Fibrinogen-marker or mediator of vascular disease? Vasc. Med. 2003, 8, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, N.; Tousoulis, D.; Siasos, G.; Stefanadis, C. Is fibrinogen a marker of inflammation in coronary artery disease. Hellenic. J. Cardiol. 2010, 51, 1–9. [Google Scholar]
- Tousoulis, D.; Papageorgiou, N.; Androulakis, E.; Briasoulis, A.; Antoniades, C.; Stefanadis, C. Fibrinogen and cardiovascular disease: Genetics and biomarkers. Blood Rev. 2011, 25, 239–245. [Google Scholar] [CrossRef]
- Buljubasic, N.; Akkerhuis, K.M.; Cheng, J.M.; Oemrawsingh, R.M.; Garcia-Garcia, H.M.; de Boer, S.P.; Regar, E.; van Geuns, R.M.; Serruys, P.W.; Boersma, E.; et al. Fibrinogen in relation to degree and composition of coronary plaque on intravascular ultrasound in patients undergoing coronary angiography. Coron. Artery Dis. 2017, 28, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Lu, Q.; Zhu, W.J.; Wang, T.Z.; Du, Y.; Bai, L. Correlations of degree of coronary artery stenosis with blood lipid, CRP, Hcy, GGT, SCD36 and fibrinogen levels in elderly patients with coronary heart disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9582–9589. [Google Scholar] [PubMed]
- Wang, J.; Jia, L.; Li, X.; Jin, S.; Li, X.; Liu, F.; Shan, C.; Zhang, Y.; Yang, Y. New Insights into the Association between Fibrinogen and Coronary Atherosclerotic Plaque Vulnerability: An Intravascular Optical Coherence Tomography Study. Cardiovasc. Ther. 2019, 2019, 8563717. [Google Scholar] [CrossRef] [PubMed]
- Tabakci, M.M.; Gerin, F.; Sunbul, M.; Toprak, C.; Durmus, H.I.; Demir, S.; Arslantas, U.; Cersit, S.; Batgerel, U.; Kargin, R. Relation of Plasma Fibrinogen Level with the Presence, Severity, and Complexity of Coronary Artery Disease. Clin. Appl. Thromb. Hemost. 2017, 23, 638–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Wang, H.; Li, Y.M.; Huang, B.T.; Huang, F.Y.; Xia, T.L.; Chai, H.; Wang, P.J.; Liu, W.; Zhang, C.; et al. Relation between admission plasma fibrinogen levels and mortality in Chinese patients with coronary artery disease. Sci. Rep. 2016, 6, 30506. [Google Scholar] [CrossRef] [Green Version]
- Lovely, R.S.; Falls, L.A.; Al-Mondhiry, H.A.; Chambers, C.E.; Sexton, G.J.; Ni, H.; Farrell, D.H. Association of γA/γ’fibrinogen levels and coronary artery disease. Thromb. Haemost. 2002, 88, 26–31. [Google Scholar] [CrossRef]
- Mannila, M.N.; Lovely, R.; Kazmierczak, S.; Eriksson, P.; Samnegård, A.; Farrell, D.; Hamsten, A.; Silveira, A. Elevated plasma fibrinogen γ′ concentration is associated with myocardial infarction: Effects of variation in fibrinogen genes and environmental factors. J. Thromb. Haemost. 2007, 5, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.Y.; Uitte de Willige, S.; Vos, H.L.; Leebeek, F.W.; Dippel, D.W.; Bertina, R.M.; de Maat, M.P. Fibrinogen gamma’ in ischemic stroke: A case-control study. Stroke 2008, 39, 1033–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appiah, D.; Schreiner, P.J.; MacLehose, R.F.; Folsom, A.R. Association of Plasma gamma’ Fibrinogen with Incident Cardiovascular Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2700–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shridas, P.; Tannock, L.R. Role of serum amyloid A in atherosclerosis. Curr. Opin. Lipidol. 2019, 30, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Harb, T.S.; Zareba, W.; Moss, A.J.; Ridker, P.M.; Marder, V.J.; Rifai, N.; Miller Watelet, L.F.; Arora, R.; Brown, M.W.; Case, R.B.; et al. Association of C-reactive protein and serum amyloid A with recurrent coronary events in stable patients after healing of acute myocardial infarction. Am. J. Cardiol. 2002, 89, 216–221. [Google Scholar] [CrossRef]
- Johnson, B.D.; Kip, K.E.; Marroquin, O.C.; Ridker, P.M.; Kelsey, S.F.; Shaw, L.J.; Pepine, C.J.; Sharaf, B.; Bairey Merz, C.N.; Sopko, G.; et al. Serum amyloid A as a predictor of coronary artery disease and cardiovascular outcome in women: The National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004, 109, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, K.; Mashiba, S.; Wada, Y.; Sahara, M.; Uchida, K.; Aizawa, T.; Kodama, T. A serum amyloid A and LDL complex as a new prognostic marker in stable coronary artery disease. Atherosclerosis 2004, 174, 349–356. [Google Scholar] [CrossRef]
- Chang, C.; Pan, Y.; Du, H.; Wang, X.; Li, X. Serum amyloid A1 can be a novel biomarker for evaluating the presence and severity of acute coronary syndrome. Clin. Biochem. 2020, 85, 27–32. [Google Scholar] [CrossRef]
- Kalsch, A.I.; Scharnagl, H.; Kleber, M.E.; Windpassinger, C.; Sattler, W.; Leipe, J.; Kramer, B.K.; Marz, W.; Malle, E. Long- and short-term association of low-grade systemic inflammation with cardiovascular mortality in the LURIC study. Clin. Res. Cardiol. 2020, 109, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF receptor superfamily: Structure-function relationship (s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naya, M.; Tsukamoto, T.; Morita, K.; Katoh, C.; Furumoto, T.; Fujii, S.; Tamaki, N.; Tsutsui, H. Plasma interleukin-6 and tumor necrosis factor-α can predict coronary endothelial dysfunction in hypertensive patients. Hypertens. Res. 2007, 30, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoog, T.; Dichtl, W.; Boquist, S.; Skoglund-Andersson, C.; Karpe, F.; Tang, R.; Bond, M.G.; de Faire, U.; Nilsson, J.; Eriksson, P.; et al. Plasma tumour necrosis factor-alpha and early carotid atherosclerosis in healthy middle-aged men. Eur. Heart J. 2002, 23, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kaptoge, S.; Seshasai, S.R.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safranow, K.; Dziedziejko, V.; Rzeuski, R.; Czyzycka, E.; Wojtarowicz, A.; Binczak-Kuleta, A.; Jakubowska, K.; Olszewska, M.; Ciechanowicz, A.; Kornacewicz-Jach, Z.; et al. Plasma concentrations of TNF-alpha and its soluble receptors sTNFR1 and sTNFR2 in patients with coronary artery disease. Tissue Antigens 2009, 74, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lei, L.; Zhu, Z.; Li, Q.; Guo, D.; Xu, J.; Chen, J.; Sha, H.; Zhang, X.; Yang, X.; et al. Excess TNF-alpha in the blood activates monocytes with the potential to directly form cholesteryl ester-laden cells. Acta Biochim. Biophys. Sin. 2015, 47, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.O.; Simpson-Haidaris, P.J. Functional analysis of interleukin 6 response elements (IL-6REs) on the human gamma-fibrinogen promoter: Binding of hepatic Stat3 correlates negatively with transactivation potential of type II IL-6REs. J. Biol. Chem. 2003, 278, 41270–41281. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, B.C.; Lopes, A.L.; Macedo, R.C.O.; Correa, C.S.; Ramis, T.R.; Ribeiro, J.L.; Reischak-Oliveira, A. Inflammatory markers, endothelial function and cardiovascular risk. J. Vasc. Bras. 2014, 13, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Koyama, K.; Yoneyama, K.; Mitarai, T.; Ishibashi, Y.; Takahashi, E.; Kongoji, K.; Harada, T.; Akashi, Y.J. Association between inflammatory biomarkers and thin-cap fibroatheroma detected by optical coherence tomography in patients with coronary heart disease. Arch. Med. Sci. 2015, 11, 505–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Held, C.; White, H.D.; Stewart, R.A.H.; Budaj, A.; Cannon, C.P.; Hochman, J.S.; Koenig, W.; Siegbahn, A.; Steg, P.G.; Soffer, J.; et al. Inflammatory Biomarkers Interleukin-6 and C-Reactive Protein and Outcomes in Stable Coronary Heart Disease: Experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J. Am. Heart Assoc. 2017, 6, e005077. [Google Scholar] [CrossRef] [Green Version]
- Boekholdt, S.M.; Peters, R.J.; Hack, C.E.; Day, N.E.; Luben, R.; Bingham, S.A.; Wareham, N.J.; Reitsma, P.H.; Khaw, K.T. IL-8 plasma concentrations and the risk of future coronary artery disease in apparently healthy men and women: The EPIC-Norfolk prospective population study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1503–1508. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Hirao, Y.; Oshima, S.; Yuasa, K.; Taniguchi, K.; Nagai, R.; Kobayashi, I. Interleukin-8 as a sensitive marker of unstable coronary artery disease. Am. J. Cardiol. 1996, 77, 304–307. [Google Scholar] [CrossRef]
- Romuk, E.; Skrzep-Poloczek, B.; Wojciechowska, C.; Tomasik, A.; Birkner, E.; Wodniecki, J.; Gabrylewicz, B.; Ochala, A.; Tendera, M. Selectin-P and interleukin-8 plasma levels in coronary heart disease patients. Eur. J. Clin. Investig. 2002, 32, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Komoda, H.; Nonaka, M.; Kameda, M.; Uchida, T.; Node, K. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease. Int. J. Cardiol. 2008, 124, 319–325. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [Green Version]
- Mallat, Z.; Corbaz, A.; Scoazec, A.; Besnard, S.; Leseche, G.; Chvatchko, Y.; Tedgui, A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 2001, 104, 1598–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulthe, J.; McPheat, W.; Samnegard, A.; Tornvall, P.; Hamsten, A.; Eriksson, P. Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6. Atherosclerosis 2006, 188, 450–454. [Google Scholar] [CrossRef]
- Blankenberg, S.; Tiret, L.; Bickel, C.; Peetz, D.; Cambien, F.; Meyer, J.; Rupprecht, H.J.; AtheroGene, I. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 2002, 106, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, D.Y.; Liu, C.L.; Tang, J.N.; Zhu, Z.Z.; Xuan, X.X.; Zhu, X.D.; Wang, Y.Z.; Zhang, T.X.; Shen, D.L.; Wang, X.F.; et al. Interleukin-18, matrix metalloproteinase-22 and -29 are independent risk factors of human coronary heart disease. J. Zhejiang Univ. Sci. B 2017, 18, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Blankenberg, S.; Luc, G.; Ducimetiere, P.; Arveiler, D.; Ferrieres, J.; Amouyel, P.; Evans, A.; Cambien, F.; Tiret, L.; Group, P.S. Interleukin-18 and the risk of coronary heart disease in European men: The Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation 2003, 108, 2453–2459. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, B.J.; Papacosta, O.; Owen, C.G.; Wannamethee, S.G.; Humphries, S.E.; Woodward, M.; Lennon, L.T.; Thomson, A.; Welsh, P.; Rumley, A.; et al. Interleukin 18 and coronary heart disease: Prospective study and systematic review. Atherosclerosis 2011, 217, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Tang, Q.; Zhu, X.; Yang, X. IL-37 increased in patients with acute coronary syndrome and associated with a worse clinical outcome after ST-segment elevation acute myocardial infarction. Clin. Chim. Acta 2017, 468, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Semaan, H.B.; Gurbel, P.A.; Anderson, J.L.; Muhlestein, J.B.; Carlquist, J.F.; Horne, B.D.; Serebruany, V.L. Soluble VCAM-1 and E-selectin, but not ICAM-1 discriminate endothelial injury in patients with documented coronary artery disease. Cardiology 2000, 93, 7–10. [Google Scholar] [CrossRef]
- Leite, A.R.; Borges-Canha, M.; Cardoso, R.; Neves, J.S.; Castro-Ferreira, R.; Leite-Moreira, A. Novel Biomarkers for Evaluation of Endothelial Dysfunction. Angiology 2020, 71, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.; Nawroth, P.; Conradt, C.; Nordt, T.; Weiss, T.; Boehme, M.; Wunsch, A.; Allenberg, J.; Kubler, W.; Bode, C. Circulating vascular cell adhesion molecule-1 correlates with the extent of human atherosclerosis in contrast to circulating intercellular adhesion molecule-1, E-selectin, P-selectin, and thrombomodulin. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, N.; Foley, J.; Murphy, R.; Curtin, R.; Crean, P.; Walsh, M. Risk stratification in unstable angina and non-Q wave myocardial infarction using soluble cell adhesion molecules. Heart 2001, 85, 623–627. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; Hafner, G.; Tiret, L.; Meyer, J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001, 104, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Hillis, G.S.; Terregino, C.; Taggart, P.; Killian, A.; Zhao, N.; Dalsey, W.C.; Mangione, A. Elevated soluble P-selectin levels are associated with an increased risk of early adverse events in patients with presumed myocardial ischemia. Am. Heart J. 2002, 143, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Nasuno, A.; Matsubara, T.; Hori, T.; Higuchi, K.; Imai, S.; Nakagawa, I.; Tsuchida, K.; Ozaki, K.; Mezaki, T.; Tanaka, T.; et al. Levels of soluble E-selectin and ICAM-1 in the coronary circulation of patients with stable coronary artery disease: Association with the severity of coronary atherosclerosis. Jpn. Heart J. 2002, 43, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Haim, M.; Tanne, D.; Boyko, V.; Reshef, T.; Goldbourt, U.; Leor, J.; Mekori, Y.A.; Behar, S. Soluble intercellular adhesion molecule-1 and long-term risk of acute coronary events in patients with chronic coronary heart disease. Data from the Bezafibrate Infarction Prevention (BIP) Study. J. Am. Coll. Cardiol. 2002, 39, 1133–1138. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, A.; Seljeflot, I.; Sommervoll, L.; Christensen, B.; Otterstad, J.E.; Arnesen, H. Increased levels of markers of vascular inflammation in patients with coronary heart disease. Scand. J. Clin. Lab. Investig. 2002, 62, 59–68. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Gika, H.I.; Zolindaki, M.G.; Xydas, T.A.; Paravolidakis, K.E.; Velissaridou, A.H. Usefulness of elevated levels of soluble vascular cell adhesion molecule-1 in predicting in-hospital prognosis in patients with unstable angina pectoris. Am. J. Cardiol. 2003, 92, 1195–1197. [Google Scholar] [CrossRef]
- Jha, H.C.; Divya, A.; Prasad, J.; Mittal, A. Plasma circulatory markers in male and female patients with coronary artery disease. Heart Lung 2010, 39, 296–303. [Google Scholar] [CrossRef]
- Liang, K.W.; Sheu, W.H.; Lee, W.J.; Lee, W.L.; Fu, C.P.; Wang, J.S. Differential expression of circulating vascular cell adhesion molecule-1 in subjects with coronary artery disease and cardiac syndrome X without known diabetes mellitus. Biomarkers 2017, 22, 798–804. [Google Scholar] [CrossRef]
- Nozaki, T.; Sugiyama, S.; Koga, H.; Sugamura, K.; Ohba, K.; Matsuzawa, Y.; Sumida, H.; Matsui, K.; Jinnouchi, H.; Ogawa, H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J. Am. Coll. Cardiol. 2009, 54, 601–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blann, A.D. A reliable marker of vascular function: Does it exist? Trends Cardiovasc. Med. 2015, 25, 588–591. [Google Scholar] [CrossRef] [Green Version]
- Werner, N.; Wassmann, S.; Ahlers, P.; Kosiol, S.; Nickenig, G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, J.; Chen, X.; Sun, H.; Peng, S.; Kuang, Y.; Pi, J.; Zhuang, T.; Zhang, L.; Yu, Z.; et al. Dysfunctional endothelial-derived microparticles promote inflammatory macrophage formation via NF-small ka, CyrillicB and IL-1beta signal pathways. J. Cell Mol. Med. 2019, 23, 476–486. [Google Scholar] [CrossRef]
- Radecke, C.E.; Warrick, A.E.; Singh, G.D.; Rogers, J.H.; Simon, S.I.; Armstrong, E.J. Coronary artery endothelial cells and microparticles increase expression of VCAM-1 in myocardial infarction. Thromb. Haemost. 2015, 113, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Gkaliagkousi, E.; Gavriilaki, E.; Vasileiadis, I.; Nikolaidou, B.; Yiannaki, E.; Lazaridis, A.; Triantafyllou, A.; Anyfanti, P.; Markala, D.; Zarifis, I.; et al. Endothelial Microvesicles Circulating in Peripheral and Coronary Circulation Are Associated with Central Blood Pressure in Coronary Artery Disease. Am. J. Hypertens. 2019, 32, 1199–1205. [Google Scholar] [CrossRef]
- Yamamoto, E.; Sugiyama, S.; Hirata, Y.; Tokitsu, T.; Tabata, N.; Fujisue, K.; Sugamura, K.; Sakamoto, K.; Tsujita, K.; Matsumura, T.; et al. Prognostic significance of circulating leukocyte subtype counts in patients with coronary artery disease. Atherosclerosis 2016, 255, 210–216. [Google Scholar] [CrossRef]
- Urbanski, K.; Ludew, D.; Filip, G.; Filip, M.; Sagan, A.; Szczepaniak, P.; Grudzien, G.; Sadowski, J.; Jasiewicz-Honkisz, B.; Sliwa, T.; et al. CD14(+)CD16(++) “nonclassical” monocytes are associated with endothelial dysfunction in patients with coronary artery disease. Thromb. Haemost. 2017, 117, 971–980. [Google Scholar] [CrossRef]
- Arnold, K.A.; Blair, J.E.; Paul, J.D.; Shah, A.P.; Nathan, S.; Alenghat, F.J. Monocyte and macrophage subtypes as paired cell biomarkers for coronary artery disease. Exp. Physiol. 2019, 104, 1343–1352. [Google Scholar] [CrossRef]
- Leone, A.; Moncada, S.; Vallance, P.; Calver, A.; Collier, J. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Sandoo, A.; Kitas, G.D. Asymmetric dimethylarginine as a surrogate marker of endothelial dysfunction and cardiovascular risk in patients with systemic rheumatic diseases. Int. J. Mol. Sci. 2012, 13, 12315–12335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitroulas, T.; Giannakoulas, G.; Papadopoulou, K.; Sfetsios, T.; Karvounis, H.; Dimitroula, H.; Parcharidou, D.; Koliakos, G.; Garyfallos, A.; Styliadis, I.; et al. Left atrial volume and N-terminal pro-B type natriuretic peptide are associated with elevated pulmonary artery pressure in patients with systemic sclerosis. Clin. Rheumatol. 2010, 29, 957–964. [Google Scholar] [CrossRef]
- Dimitroulas, T.; Hodson, J.; Sandoo, A.; Smith, J.; Kitas, G.D. Endothelial injury in rheumatoid arthritis: A crosstalk between dimethylarginines and systemic inflammation. Arthritis Res. Ther. 2017, 19, 32. [Google Scholar] [CrossRef] [Green Version]
- Surdacki, A.; Nowicki, M.; Sandmann, J.; Tsikas, D.; Boeger, R.H.; Bode-Boeger, S.M.; Kruszelnicka-Kwiatkowska, O.; Kokot, F.; Dubiel, J.S.; Froelich, J.C. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J. Cardiovasc. Pharmacol. 1999, 33, 652–658. [Google Scholar] [CrossRef]
- Eid, H.M.; Arnesen, H.; Hjerkinn, E.M.; Lyberg, T.; Seljeflot, I. Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Metabolism 2004, 53, 1574–1579. [Google Scholar] [CrossRef]
- Lundman, P.; Eriksson, M.J.; Stühlinger, M.; Cooke, J.P.; Hamsten, A.; Tornvall, P. Mild-to-moderate hypertriglyceridemia in young men is associated with endothelial dysfunction and increased plasma concentrations of asymmetric dimethylarginine. J. Am. Coll. Cardiol. 2001, 38, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Altinova, A.E.; Arslan, M.; Sepici-Dincel, A.; Akturk, M.; Altan, N.; Toruner, F.B. Uncomplicated type 1 diabetes is associated with increased asymmetric dimethylarginine concentrations. J. Clin. Endocrinol. Metab. 2007, 92, 1881–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, F.; Asagmi, T.; Cooke, J.P.; Lamendola, C.; McLaughlin, T.; Reaven, G.M.; Stuehlinger, M.; Tsao, P.S. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am. J. Cardiol. 2001, 88, 1201–1203. [Google Scholar] [CrossRef]
- Leong, T.; Zylberstein, D.; Graham, I.; Lissner, L.; Ward, D.; Fogarty, J.; Bengtsson, C.; Bjorkelund, C.; Thelle, D.; Swedish-Irish-Norwegian, C. Asymmetric dimethylarginine independently predicts fatal and nonfatal myocardial infarction and stroke in women: 24-year follow-up of the population study of women in Gothenburg. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.H.; Lee, S.C. Elevated levels of plasma homocyst(e)ine and asymmetric dimethylarginine in elderly patients with stroke. Atherosclerosis 2001, 158, 425–430. [Google Scholar] [CrossRef]
- Dowsett, L.; Higgins, E.; Alanazi, S.; Alshuwayer, N.A.; Leiper, F.C.; Leiper, J. ADMA: A Key Player in the Relationship between Vascular Dysfunction and Inflammation in Atherosclerosis. J. Clin. Med. 2020, 9, 3026. [Google Scholar] [CrossRef]
- Mangiacapra, F.; Conte, M.; Demartini, C.; Muller, O.; Delrue, L.; Dierickx, K.; Di Sciascio, G.; Trimarco, B.; De Bruyne, B.; Wijns, W.; et al. Relationship of asymmetric dimethylarginine (ADMA) with extent and functional severity of coronary atherosclerosis. Int. J. Cardiol. 2016, 220, 629–633. [Google Scholar] [CrossRef]
- Jarzebska, N.; Mangoni, A.A.; Martens-Lobenhoffer, J.; Bode-Boger, S.M.; Rodionov, R.N. The Second Life of Methylarginines as Cardiovascular Targets. Int. J. Mol. Sci. 2019, 20, 4592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielstein, J.T.; Martens-Lobenhoffer, J.; Vollmer, S.; Bode-Boger, S.M. L-Arginine, ADMA, SDMA, creatinine, MDRD formula: Detour to renal function testing. J. Nephrol. 2008, 21, 959–961. [Google Scholar]
- Schulze, F.; Carter, A.M.; Schwedhelm, E.; Ajjan, R.; Maas, R.; von Holten, R.A.; Atzler, D.; Grant, P.J.; Boger, R.H. Symmetric dimethylarginine predicts all-cause mortality following ischemic stroke. Atherosclerosis 2010, 208, 518–523. [Google Scholar] [CrossRef]
- Speer, T.; Rohrer, L.; Blyszczuk, P.; Shroff, R.; Kuschnerus, K.; Krankel, N.; Kania, G.; Zewinger, S.; Akhmedov, A.; Shi, Y.; et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013, 38, 754–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jud, P.; Hafner, F.; Verheyen, N.; Meinitzer, A.; Gary, T.; Brodmann, M.; Seinost, G.; Hackl, G. Homoarginine/ADMA ratio and homoarginine/SDMA ratio as independent predictors of cardiovascular mortality and cardiovascular events in lower extremity arterial disease. Sci. Rep. 2018, 8, 14197. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, E.; Serra-Planas, E.; Granada, M.L.; Pellitero, S.; Reverter, J.L.; Alonso, N.; Soldevila, B.; Mauricio, D.; Puig-Domingo, M. Relationship of YKL-40 and adiponectin and subclinical atherosclerosis in asymptomatic patients with type 1 diabetes mellitus from a European Mediterranean population. Cardiovasc. Diabetol. 2015, 14, 121. [Google Scholar] [CrossRef] [Green Version]
- Fadaei, R.; Shateri, H.; DiStefano, J.K.; Moradi, N.; Mohammadi, M.; Emami, F.; Aghajani, H.; Ziamajidi, N. Higher circulating levels of ANGPTL8 are associated with body mass index, triglycerides, and endothelial dysfunction in patients with coronary artery disease. Mol. Cell Biochem. 2020, 469, 29–39. [Google Scholar] [CrossRef]
- Moradi, N.; Fadaei, R.; Emamgholipour, S.; Kazemian, E.; Panahi, G.; Vahedi, S.; Saed, L.; Fallah, S. Association of circulating CTRP9 with soluble adhesion molecules and inflammatory markers in patients with type 2 diabetes mellitus and coronary artery disease. PLoS ONE 2018, 13, e0192159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Qian, X.; Li, J.; Li, Y.; Li, Y.; Luo, Y. Evaluation of serum cysteine-rich protein 61 levels in patients with coronary artery disease. Biomark. Med. 2018, 12, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Turan, T.; Akyuz, A.R.; Aykan, A.C.; Kul, S.; Cirakoglu, O.F.; Aslan, A.O.; Gul, I.; Ucar, U.; Demir, S.; Celik, S. Plasma Endocan Levels in Patients with Isolated Coronary Artery Ectasia. Angiology 2016, 67, 932–936. [Google Scholar] [CrossRef]
- Efe, S.C.; Demirci, K.; Ozturk, S.; Gurbuz, A.S.; Poci, N.; Kilicgedik, A.; Guler, A.; Yilmaz, M.F.; Izgi, I.A.; Kirma, C. Serum endocan levels in patients with cardiac syndrome X. Herz 2018, 43, 359–363. [Google Scholar] [CrossRef]
- Aksan, G.; Gedikli, O.; Keskin, K.; Nar, G.; Inci, S.; Yildiz, S.S.; Kaplan, O.; Soylu, K.; Kilickesmez, K.O.; Sahin, M. Is galectin-3 a biomarker, a player-or both-in the presence of coronary atherosclerosis? J. Investig. Med. 2016, 64, 764–770. [Google Scholar] [CrossRef]
- Maneerat, Y.; Prasongsukarn, K.; Benjathummarak, S.; Dechkhajorn, W.; Chaisri, U. Increased alpha-defensin expression is associated with risk of coronary heart disease: A feasible predictive inflammatory biomarker of coronary heart disease in hyperlipidemia patients. Lipids Health Dis. 2016, 15, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, C.; Hu, W.; Wang, M.; Lv, W.; Jia, T.; Xiao, Y. Irisin as a mediator between obesity and vascular inflammation in Chinese children and adolescents. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 320–329. [Google Scholar] [CrossRef]
- Huerta-Delgado, A.S.; Roffe-Vazquez, D.N.; Gonzalez-Gil, A.M.; Villarreal-Calderon, J.R.; Tamez-Rivera, O.; Rodriguez-Gutierrez, N.A.; Castillo, E.C.; Silva-Platas, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Serum Irisin Levels, Endothelial Dysfunction, and Inflammation in Pediatric Patients with Type 2 Diabetes Mellitus and Metabolic Syndrome. J. Diabetes Res. 2020, 2020, 1949415. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, B.; Wang, X. Lower irisin levels in coronary artery disease: A meta-analysis. Minerva Endocrinol. 2020, 45, 61–69. [Google Scholar] [CrossRef]
- Sairam, S.G.; Sola, S.; Barooah, A.; Javvaji, S.K.; Jaipuria, J.; Venkateshan, V.; Chelli, J.; Sanjeevi, C.B. The role of Lp-PLA2 and biochemistry parameters as potential biomarkers of coronary artery disease in Asian South-Indians: A case-control study. Cardiovasc. Diagn. Ther. 2017, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Naka, K.K.; Papathanassiou, K.; Bechlioulis, A.; Pappas, K.; Tigas, S.; Makriyiannis, D.; Antoniou, S.; Kazakos, N.; Margeli, A.; Papassotiriou, I.; et al. Association of vascular indices with novel circulating biomarkers as prognostic factors for cardiovascular complications in patients with type 2 diabetes mellitus. Clin. Biochem. 2018, 53, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Grzywocz, P.; Mizia-Stec, K.; Wybraniec, M.; Chudek, J. Adipokines and endothelial dysfunction in acute myocardial infarction and the risk of recurrent cardiovascular events. J. Cardiovasc. Med. 2015, 16, 37–44. [Google Scholar]
- Ashraf, H.; Soltani, D.; Sobh-Rakhshankhah, A.; Jafari, S.; Boroumand, M.A.; Goudarzi, V.; Vasheghani Farahani, A.; Masoudkabir, F. Visfatin as marker of isolated coronary artery ectasia and its severity. Cytokine 2019, 113, 216–220. [Google Scholar] [CrossRef]
- Safdar, B.; Guo, X.; Johnson, C.; D’Onofrio, G.; Dziura, J.; Sinusas, A.J.; Testani, J.; Rao, V.; Desir, G. Elevated renalase levels in patients with acute coronary microvascular dysfunction—A possible biomarker for ischemia. Int. J. Cardiol. 2019, 279, 155–161. [Google Scholar] [CrossRef]
- Han, W.; Wei, Z.; Zhang, H.; Geng, C.; Dang, R.; Yang, M.; Zhang, J.; Wang, C.; Jiang, P. The Association Between Sortilin and Inflammation in Patients with Coronary Heart Disease. J. Inflamm. Res. 2020, 13, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Mekonnen, G.; Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Eapen, D.J.; Al-Kassem, H.; Rasoul-Arzrumly, E.; Gogas, B.D.; McDaniel, M.C.; Pielak, T.; et al. Plasma soluble urokinase-type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non-obstructive coronary artery disease. Atherosclerosis 2015, 239, 55–60. [Google Scholar] [CrossRef]
- Leucker, T.M.; Weiss, R.G.; Schar, M.; Bonanno, G.; Mathews, L.; Jones, S.R.; Brown, T.T.; Moore, R.; Afework, Y.; Gerstenblith, G.; et al. Coronary Endothelial Dysfunction Is Associated with Elevated Serum PCSK9 Levels in People With HIV Independent of Low-Density Lipoprotein Cholesterol. J. Am. Heart Assoc. 2018, 7, e009996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caselli, C.; Del Turco, S.; Ragusa, R.; Lorenzoni, V.; De Graaf, M.; Basta, G.; Scholte, A.; De Caterina, R.; Neglia, D. Association of PCSK9 plasma levels with metabolic patterns and coronary atherosclerosis in patients with stable angina. Cardiovasc. Diabetol. 2019, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Jakobson, M.; Lieberg, J.; Zilmer, M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 44–52. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wang, Y.; Ding, H.; Xue, S.; Yu, H.; Hu, L.; Qi, H.; Wang, Y.; Zhu, W.; et al. KCNQ1OT1, HIF1A-AS2 and APOA1-AS are promising novel biomarkers for diagnosis of coronary artery disease. Clin. Exp. Pharmacol. Physiol. 2019, 46, 635–642. [Google Scholar] [CrossRef]
- Xu, Y.; Shao, B. Circulating lncRNA IFNG-AS1 expression correlates with increased disease risk, higher disease severity and elevated inflammation in patients with coronary artery disease. J. Clin. Lab. Anal. 2018, 32, e22452. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Yang, Q.; Xi, R.; Li, L.; Shi, D.; Chen, K. miR-941 as a promising biomarker for acute coronary syndrome. BMC Cardiovasc. Disord. 2017, 17, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, L.L.; Shah, S.A.V.; Ponde, C.K.; Rajani, R.M.; Ashavaid, T.F. Circulating miRNA-33: A potential biomarker in patients with coronary artery disease. Biomarkers 2019, 24, 36–42. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Zheng, Y.; Yan, M.; Cui, Y.; Jiang, J. Circulating microRNA-92a level predicts acute coronary syndrome in diabetic patients with coronary heart disease. Lipids Health Dis. 2019, 18, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, M.; Horvathova, V.; Hajek, P.; Stechovsky, C.; Honek, J.; Senolt, L.; Veselka, J. MicroRNA-331 and microRNA-151-3p as biomarkers in patients with ST-segment elevation myocardial infarction. Sci. Rep. 2020, 10, 5845. [Google Scholar] [CrossRef] [Green Version]
- Ling, H.; Guo, Z.; Shi, Y.; Zhang, L.; Song, C. Serum Exosomal MicroRNA-21, MicroRNA-126, and PTEN Are Novel Biomarkers for Diagnosis of Acute Coronary Syndrome. Front. Physiol. 2020, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Sun, H.; Sun, B. MicroRNA-145 is involved in endothelial cell dysfunction and acts as a promising biomarker of acute coronary syndrome. Eur. J. Med. Res. 2020, 25, 2. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wang, Z.Y.; Li, C.C. miRNA-22 as a Candidate Diagnostic Biomarker for Coronary Slow Flow. Cardiol. Res. Pract. 2020, 2020, 7490942. [Google Scholar] [CrossRef]
- Danaii, S.; Shiri, S.; Dolati, S.; Ahmadi, M.; Ghahremani-Nasab, L.; Amiri, A.; Kamrani, A.; Samadi Kafil, H.; Chakari-Khiavi, F.; Hojjat-Farsangi, M.; et al. The Association between Inflammatory Cytokines and miRNAs with Slow Coronary Flow Phenomenon. Iran. J. Allergy Asthma Immunol. 2020, 19, 56–64. [Google Scholar] [CrossRef]
- Vita, J.A. Endothelial function. Circulation 2011, 124, e906–e912. [Google Scholar] [CrossRef] [Green Version]
- Cooper, S.; Teoh, H.; Campeau, M.A.; Verma, S.; Leask, R.L. Empagliflozin restores the integrity of the endothelial glycocalyx in vitro. Mol. Cell Biochem. 2019, 459, 121–130. [Google Scholar] [CrossRef]
- Aini, K.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Yagi, S.; Kusunose, K.; Yamada, H.; Soeki, T.; Sata, M. Vildagliptin, a DPP-4 Inhibitor, Attenuates Endothelial Dysfunction and Atherogenesis in Nondiabetic Apolipoprotein E-Deficient Mice. Int. Heart J. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients with Prediabetes with Stable Angina and Nonobstructive Coronary Artery Stenosis: The CODYCE Multicenter Prospective Study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Du, X.; Wu, Y.; Hua, L.; Wan, L.; Yan, N. Simvastatin promotes endothelial dysfunction by activating the Wnt/betacatenin pathway under oxidative stress. Int. J. Mol. Med. 2019, 44, 1289–1298. [Google Scholar] [PubMed] [Green Version]
- Li, X.; Xiao, H.; Lin, C.; Sun, W.; Wu, T.; Wang, J.; Chen, B.; Chen, X.; Cheng, D. Synergistic effects of liposomes encapsulating atorvastatin calcium and curcumin and targeting dysfunctional endothelial cells in reducing atherosclerosis. Int. J. Nanomed. 2019, 14, 649–665. [Google Scholar] [CrossRef] [Green Version]
- Bacchiega, B.C.; Bacchiega, A.B.; Usnayo, M.J.; Bedirian, R.; Singh, G.; Pinheiro, G.D. Interleukin 6 Inhibition and Coronary Artery Disease in a High-Risk Population: A Prospective Community-Based Clinical Study. J. Am. Heart Assoc. 2017, 6, e005038. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Katsimbri, P.; Lambadiari, V.; Parissis, J.; Andreadou, I.; Tsoumani, M.; Boumpas, D.; Kouretas, D.; Iliodromitis, E. Tocilizumab improves oxidative stress and endothelial glycocalyx: A mechanism that may explain the effects of biological treatment on COVID-19. Food Chem. Toxicol. 2020, 145, 111694. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wan, M.; Cheng, Z.; Wang, Z.; Wu, Q. Tofacitinib inhibits ox-LDL-induced adhesion of THP-1 monocytes to endothelial cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2775–2782. [Google Scholar] [CrossRef]
- Ashry, N.A.; Abdelaziz, R.R.; Suddek, G.M. The potential effect of imatinib against hypercholesterolemia induced atherosclerosis, endothelial dysfunction and hepatic injury in rabbits. Life Sci. 2020, 243, 117275. [Google Scholar] [CrossRef]
- Zhou, X.; Cai, J.; Liu, W.; Wu, X.; Gao, C. Cysteinyl leukotriene receptor type 1 (CysLT1R) antagonist zafirlukast protects against TNF-alpha-induced endothelial inflammation. Biomed. Pharmacother. 2019, 111, 452–459. [Google Scholar] [CrossRef]
- Pang, J.; Hu, P.; Wang, J.; Jiang, J.; Lai, J. Vorapaxar stabilizes permeability of the endothelial barrier under cholesterol stimulation via the AKT/JNK and NFkappaB signaling pathways. Mol. Med. Rep. 2019, 19, 5291–5300. [Google Scholar] [PubMed] [Green Version]
- Campo, G.; Vieceli Dalla Sega, F.; Pavasini, R.; Aquila, G.; Gallo, F.; Fortini, F.; Tonet, E.; Cimaglia, P.; Del Franco, A.; Pestelli, G.; et al. Biological effects of ticagrelor over clopidogrel in patients with stable coronary artery disease and chronic obstructive pulmonary disease. Thromb. Haemost. 2017, 117, 1208–1216. [Google Scholar] [CrossRef] [Green Version]
- Vieceli Dalla Sega, F.; Fortini, F.; Aquila, G.; Pavasini, R.; Biscaglia, S.; Bernucci, D.; Del Franco, A.; Tonet, E.; Rizzo, P.; Ferrari, R.; et al. Ticagrelor Improves Endothelial Function by Decreasing Circulating Epidermal Growth Factor (EGF). Front. Physiol. 2018, 9, 337. [Google Scholar] [CrossRef]
- Aquila, G.; Vieceli Dalla Sega, F.; Marracino, L.; Pavasini, R.; Cardelli, L.S.; Piredda, A.; Scoccia, A.; Martino, V.; Fortini, F.; Bononi, I.; et al. Ticagrelor Increases SIRT1 and HES1 mRNA Levels in Peripheral Blood Cells from Patients with Stable Coronary Artery Disease and Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2020, 21, 1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, T.W.; Sebastian, M.N.; Battaglia, D.M.; Foster, T.P.; Maillet, E.L.; Nichols, C.D. Activation of 5-HT 2 receptors reduces inflammation in vascular tissue and cholesterol levels in high-fat diet-fed apolipoprotein E knockout mice. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, R.; An, D.; Li, Y.; Fu, J.; Huang, F.; Zhu, Q. Fenofibrate improves vascular endothelial function in diabetic mice. Biomed. Pharmacother. 2019, 112, 108722. [Google Scholar] [CrossRef]
- Kubo, M.; Kiyohara, Y.; Kato, I.; Tanizaki, Y.; Arima, H.; Tanaka, K.; Nakamura, H.; Okubo, K.; Iida, M. Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: The Hisayama study. Stroke 2003, 34, 2349–2354. [Google Scholar] [CrossRef] [Green Version]
- Adams, V.; Reich, B.; Uhlemann, M.; Niebauer, J. Molecular effects of exercise training in patients with cardiovascular disease: Focus on skeletal muscle, endothelium, and myocardium. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H72–H88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Prevention, Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, A.; Yamaoka-Tojo, M.; Obara, S.; Shimizu, E.; Fujiyoshi, K.; Noda, C.; Matsunaga, A.; Ako, J. Acute Effects of Whole-Body Vibration Training on Endothelial Function and Cardiovascular Response in Elderly Patients with Cardiovascular Disease A Single-Arm Pilot Study. Int. Heart J. 2019, 60, 854–861. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S. Changes in vascular and inflammatory biomarkers after exercise rehabilitation in patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2019, 70, 1280–1290. [Google Scholar] [CrossRef]
- Pedralli, M.L.; Marschner, R.A.; Kollet, D.P.; Neto, S.G.; Eibel, B.; Tanaka, H.; Lehnen, A.M. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: A randomized clinical trial. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Nagy, E.E.; Moreau, K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019, 597, 4901–4914. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, S.; Serviente, C. Endothelial dysfunction and menopause: Is exercise an effective countermeasure? Climacteric 2018, 21, 267–275. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [Green Version]
- DeSalvo, K.B.; Olson, R.; Casavale, K.O. Dietary guidelines for Americans. JAMA 2016, 315, 457–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, C.R.; Hodgson, J.M.; Woodman, R.; Bryan, J.; Wilson, C.; Murphy, K.J. A Mediterranean diet lowers blood pressure and improves endothelial function: Results from the MedLey randomized intervention trial. Am. J. Clin. Nutr. 2017, 105, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, D.; Benetou, V.; Trichopoulou, A.; La Vecchia, C.; Bamia, C. Mediterranean diet and its components in relation to all-cause mortality: Meta-analysis. Br. J. Nutr. 2018, 120, 1081–1097. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: A systematic review and meta-analysis of observational studies. Int. J. Cancer 2014, 135, 1884–1897. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuniga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Pena, J.D.; Marin, C.; Lopez-Moreno, J.; Castano, J.P.; Delgado-Lista, J.; et al. Mediterranean diet and endothelial function in patients with coronary heart disease: An analysis of the CORDIOPREV randomized controlled trial. PLoS Med. 2020, 17, e1003282. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, A.M.; Almeida, M.D.V.; Parisi, S. Chemistry of the Mediterranean Diet; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- WHO. WHO Global Report on Trends in Prevalence of Tobacco Use 2000-2025; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Golbidi, S.; Edvinsson, L.; Laher, I. Smoking and Endothelial Dysfunction. Curr. Vasc. Pharmacol. 2020, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.; Morris, J.K.; Boniface, S.; Tang, J.L.; Milenkovic, D. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018, 360, j5855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevale, R.; Sciarretta, S.; Violi, F.; Nocella, C.; Loffredo, L.; Perri, L.; Peruzzi, M.; Marullo, A.G.; De Falco, E.; Chimenti, I.; et al. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest 2016, 150, 606–612. [Google Scholar] [CrossRef]
- Skotsimara, G.; Antonopoulos, A.S.; Oikonomou, E.; Siasos, G.; Ioakeimidis, N.; Tsalamandris, S.; Charalambous, G.; Galiatsatos, N.; Vlachopoulos, C.; Tousoulis, D. Cardiovascular effects of electronic cigarettes: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 1219–1228. [Google Scholar] [CrossRef]
- Xue, C.; Chen, Q.Z.; Bian, L.; Yin, Z.F.; Xu, Z.J.; Zhang, A.L.; Xie, Y.S.; Zhang, H.L.; Du, R.; Wang, C.Q. Effects of Smoking Cessation with Nicotine Replacement Therapy on Vascular Endothelial Function, Arterial Stiffness, and Inflammation Response in Healthy Smokers. Angiology 2019, 70, 719–725. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Iwersen-Bergmann, S.; Andresen, H.; Schmoldt, A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit. Care 2012, 16, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstadter, J.; Kroller-Schon, S.; Munzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [Green Version]
- Aschauer, S.; Gouya, G.; Klickovic, U.; Storka, A.; Weisshaar, S.; Vollbracht, C.; Krick, B.; Weiss, G.; Wolzt, M. Effect of systemic high dose vitamin C therapy on forearm blood flow reactivity during endotoxemia in healthy human subjects. Vascul. Pharmacol. 2014, 61, 25–29. [Google Scholar] [CrossRef]
- Hashim Fauzy, F.; Mohd Zainudin, M.; Ismawi, H.R.; Elshami, T.F.T. Piper sarmentosum Leaves Aqueous Extract Attenuates Vascular Endothelial Dysfunction in Spontaneously Hypertensive Rats. Evid. Based Complement. Altern. Med. 2019, 2019, 7198592. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Oboh, G.; Ademiluyi, A.O.; Boligon, A.A.; Athayde, M.L. Effect of Two Ginger Varieties on Arginase Activity in Hypercholesterolemic Rats. J. Acupunct. Meridian Stud. 2016, 9, 80–87. [Google Scholar] [CrossRef]
- Yi, B.; Nguyen, M.C.; Won, M.H.; Kim, Y.M.; Ryoo, S. Arginase Inhibitor 2,3,5,4’-Tetrahydroxystilbene-2-O-beta-D-Glucoside Activates Endothelial Nitric Oxide Synthase and Improves Vascular Function. Planta Med. 2017, 83, 210–216. [Google Scholar] [PubMed]
- Usharani, P.; Merugu, P.L.; Nutalapati, C. Evaluation of the effects of a standardized aqueous extract of Phyllanthus emblica fruits on endothelial dysfunction, oxidative stress, systemic inflammation and lipid profile in subjects with metabolic syndrome: A randomised, double blind, placebo controlled clinical study. BMC Complement. Altern. Med. 2019, 19, 97. [Google Scholar]
- Usharani, P.; Fatima, N.; Kumar, C.U.; Kishan, P. Evaluation of a highly standardized Withania somnifera extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: A randomized, double blind, placebo controlled study. Int. J. Ayurveda Pharma Res. 2014, 2, 22–32. [Google Scholar]
- Pingali Usharani, N.F.; Muralidhar, N. Effects of Phyllanthus emblica extract on endothelial dysfunction and biomarkers of oxidative stress in patients with type 2 diabetes mellitus: A randomized, double-blind, controlled study. Diabetes Metab. Syndr. Obes. 2013, 6, 275. [Google Scholar]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre) hypertensive adults: A randomized double-blind, placebo-controlled, crossover trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, B.; Trindade, M.; Aquino, J.; Cunha, A.; Gismondi, R.; Neves, M.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef] [PubMed]

| Biomarker | Model | Implication or Considerations | Study |
|---|---|---|---|
| Adiponectin | Cohort of T1DM patients | ↑Adiponectin serum levels in T1DM patients are proposed as an early marker of subclinical atherosclerosis. | [168] |
| ANGPTL8 | Cohort of CAD patients | CAD patients had significantly higher serum ANGPTL8 levels and ANGPTL8 was independently associated with TG and ICAM-1 in CAD patients. | [169] |
| CTRP9 | Cohort of patients with CAD and T2DM | ↑expression of CTRP9 in patients with CAD and T2DM. | [170] |
| Cyr61 | Cohort of CAD patients | Serum Cyr61 levels were higher in CAD patients than in controls and correlated positively with Gensini score and CRP levels. | [171] |
| Endocan | Cohort of patients with isolated CAE Cohort of patients with CSX | Plasma endocan levels were increased in patients with isolated CAE as compared to controls. Endocan serum levels were higher in CSX patients than in controls and are proposed as an acute marker of microvascular disease. | [172] [173] |
| Galectin-3 | Cohort of CAD patients | Serum galectin-3 levels were higher in CAD patients than controls and were associated with severity of CAD. | [174] |
| Human neutrophil peptides or α-defensin | Cohort of CAD patients, hyperlipidemic patients and controls | Patients with hyperlipidemia and CAD have showed increased α-defensin in blood. α-defensin is proposed as a potential inflammation marker that may predict the risk of CAD. | [175] |
| Irisin | Cohort of obese children. | Obese children showed a decreased level of Irisin as compared to lean children. ↓Irisin levels correlated inversely with several markers of inflammation and endothelial dysfunction in obese children. | [176] [177] [178] |
| Cohort of children and adolescents with T2DM, MS and controls | T2DM and MS patients showed decreased levels of Irisin as compared to healthy controls. Irisin levels showed a negative correlation with sVCAM-1, sICAM-2 and MCP-1 in the total population of children and adolescents. | ||
| Meta-analysis of 7 case-control studies involving 867 patients of CAD and 700 controls | Circulating irisin concentrations were 18.10 ng/mL lower in patients with CAD than in healthy controls. | ||
| Lp-PLA2 | Cohort of patients with CAD + ASC | Circulating plasma Lp-PLA2 levels were higher CAD+ACS patients than in controls and showed a positive association with CAD risk. | [179] |
| NGAL and YKL-40 | Prospective study of T2DM patients. | NGAL and YKL-40 serum levels are increased in T2DM subjects with subclinical CAD and are associated with the risk of future cardiovascular events. | [180] |
| Resistin and Visfatin | Cohort of CAD patients. | Resistin and visfatin serum levels were higher in patients with acute myocardial infarction than in patients with stable angina. | [181] [182] |
| Cohort of patients with CAE. | Visfatin serum levels were higher in patients with both CAE + CAD and are proposed as an independent marker for severity of coronary ectasia in both isolated CAE and CAD coexisting with CAE groups. | ||
| Renalase | Patients presenting to the emergency room with acute chest pain, with diagnostic workup including PET to identify CMD. | ↑Renalase serum levels were associated with symptomatic CMD in patients presenting with acute chest pain, increased peripheral renalase blood levels are proposed as a biomarker for CMD. | [183] |
| Sortilin | Cohort of CAD patients | Sortilin serum levels were higher in CAD patients than in controls and correlated with inflammatory cytokine levels. | [184] |
| suPAR | Cohort of patients with non-obstructive CAD. | In patients with non-obstructive CAD, plasma suPAR levels correlated negatively with coronary flow reserve. suPAR levels are proposed as an independent risk predictor of coronary microvascular function. | [185] |
| PCSK9 | Cohort of HIV+ patients under retroviral therapy | PCSK9 serum levels were higher in HIV+ than in age and LDL-C level matched HIV-patients and were inversely associated with coronary endothelial function measured by magnetic resonance. | [186] [187] |
| Cohort of patients with suspected CAD | Low PCSK9 plasma levels were associated with unfavorable metabolic profile and with diffuse non-obstructive coronary atherosclerosis as determinated by coronary computed tomography angiography. | ||
| Phosphatidylcholine and lysophosphatidylcholine | Cohort of patients with CAD and PAD | Serum of phosphatidylcholine and lysophosphatidylcholine levels were lower in CAD and PAD patients than in controls. | [188] |
| lncRNA KCNQ1OT1, HIF1A-AS2 and APOA1-AS | Cohort of patients with CAD | KCNQ1OT1, HIF1A-AS2 and APOA1-AS in patients with CAD. ROC analysis confirmed their suitability as biomarkers of CAD. | [189] |
| Circulating lncRNA IFNGAS1 | Cohort of patients with CAD | Increased lncRNA IFNGAS1 plasma levels were associated with CAD risk and severity assessed by coronary angiography. | [190] |
| Circulating microRNA-941 | Cohort of patients with ACS. | microRNA-941 plasma levels were higher in patients with ACS and ST-elevation myocardial infarction than in controls. | [191] |
| Circulating microRNA-33 | Cohort of patients with CAD. | microRNA-33 expression is higher in CAD patients than controls. | [192] |
| Circulating microRNA-92a | Cohort of patients with T2DM + CAD | ↑ Expression of microRNA-92a, was significantly associated with T risk of acute coronary T2DM. miR-92a levels were identified as an independent predictive factor for ACS events in the patients with T2DM. | [193] |
| Circulating microRNAs-331, 151-3p | Cohort of patients with STEMI. | MicroRNAs-331 and 151-3p were significantly up-regulated in patients with STEMI as compared to patients with stable angina and controls. These miRNAs are proposed as suitable biomarkers than may be associated with plaque rupture. | [194] |
| Serum exosomal microRNA-21, 126 and PTEN. | Cohort of patients with ACS. | Serum levels of exosomal microRNAs-21, 126 and PTEN were higher in patients with ACS than in controls. Exosomal microRNA-126 showed a positive correlation with coronary artery stenosis severity. | [195] |
| Circulating microRNA-145 | Cohort of patients with ACS. | ↓ Expression of microRNA-145 in ACS patients as compared to controls. microRNA-145 levels correlated with other endothelial inflammation and damage markers. | [196] |
| ACS coronary ligation rat model | microRNA-145 overexpression in an ACS rat model improved endothelial injury and abnormal inflammation, suggesting it may be a therapeutic target. | ||
| Circulating microRNA-22 | Cohort of patients with CSF. | microRNA-22 expression was increased in patients with CSF as compared to those with normal coronary flow. Increased microRNA-22 circulating levels are proposed as a suitable biomarker of CSF. | [197] |
| microRNA signature | Cohort of patients with CSF. | Expression levels of miR-1, miR-133, miR-208a, miR-206, miR-17, miR-29, miR-223, miR-326, and 155 in PBMCs were significantly increased in SCF patients as compared to controls. | [198] |
| Expression levels of microRNAs: miR-15a, miR-21, miR-25, miR-126, miR-16, and miR-18a were significantly decreased in patients with SCF patients as compared to control group. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. https://doi.org/10.3390/ijms22083850
Medina-Leyte DJ, Zepeda-García O, Domínguez-Pérez M, González-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. International Journal of Molecular Sciences. 2021; 22(8):3850. https://doi.org/10.3390/ijms22083850
Chicago/Turabian StyleMedina-Leyte, Diana Jhoseline, Oscar Zepeda-García, Mayra Domínguez-Pérez, Antonia González-Garrido, Teresa Villarreal-Molina, and Leonor Jacobo-Albavera. 2021. "Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches" International Journal of Molecular Sciences 22, no. 8: 3850. https://doi.org/10.3390/ijms22083850
APA StyleMedina-Leyte, D. J., Zepeda-García, O., Domínguez-Pérez, M., González-Garrido, A., Villarreal-Molina, T., & Jacobo-Albavera, L. (2021). Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. International Journal of Molecular Sciences, 22(8), 3850. https://doi.org/10.3390/ijms22083850









