Healthy Subcutaneous and Omental Adipose Tissue Is Associated with High Expression of Extracellular Matrix Components
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
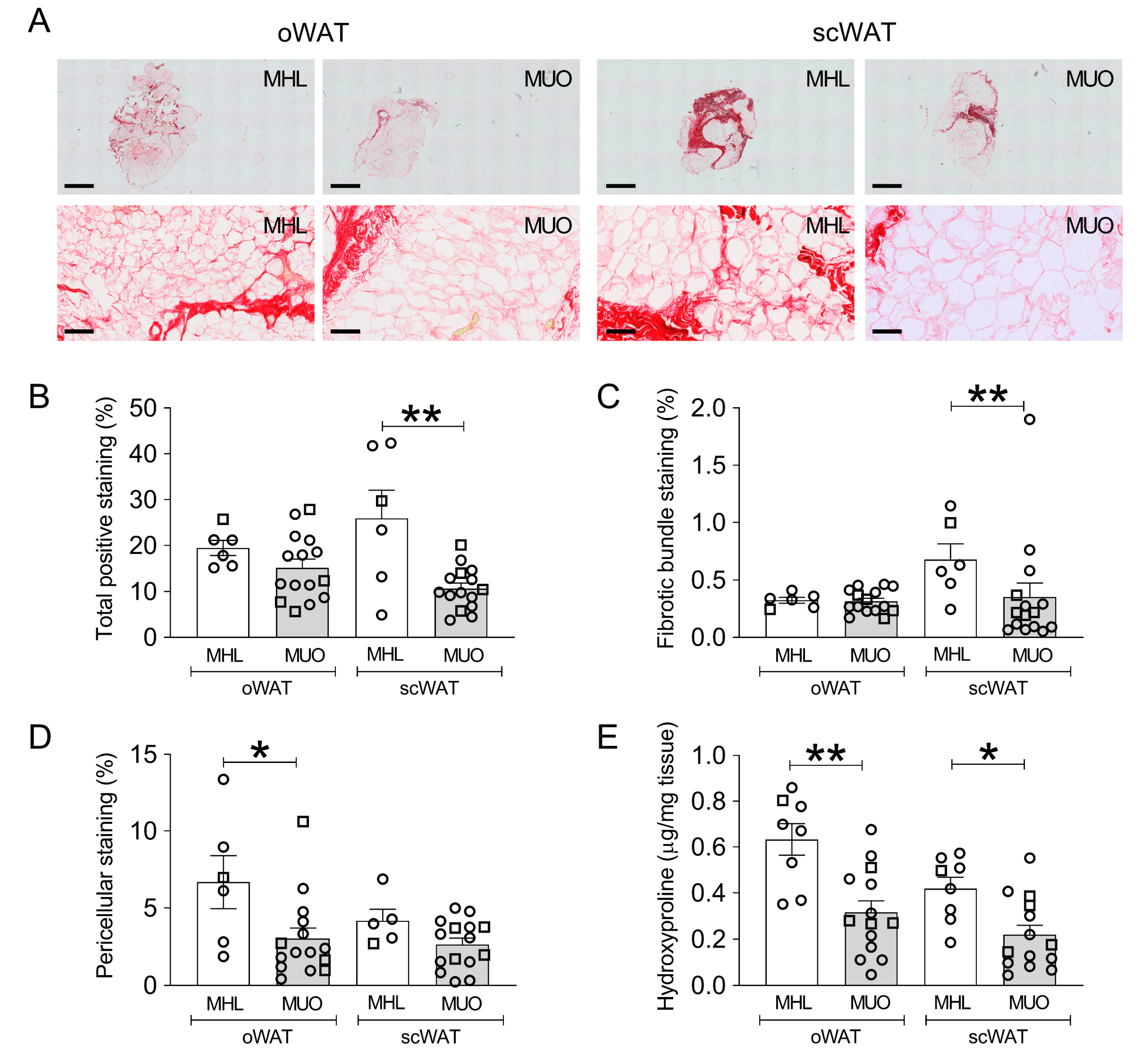
2.2. Histological Staining of Collagen in oWAT and scWAT of MHL and MUO Subjects
2.3. Total Collagen Content Assessed by Quantification of Hydroxyproline Content
2.4. mRNA Expression of Extracellular Matrix Genes
2.5. Correlation between Collagen Content, Clinical Parameters and mRNA Expression
3. Discussion
3.1. ECM Specific Differences between Metabolic Groups
3.2. Differences between oWAT and scWAT
3.3. Summary
3.4. Study Limitations
4. Materials and Methods
4.1. Study Participants
4.2. Plasma and Serum Preparation and Biochemical Laboratory Analyses
4.3. Adipose Tissue Samples
4.4. Immunohistochemistry Analysis
4.5. Hydroxyproline Assay
4.6. RNA Isolation
4.7. Reverse Transcription and Droplet Digital Polymerase Chain Reaction
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chait, A.; den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Gulati, P.; Day, F.R.; Payne, F.; Ongen, H.; van de Bunt, M.; Gaulton, K.J.; Eicher, J.D.; Sharp, S.J.; Luan, J.; et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 2017, 49, 17–26. [Google Scholar] [CrossRef]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. 2015, 26, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ojeda, F.J.; Mendez-Gutierrez, A.; Aguilera, C.M.; Plaza-Diaz, J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef] [Green Version]
- Hammarstedt, A.; Gogg, S.; Hedjazifar, S.; Nerstedt, A.; Smith, U. Impaired adipogenesis and dysfunctional adipose tissue in human hypertrophic obesity. Physiol. Rev. 2018, 98, 1911–1941. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [Green Version]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Divoux, A.; Tordjman, J.; Lacasa, D.; Veyrie, N.; Hugol, D.; Aissat, A.; Basdevant, A.; Guerre-Millo, M.; Poitou, C.; Zucker, J.D.; et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010, 59, 2817–2825. [Google Scholar] [CrossRef] [Green Version]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol. Cell Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E., Jr.; Peterson, C.A.; Kern, P.A. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaud, A.; Tordjman, J.; Pelletier, M.; Liu, Y.; Laforest, S.; Noel, S.; Le Naour, G.; Bouchard, C.; Clement, K.; Tchernof, A. Relevance of omental pericellular adipose tissue collagen in the pathophysiology of human abdominal obesity and related cardiometabolic risk. Int. J. Obes. 2016, 40, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Burk, D.H.; Ali, M.R.; Mostaedi, R.; Smith, W.H.; Park, J.; Scherer, P.E.; Seay, S.A.; McCoin, C.S.; Bonaldo, P.; et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E233–E246. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, L.J.; Rawling, T.J.; Sjoholm, K.; Franck, N.; Dankel, S.N.; Price, E.J.; Knight, B.; Liversedge, N.H.; Mellgren, G.; Nystrom, F.; et al. COL6A3 is regulated by leptin in human adipose tissue and reduced in obesity. Endocrinology 2015, 156, 134–146. [Google Scholar] [CrossRef] [Green Version]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Armstrong, L.C.; Bornstein, P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003, 22, 63–71. [Google Scholar] [CrossRef]
- Guglielmi, V.; Cardellini, M.; Cinti, F.; Corgosinho, F.; Cardolini, I.; D’Adamo, M.; Zingaretti, M.C.; Bellia, A.; Lauro, D.; Gentileschi, P.; et al. Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr. Diabetes 2015, 5, e175. [Google Scholar] [CrossRef] [Green Version]
- Guzman-Ruiz, R.; Tercero-Alcazar, C.; Rabanal-Ruiz, Y.; Diaz-Ruiz, A.; El Bekay, R.; Rangel-Zuniga, O.A.; Navarro-Ruiz, M.C.; Molero, L.; Membrives, A.; Ruiz-Rabelo, J.F.; et al. Adipose tissue depot-specific intracellular and extracellular cues contributing to insulin resistance in obese individuals. FASEB J. 2020, 34, 7520–7539. [Google Scholar] [CrossRef] [Green Version]
- Datta, R.; Podolsky, M.J.; Atabai, K. Fat fibrosis: Friend or foe? JCI Insight 2018, 3, e122289. [Google Scholar] [CrossRef] [PubMed]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-Inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chu, M. Differential roles of VEGF: Relevance to tissue fibrosis. J. Cell Biochem. 2019, 120, 10945–10951. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Krautbauer, S.; Eisinger, K. Adipose tissue fibrosis. World J. Diabetes 2015, 6, 548–553. [Google Scholar] [CrossRef]
- Pasarica, M.; Gowronska-Kozak, B.; Burk, D.; Remedios, I.; Hymel, D.; Gimble, J.; Ravussin, E.; Bray, G.A.; Smith, S.R. Adipose tissue collagen VI in obesity. J. Clin. Endocrinol. Metab. 2009, 94, 5155–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, M.; Yao-Borengasser, A.; Unal, R.; Rasouli, N.; Gurley, C.M.; Zhu, B.; Peterson, C.A.; Kern, P.A. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E1016–E1027. [Google Scholar] [CrossRef]
- Abdennour, M.; Reggio, S.; Le Naour, G.; Liu, Y.; Poitou, C.; Aron-Wisnewsky, J.; Charlotte, F.; Bouillot, J.L.; Torcivia, A.; Sasso, M.; et al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: Links with diabetes and BMI loss after gastric bypass. J. Clin. Endocrinol. Metab. 2014, 99, 898–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeBari, M.K.; Abbott, R.D. Adipose tissue fibrosis: Mechanisms, models, and importance. Int. J. Mol. Sci. 2020, 21, 6030. [Google Scholar] [CrossRef]
- Huang, A.; Lin, Y.S.; Kao, L.Z.; Chiou, Y.W.; Lee, G.H.; Lin, H.H.; Wu, C.H.; Chang, C.S.; Lee, K.T.; Hsueh, Y.Y.; et al. Inflammation-Induced macrophage lysyl oxidase in adipose stiffening and dysfunction in obesity. Clin. Transl. Med. 2021, 11, e543. [Google Scholar] [CrossRef]
- Creemers, E.E.; Cleutjens, J.P.; Smits, J.F.; Daemen, M.J. Matrix metalloproteinase inhibition after myocardial infarction: A new approach to prevent heart failure? Circ. Res. 2001, 89, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Karsdal, M.A.; Nielsen, S.H.; Leeming, D.J.; Langholm, L.L.; Nielsen, M.J.; Manon-Jensen, T.; Siebuhr, A.; Gudmann, N.S.; Ronnow, S.; Sand, J.M.; et al. The good and the bad collagens of fibrosis—Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017, 121, 43–56. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuna, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and sex differences in adipose tissue. Curr. Diab. Rep. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Newell-Fugate, A.E. The role of sex steroids in white adipose tissue adipocyte function. Reproduction 2017, 153, R133–R149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabrielsson, B.G.; Olofsson, L.E.; Sjogren, A.; Jernas, M.; Elander, A.; Lonn, M.; Rudemo, M.; Carlsson, L.M. Evaluation of reference genes for studies of gene expression in human adipose tissue. Obes Res. 2005, 13, 649–652. [Google Scholar] [CrossRef]
- Sun, K.; Park, J.; Gupta, O.T.; Holland, W.L.; Auerbach, P.; Zhang, N.; Goncalves Marangoni, R.; Nicoloro, S.M.; Czech, M.P.; Varga, J.; et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nat. Commun. 2014, 5, 3485. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Aron-Wisnewsky, J.; Marcelin, G.; Genser, L.; Le Naour, G.; Torcivia, A.; Bauvois, B.; Bouchet, S.; Pelloux, V.; Sasso, M.; et al. Accumulation and changes in composition of collagens in subcutaneous adipose tissue after bariatric surgery. J. Clin. Endocrinol. Metab. 2016, 101, 293–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajan, M.R.; Sotak, M.; Barrenas, F.; Shen, T.; Borkowski, K.; Ashton, N.J.; Biorserud, C.; Lindahl, T.L.; Ramstrom, S.; Scholl, M.; et al. Comparative analysis of obesity-related cardiometabolic and renal biomarkers in human plasma and serum. Sci. Rep. 2019, 9, 15385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variables | MHL | MUO | p Values | Significance |
|---|---|---|---|---|
| Characteristics | ||||
| n | 8 | 15 | ||
| Sex | 1 ♂/7 ♀ | 4 ♂/11 ♀ | ||
| Age (years) | 40.0 (33.8–47.2) | 47.0 (35.0–54.0) | 0.583 | |
| Anthropometrics | ||||
| Body weight (kg) | 64.0 (59.2–65.5) | 131.5 (113.8–138.0) | 0.0001 | *** |
| BMI (kg/m2) | 22.2 (21.7–23.2) | 43.2 (39.6–44.9) | <0.0001 | *** |
| Waist circumference (cm) | 80.0 (77.5–88.0) | 124.5 (120.0–129.5) | 0.0001 | *** |
| Clinical measurements | ||||
| BP diastolic (mm Hg) | 72.5 (69.5–77.0) | 88.0 (81.0–96.0) | 0.002 | ** |
| BP systolic (mm Hg) | 119.0 (113.5–123.5) | 139.0 (132.5–148.5) | 0.0009 | *** |
| Heart Rate (bpm) | 79.5 (76.5–81.8) | 83.0 (69.5–89.5) | 0.815 | |
| Triglycerides (mmol/L) | 0.8 (0.7–0.9) | 1.2 (1.0–1.6) | 0.024 | * |
| Cholesterol (mmol/L) | 4.6 (4.5–5.2) | 4.8 (4.2–5.0) | 0.569 | |
| HDL-cholesterol (mmol/L) | 1.4 (1.3–1.6) | 1.2 (1.0–1.2) | 0.122 | |
| LDL-cholesterol (mmol/L) | 3.3 (3.1–3.6) | 3.0 (2.8–3.2) | 0.227 | |
| Glucose (mmol/L) | 5.6 (5.1–5.9) | 6.5 (5.9–7.0) | 0.012 | * |
| HbA1c (mmol/mol) | 30.0 (27.5–31.5) | 37.0 (34.0–42.0) | 0.024 | * |
| ALT (µkat/L) | 0.3 (0.3–0.4) | 0.5 (0.4–0.7) | 0.033 | * |
| ALP (µkat/L) | 1.1 (1.0–1.4) | 1.3 (1.0–1.6) | 0.456 | |
| AST (µkat/L) | 0.3 (0.3–0.4) | 0.4 (0.3–0.4) | 0.608 | |
| GGT (µkat/L) | 0.3 (0.2–0.4) | 0.5 (0.4–1.0) | 0.100 | |
| CRP (mg/L) | 1.0 (1.0–1.0) # | 5.5 (4.0–7.5) | 0.025 | * |
| Total leukocytes (109/L) | 7.4 (5.6–7.7) | 6.5 (6.1–8.2) | 0.906 | |
| Platelets (109/L) | 235.0 (196.0–253.5) | 255.0 (229.5–299.0) | 0.286 | |
| APTT (sec) | 27.0 (25.8–29.5) | 29.0 (26.0–31.0) | 0.913 | |
| Prothrombin complex (INR) | 1.0 (0.9–1.1) | 0.9 (0.9–1.0) | 0.118 | |
| Hemoglobin (g/L) | 140.5 (133.0–143.0) | 142.0 (129.0–145.5) | 0.796 | |
| Bilirubin (µmol/L) | 6.1 (3.9–9.8) | 5.3 (4.3–6.4) | 0.675 | |
| Creatinine (µmol/L) | 63.0 (59.0–65.5) | 68.0 (61.0–79.5) | 0.441 | |
| Adipocyte size oWAT (µm2) | 1838 (1658–2004) | 5041 (4582–5640) | <0.0001 | *** |
| Adipocyte size scWAT (µm2) | 3327 (1938–3987) | 5510 (4776–6063) | 0.0006 | *** |
| Medications | ||||
| Diabetes medications | 0% (0/8) | 13.3% (2/15) | 0.526 | |
| Anti-hypertensive medications | 0% (0/8) | 46.7% (7/15) | 0.052 | |
| Lipid lowering medications | 0% (0/8) | 13.3% (2/15) | 0.526 | |
| oWAT ECM | scWAT ECM | |||
|---|---|---|---|---|
| rs | p Value | rs | p Value | |
| Age | 0.238 | 0.286 | 0.055 | 0.806 |
| Body weight | −0.585 | 0.004 | −0.465 | 0.029 |
| BMI | −0.621 | 0.003 | −0.461 | 0.032 |
| Waist circumference | −0.569 | 0.006 | −0.419 | 0.052 |
| BP systolic | −0.414 | 0.055 | −0.252 | 0.258 |
| BP diastolic | −0.792 | <0.001 | −0.504 | 0.017 |
| Heart Rate | −0.311 | 0.183 | −0.308 | 0.186 |
| Glucose | −0.176 | 0.471 | −0.271 | 0.262 |
| HbA1C | −0.128 | 0.625 | 0.169 | 0.518 |
| Cholesterol | 0.365 | 0.150 | 0.180 | 0.490 |
| HDL-cholesterol | 0.481 | 0.050 | 0.163 | 0.532 |
| LDL-cholesterol | 0.333 | 0.192 | 0.203 | 0.435 |
| Triglycerides | −0.269 | 0.297 | −0.025 | 0.926 |
| CRP | −0.269 | 0.296 | −0.090 | 0.732 |
| ALP | −0.150 | 0.506 | −0.044 | 0.845 |
| AST | 0.018 | 0.936 | 0.164 | 0.465 |
| ALT | −0.293 | 0.185 | −0.126 | 0.576 |
| GGT | −0.160 | 0.540 | −0.053 | 0.840 |
| Total leukocytes | −0.206 | 0.427 | 0.026 | 0.922 |
| Platelets | −0.372 | 0.142 | −0.343 | 0.177 |
| APTT | −0.061 | 0.800 | −0.211 | 0.372 |
| Prothrombin complex | 0.164 | 0.489 | 0.156 | 0.512 |
| Hemoglobin | −0.058 | 0.797 | −0.226 | 0.312 |
| Bilirubin | 0.011 | 0.960 | −0.291 | 0.189 |
| Creatinine | −0.023 | 0.929 | −0.177 | 0.497 |
| Adipocyte size (median) | −0.629 | 0.004 | −0.388 | 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soták, M.; Rajan, M.R.; Clark, M.; Biörserud, C.; Wallenius, V.; Hagberg, C.E.; Börgeson, E. Healthy Subcutaneous and Omental Adipose Tissue Is Associated with High Expression of Extracellular Matrix Components. Int. J. Mol. Sci. 2022, 23, 520. https://doi.org/10.3390/ijms23010520
Soták M, Rajan MR, Clark M, Biörserud C, Wallenius V, Hagberg CE, Börgeson E. Healthy Subcutaneous and Omental Adipose Tissue Is Associated with High Expression of Extracellular Matrix Components. International Journal of Molecular Sciences. 2022; 23(1):520. https://doi.org/10.3390/ijms23010520
Chicago/Turabian StyleSoták, Matúš, Meenu Rohini Rajan, Madison Clark, Christina Biörserud, Ville Wallenius, Carolina E. Hagberg, and Emma Börgeson. 2022. "Healthy Subcutaneous and Omental Adipose Tissue Is Associated with High Expression of Extracellular Matrix Components" International Journal of Molecular Sciences 23, no. 1: 520. https://doi.org/10.3390/ijms23010520
APA StyleSoták, M., Rajan, M. R., Clark, M., Biörserud, C., Wallenius, V., Hagberg, C. E., & Börgeson, E. (2022). Healthy Subcutaneous and Omental Adipose Tissue Is Associated with High Expression of Extracellular Matrix Components. International Journal of Molecular Sciences, 23(1), 520. https://doi.org/10.3390/ijms23010520






