Chemogenetic Activation of Astrocytes in the Basolateral Amygdala Contributes to Fear Memory Formation by Modulating the Amygdala–Prefrontal Cortex Communication
Abstract
:1. Introduction
2. Methods and Materials
2.1. Animal and Ethical Consideration
2.2. Stereotactic Surgery and Virus Injection
2.3. Immunohistochemistry (IHC)
2.4. Behavioral Testing
2.4.1. Fear Conditioning (FC)
2.4.2. Non-Associative Place Recognition (NAPR)
2.4.3. Open Field Test (OFT)
2.4.4. Elevated Zero Maze (EZM)
2.4.5. Chemogenetic Manipulation: CNO Administration
2.5. Electrophysiology Recordings
Electrode Implantation
2.6. Data Acquisition
2.7. Data Analyses
2.8. Quantification and Statistical Analysis
3. Results
3.1. Astrocytic Gq Pathway Modulation Promotes Auditorily Cued Fear Memory
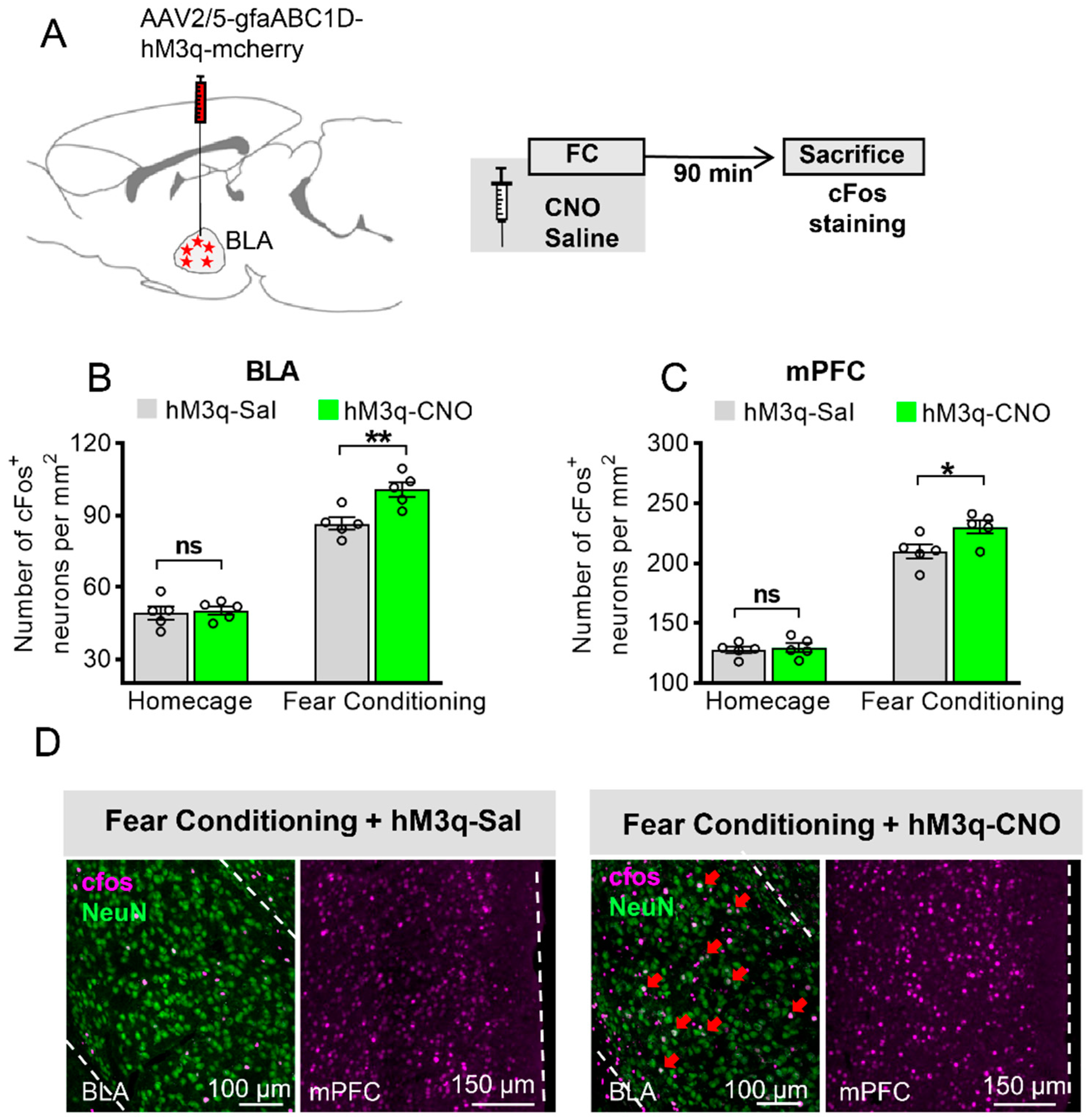
3.2. Astrocytic Gq Activation Increases c-Fos Expression in the BLA and the mPFC in Fear Conditioning, but Not in the Home Cage
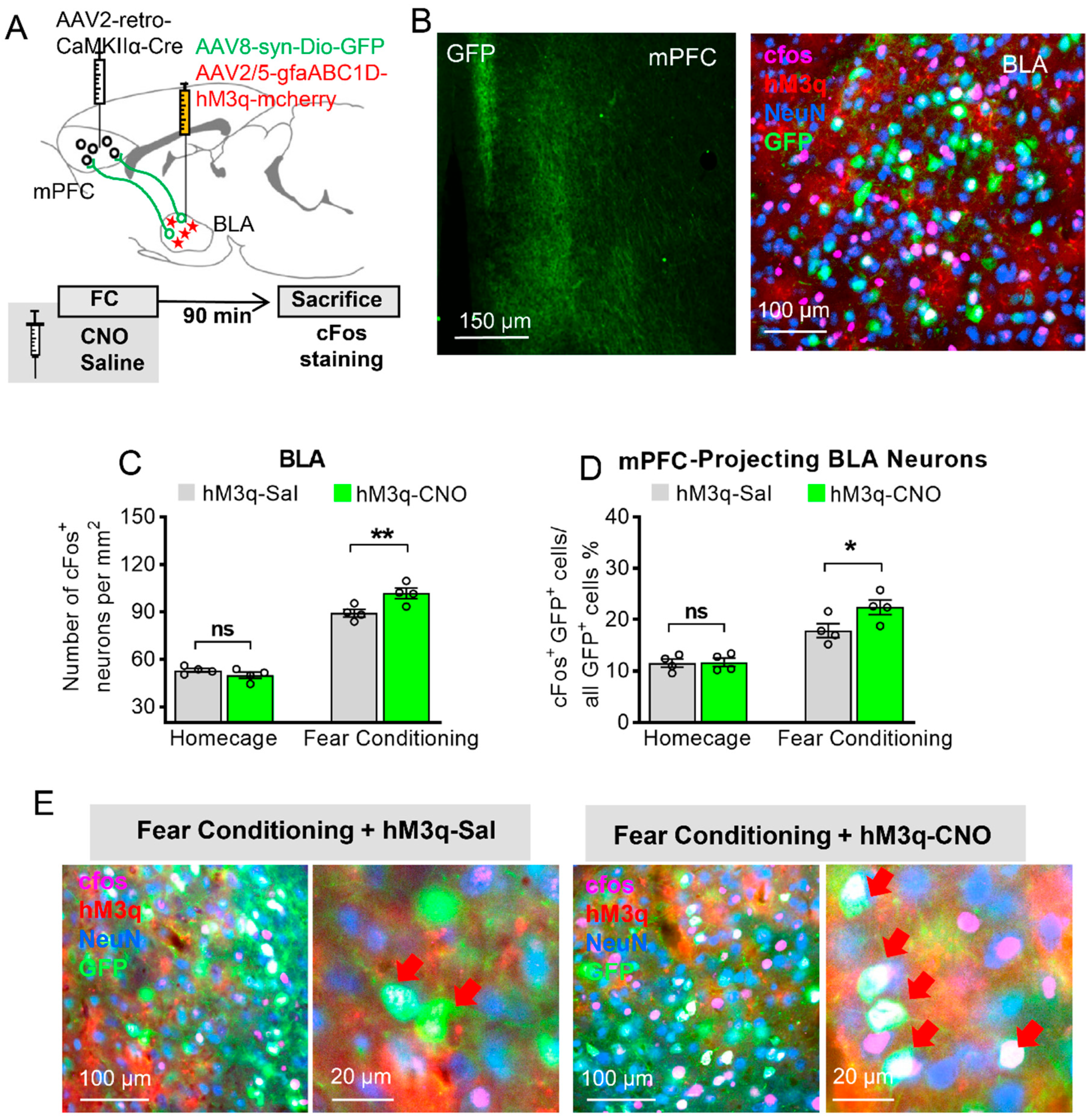
3.3. Astrocytic Gq Modulation Induced a Projection-Specific Enhancement of BLA–mPFC Neurons during Fear Learning
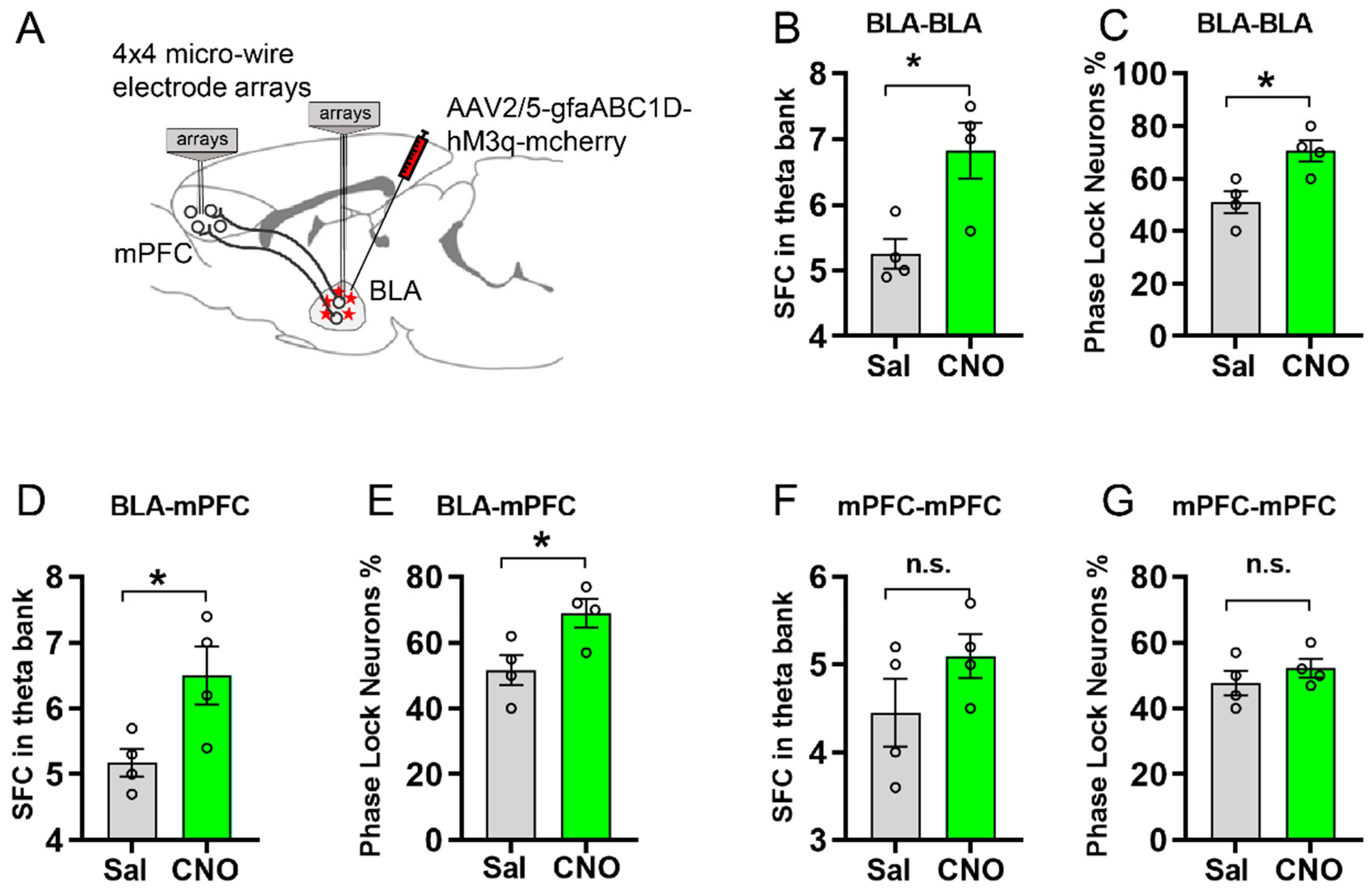
3.4. Activating Astrocytic Gq Pathway in BLA Facilitated Spike-Field Coherence and Phase Locking within BLA and between BLA and mPFC
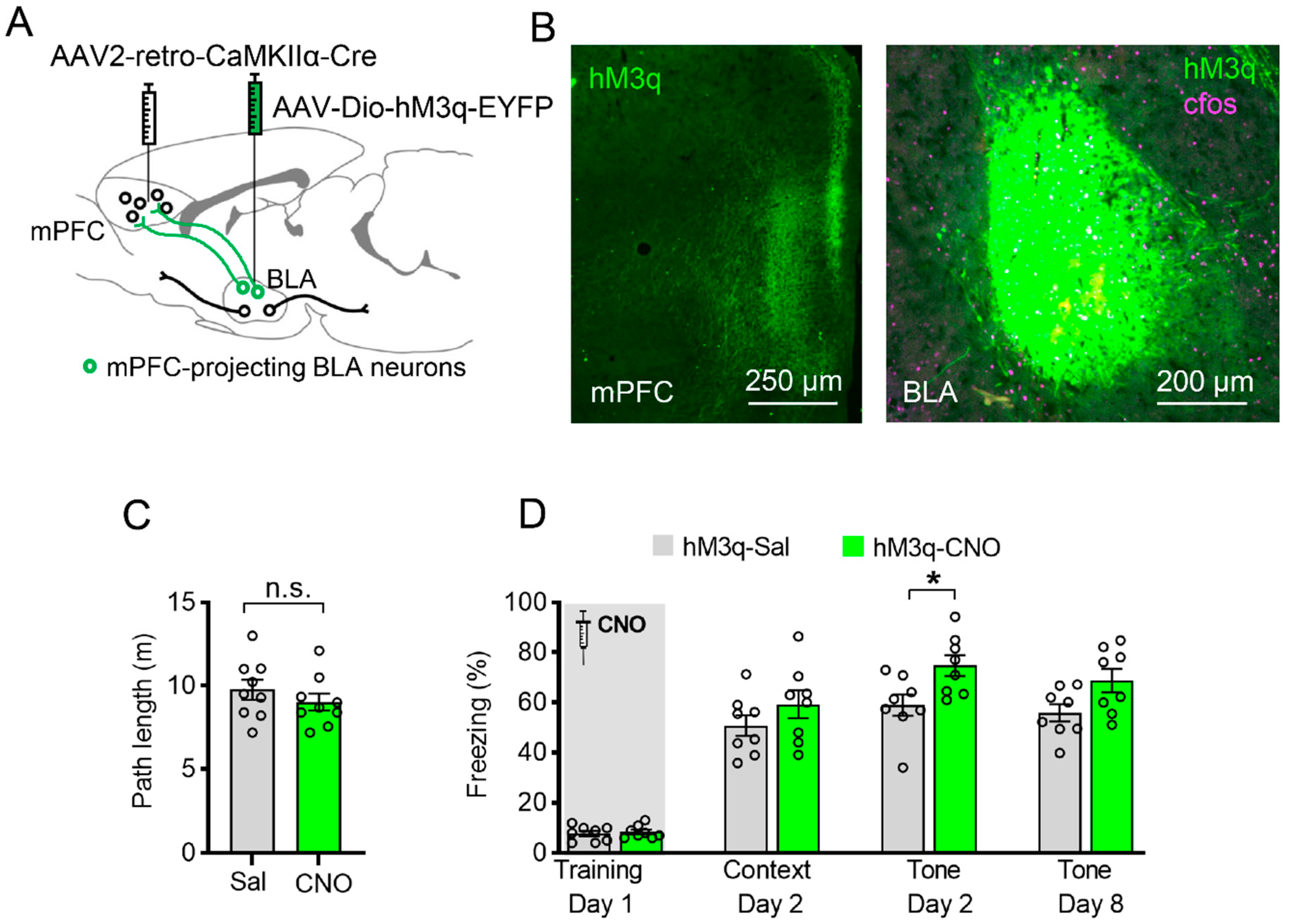
3.5. Specific Chemogenetic Activation of BLA-to-mPFC Projection during Learning Enhances Cued Fear Memory
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Letzkus, J.J.; Wolff, S.B.E.; Meyer, E.M.M.; Tovote, P.; Courtin, J.; Herry, C.; Lüthi, A. A Disinhibitory Microcircuit for Associative Fear Learning in the Auditory Cortex. Nature 2011, 480, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Aggleton, J.P. The Amygdala: A Functional Analysis; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Bocchio, M.; Nabavi, S.; Capogna, M. Synaptic Plasticity, Engrams, and Network Oscillations in Amygdala Circuits for Storage and Retrieval of Emotional Memories. Neuron 2017, 94, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Herry, C.; Johansen, J.P. Encoding of Fear Learning and Memory in Distributed Neuronal Circuits. Nat. Neurosci. 2014, 17, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Maren, S. Synaptic Mechanisms of Associative Memory in the Amygdala. Neuron 2005, 47, 783–786. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.M.; Schafe, G.E.; LeDoux, J.E. Molecular Mechanisms Underlying Emotional Learning and Memory in the Lateral Amygdala. Neuron 2004, 44, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Rogan, M.T.; Stäubli, U.V.; LeDoux, J.E. Fear Conditioning Induces Associative Long-Term Potentiation in the Amygdala. Nature 1997, 390, 604–607. [Google Scholar] [CrossRef]
- Alberini, C.M.; Cruz, E.; Descalzi, G.; Bessières, B.; Gao, V. Astrocyte Glycogen and Lactate: New Insights into Learning and Memory Mechanisms. Glia 2018, 66, 1244–1262. [Google Scholar] [CrossRef]
- Cho, W.-H.; Barcelon, E.; Lee, S.J. Optogenetic Glia Manipulation: Possibilities and Future Prospects. Exp. Neurobiol. 2016, 25, 197. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tu, J.; Cao, B.; Mu, L.; Yang, X.; Cong, M.; Ramkrishnan, A.S.; Chan, R.H.M.; Wang, L.; Li, Y. Astrocytic L-Lactate Signaling Facilitates Amygdala-Anterior Cingulate Cortex Synchrony and Decision Making in Rats. Cell Rep. 2017, 21, 2407–2418. [Google Scholar] [CrossRef]
- Nagai, J.; Yu, X.; Papouin, T.; Cheong, E.; Freeman, M.R.; Monk, K.R.; Hastings, M.H.; Haydon, P.G.; Rowitch, D.; Shaham, S.; et al. Behaviorally Consequential Astrocytic Regulation of Neural Circuits. Neuron 2021, 109, 576–596. [Google Scholar] [CrossRef]
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte–Neuron Communication. Neuroscience 2021, 456, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Adamsky, A.; Kol, A.; Kreisel, T.; Doron, A.; Ozeri-Engelhard, N.; Melcer, T.; Refaeli, R.; Horn, H.; Regev, L.; Groysman, M.; et al. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 2018, 174, 59–71.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kol, A.; Adamsky, A.; Groysman, M.; Kreisel, T.; London, M.; Goshen, I. Astrocytes Contribute to Remote Memory Formation by Modulating Hippocampal–Cortical Communication during Learning. Nat. Neurosci. 2020, 23, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Martin-Fernandez, M.; Jamison, S.; Robin, L.M.; Zhao, Z.; Martin, E.D.; Aguilar, J.; Benneyworth, M.A.; Marsicano, G.; Araque, A. Synapse-Specific Astrocyte Gating of Amygdala-Related Behavior. Nat. Neurosci. 2017, 20, 1540–1548. [Google Scholar] [CrossRef] [Green Version]
- Stehberg, J.; Moraga-Amaro, R.; Salazar, C.; Becerra, A.; Echeverría, C.; Orellana, J.A.; Bultynck, G.; Ponsaerts, R.; Leybaert, L.; Simon, F.; et al. Release of Gliotransmitters through Astroglial Connexin 43 Hemichannels Is Necessary for Fear Memory Consolidation in the Basolateral Amygdala. FASEB J. 2012, 26, 3649–3657. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Tao, Y.; Guo, X.; Cheng, D.; Wang, F.; Liu, X.; Ma, L. Fear Conditioning Downregulates Rac1 Activity in the Basolateral Amygdala Astrocytes to Facilitate the Formation of Fear Memory. Front. Mol. Neurosci. 2017, 10, 396. [Google Scholar] [CrossRef] [Green Version]
- Kastanenka, K.V.; Moreno-Bote, R.; De Pittà, M.; Perea, G.; Eraso-Pichot, A.; Masgrau, R.; Poskanzer, K.E.; Galea, E. A Roadmap to Integrate Astrocytes into Systems Neuroscience. Glia 2019, 68, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Poskanzer, K.E.; Yuste, R. Astrocytes Regulate Cortical State Switching in Vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E2675–E2684. [Google Scholar] [CrossRef] [Green Version]
- Takata, N.; Mishima, T.; Hisatsune, C.; Nagai, T.; Ebisui, E.; Mikoshiba, K.; Hirase, H. Astrocyte Calcium Signaling Transforms Cholinergic Modulation to Cortical Plasticity In Vivo. J. Neurosci. 2011, 31, 18155–18165. [Google Scholar] [CrossRef]
- Perea, G.; Gómez, R.; Mederos, S.; Covelo, A.; Ballesteros, J.J.; Schlosser, L.; Hernández-Vivanco, A.; Martín-Fernández, M.; Quintana, R.; Rayan, A.; et al. Activity-Dependent Switch of GABAergic Inhibition into Glutamatergic Excitation in Astrocyte-Neuron Networks. Elife 2016, 5, e20362. [Google Scholar] [CrossRef] [Green Version]
- Likhtik, E.; Stujenske, J.M.; Topiwala, M.A.; Harris, A.Z.; Gordon, J.A. Prefrontal Entrainment of Amygdala Activity Signals Safety in Learned Fear and Innate Anxiety. Nat. Neurosci. 2014, 17, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popa, D.; Duvarci, S.; Popescu, A.T.; Léna, C.; Paré, D. Coherent Amygdalocortical Theta Promotes Fear Memory Consolidation during Paradoxical Sleep. Proc. Natl. Acad. Sci. USA 2010, 107, 6516–6519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardinha, V.M.; Guerra-Gomes, S.; Caetano, I.; Tavares, G.; Martins, M.; Reis, J.S.; Correia, J.S.; Teixeira-Castro, A.; Pinto, L.; Sousa, N.; et al. Astrocytic Signaling Supports Hippocampal-Prefrontal Theta Synchronization and Cognitive Function. Glia 2017, 65, 1944–1960. [Google Scholar] [CrossRef] [PubMed]
- Bouton, M.E. Context, Ambiguity, and Unlearning: Sources of Relapse after Behavioral Extinction. Biol. Psychiatry 2002, 52, 976–986. [Google Scholar] [CrossRef]
- Burgos-Robles, A.; Vidal-Gonzalez, I.; Quirk, G.J. Sustained Conditioned Responses in Prelimbic Prefrontal Neurons Are Correlated with Fear Expression and Extinction Failure. J. Neurosci. 2009, 29, 8474–8482. [Google Scholar] [CrossRef]
- Cao, B.; Wang, J.; Mu, L.; Poon, D.C.H.; Li, Y. Impairment of Decision Making Associated with Disruption of Phase-Locking in the Anterior Cingulate Cortex in Viscerally Hypersensitive Rats. Exp. Neurol. 2016, 286, 21–31. [Google Scholar] [CrossRef]
- Mu, L.; Wang, J.; Cao, B.; Jelfs, B.; Chan, R.H.M.; Xu, X.; Hasan, M.; Zhang, X.; Li, Y. Impairment of Cognitive Function by Chemotherapy: Association with the Disruption of Phase-Locking and Synchronization in Anterior Cingulate Cortex. Mol. Brain 2015, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Murugappan, S.K.; Xie, L.; Wong, H.Y.; Iqbal, Z.; Lei, Z.; Ramkrishnan, A.S.; Li, Y. Suppression of Pain in the Late Phase of Chronic Trigeminal Neuropathic Pain Failed to Rescue the Decision-Making Deficits in Rats. Int. J. Mol. Sci. 2021, 22, 7846. [Google Scholar] [CrossRef]
- Yang, L.; Qi, Y.; Yang, Y. Astrocytes Control Food Intake by Inhibiting AGRP Neuron Activity via Adenosine A1 Receptors. Cell Rep. 2015, 11, 798–807. [Google Scholar] [CrossRef] [Green Version]
- Bullitt, E. Expression OfC-Fos-like Protein as a Marker for Neuronal Activity Following Noxious Stimulation in the Rat. J. Comp. Neurol. 1990, 296, 517–530. [Google Scholar] [CrossRef]
- Durkee, C.A.; Covelo, A.; Lines, J.; Kofuji, P.; Aguilar, J.; Araque, A. G i/o Protein-Coupled Receptors Inhibit Neurons but Activate Astrocytes and Stimulate Gliotransmission. Glia 2019, 67, 1076–1093. [Google Scholar] [CrossRef] [PubMed]
- Gao, V.; Suzuki, A.; Magistretti, P.J.; Lengacher, S.; Pollonini, G.; Steinman, M.Q.; Alberini, C.M. Astrocytic Β2- Adrenergic Receptors Mediate Hippocampal Long- Term Memory Consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 8526–8531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, L.A.; Korol, D.L.; Gold, P.E. Lactate Produced by Glycogenolysis in Astrocytes Regulates Memory Processing. PLoS ONE 2011, 6, e28427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Stern, S.A.; Bozdagi, O.; Huntley, G.W.; Walker, R.H.; Magistretti, P.J.; Alberini, C.M. Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 2011, 144, 810–823. [Google Scholar] [CrossRef] [Green Version]
- Tadi, M.; Allaman, I.; Lengacher, S.; Grenningloh, G.; Magistretti, P.J. Learning-Induced Gene Expression in the Hippocampus Reveals a Role of Neuron-Astrocyte Metabolic Coupling in Long Term Memory. PLoS ONE 2015, 10, e0141568. [Google Scholar] [CrossRef]
- Han, X.; Chen, M.; Wang, F.; Windrem, M.; Wang, S.; Shanz, S.; Xu, Q.; Oberheim, N.A.; Bekar, L.; Betstadt, S.; et al. Forebrain Engraftment by Human Glial Progenitor Cells Enhances Synaptic Plasticity and Learning in Adult Mice. Cell Stem Cell 2013, 12, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Orr, A.G.; Hsiao, E.C.; Wang, M.M.; Ho, K.; Kim, D.H.; Wang, X.; Guo, W.; Kang, J.; Yu, G.-Q.; Adame, A.; et al. Astrocytic Adenosine Receptor A2A and Gs-Coupled Signaling Regulate Memory. Nat. Neurosci. 2015, 18, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, L.; Wu, J.; Zhu, Z.; Feng, X.; Qin, L.; Zhu, Y.; Sun, L.; Liu, Y.; Qiu, Z.; et al. Activation of Astrocytes in Hippocampus Decreases Fear Memory through Adenosine A1 Receptors. Elife 2020, 9, 1–25. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, X.; Tu, J. Chronic Optogenetic Manipulation of Basolateral Amygdala Astrocytes Rescues Stress-Induced Anxiety. Biochem. Biophys. Res. Commun. 2020, 533, 657–664. [Google Scholar] [CrossRef]
- Klavir, O.; Prigge, M.; Sarel, A.; Paz, R.; Yizhar, O. Manipulating Fear Associations via Optogenetic Modulation of Amygdala Inputs to Prefrontal Cortex. Nat. Neurosci. 2017, 20, 836–844. [Google Scholar] [CrossRef]
- Burgos-Robles, A.; Kimchi, E.Y.; Izadmehr, E.M.; Porzenheim, M.J.; Ramos-Guasp, W.A.; Nieh, E.H.; Felix-Ortiz, A.C.; Namburi, P.; Leppla, C.A.; Presbrey, K.N.; et al. Amygdala Inputs to Prefrontal Cortex Guide Behavior amid Conflicting Cues of Reward and Punishment. Nat. Neurosci. 2017, 20, 824–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corkrum, M.; Covelo, A.; Lines, J.; Bellocchio, L.; Pisansky, M.; Loke, K.; Quintana, R.; Rothwell, P.E.; Lujan, R.; Marsicano, G.; et al. Dopamine-Evoked Synaptic Regulation in the Nucleus Accumbens Requires Astrocyte Activity. Neuron 2020, 105, 1036–1047.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Hong, S.I.; Lee, J.; Peyton, L.; Baker, M.; Choi, S.; Kim, H.; Chang, S.Y.; Choi, D.S. Activation of Astrocytes in the Dorsomedial Striatum Facilitates Transition From Habitual to Goal-Directed Reward-Seeking Behavior. Biol. Psychiatry 2020, 88, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutras, M.J.; Buffalo, E.A. Synchronous Neural Activity and Memory Formation. Curr. Opin. Neurobiol. 2010, 20, 150–155. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Z.; Xie, L.; Li, C.H.; Lam, Y.Y.; Ramkrishnan, A.S.; Fu, Z.; Zeng, X.; Liu, S.; Iqbal, Z.; Li, Y. Chemogenetic Activation of Astrocytes in the Basolateral Amygdala Contributes to Fear Memory Formation by Modulating the Amygdala–Prefrontal Cortex Communication. Int. J. Mol. Sci. 2022, 23, 6092. https://doi.org/10.3390/ijms23116092
Lei Z, Xie L, Li CH, Lam YY, Ramkrishnan AS, Fu Z, Zeng X, Liu S, Iqbal Z, Li Y. Chemogenetic Activation of Astrocytes in the Basolateral Amygdala Contributes to Fear Memory Formation by Modulating the Amygdala–Prefrontal Cortex Communication. International Journal of Molecular Sciences. 2022; 23(11):6092. https://doi.org/10.3390/ijms23116092
Chicago/Turabian StyleLei, Zhuogui, Li Xie, Cheuk Hin Li, Yuk Yan Lam, Aruna Surendran Ramkrishnan, Zhongqi Fu, Xianlin Zeng, Shu Liu, Zafar Iqbal, and Ying Li. 2022. "Chemogenetic Activation of Astrocytes in the Basolateral Amygdala Contributes to Fear Memory Formation by Modulating the Amygdala–Prefrontal Cortex Communication" International Journal of Molecular Sciences 23, no. 11: 6092. https://doi.org/10.3390/ijms23116092
APA StyleLei, Z., Xie, L., Li, C. H., Lam, Y. Y., Ramkrishnan, A. S., Fu, Z., Zeng, X., Liu, S., Iqbal, Z., & Li, Y. (2022). Chemogenetic Activation of Astrocytes in the Basolateral Amygdala Contributes to Fear Memory Formation by Modulating the Amygdala–Prefrontal Cortex Communication. International Journal of Molecular Sciences, 23(11), 6092. https://doi.org/10.3390/ijms23116092





