IL11 Stimulates IL33 Expression and Proinflammatory Fibroblast Activation across Tissues
Abstract
:1. Introduction
2. Results
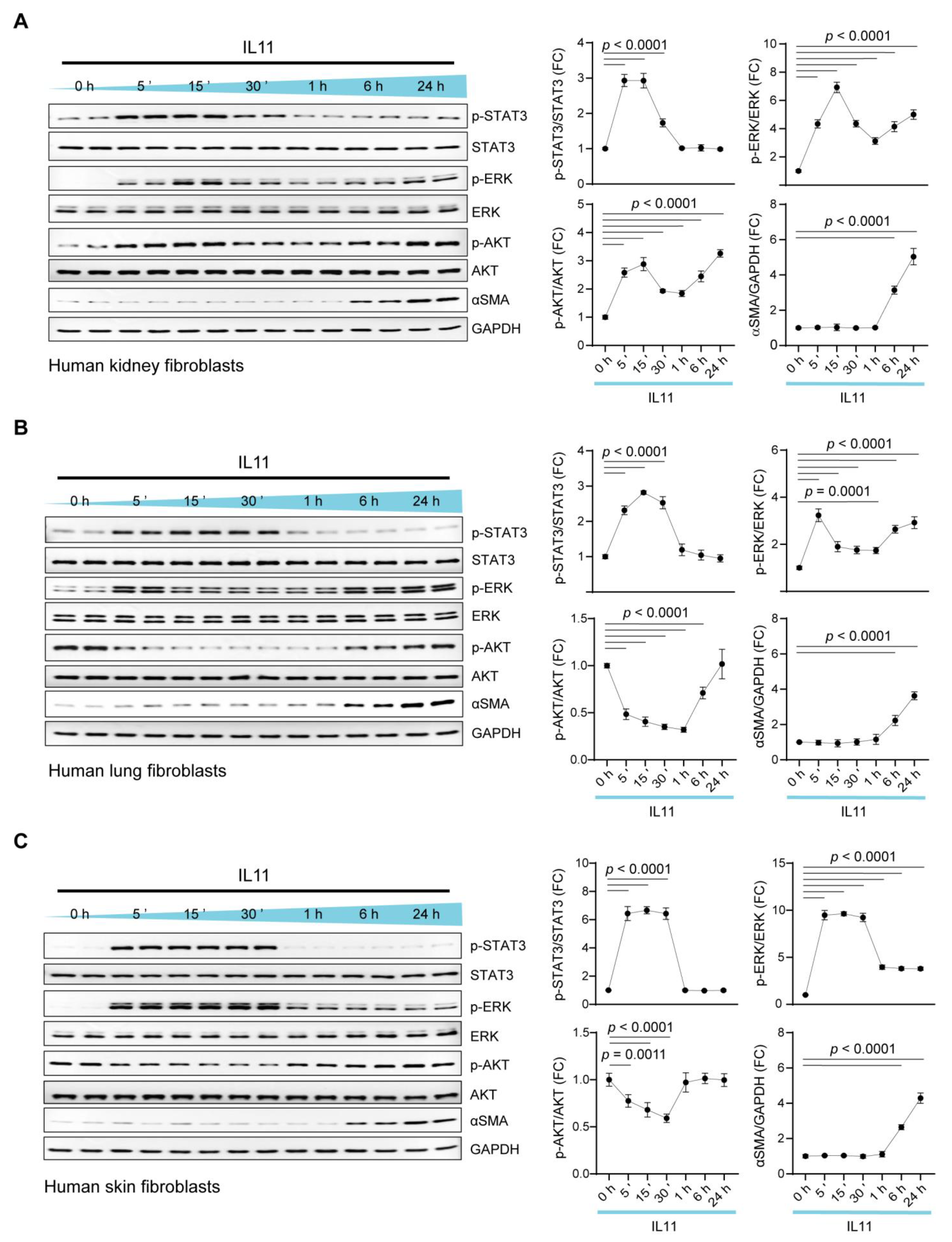
2.1. Time Course of IL11 Signaling in Fibroblasts from Three Tissues
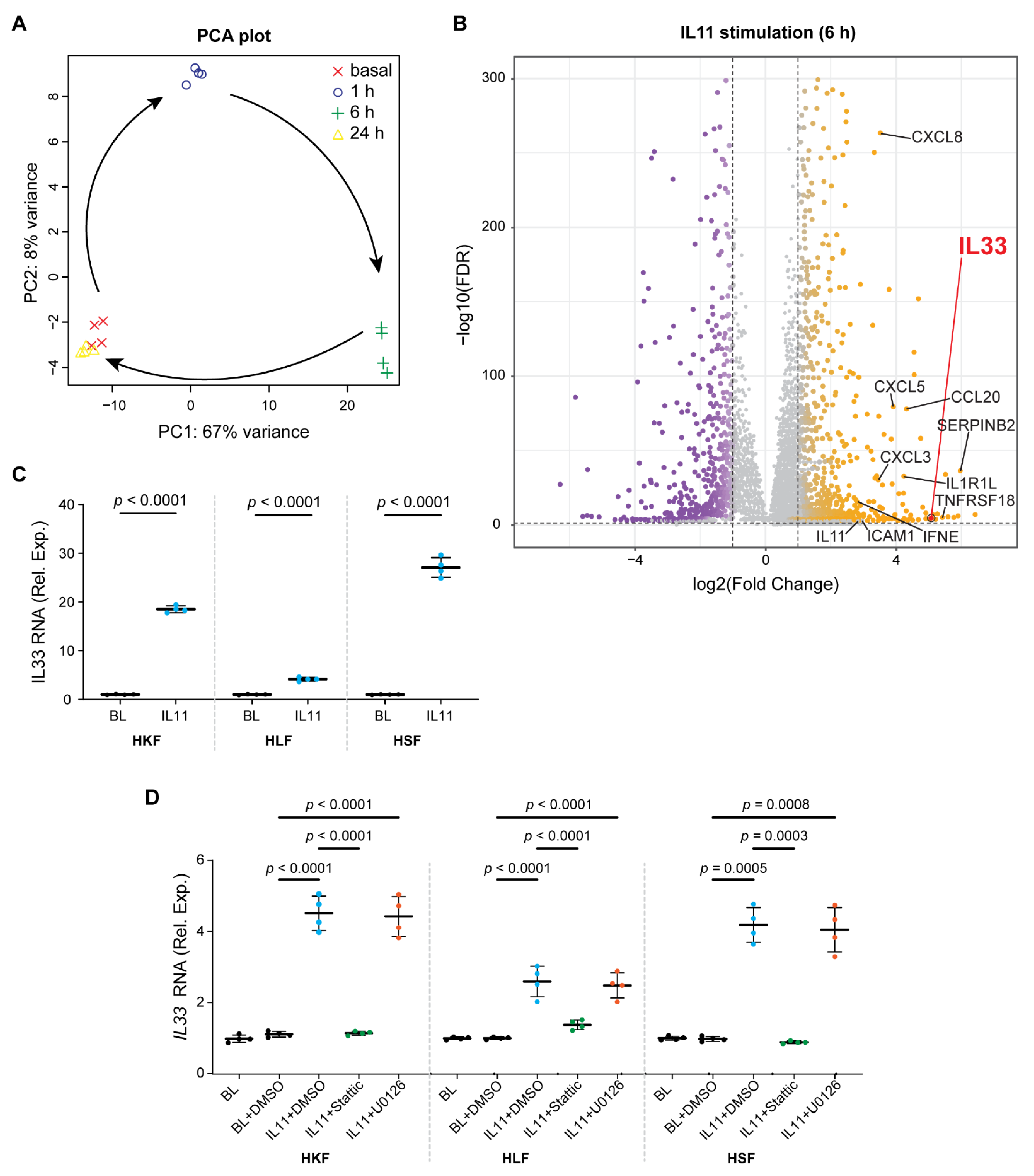
2.2. IL11 Activates a Pro-Inflammatory Transcriptional Response in Fibroblasts
2.3. Fibroblasts Stimulated with IL11 Secrete a Range of Pro-Inflammatory Factors
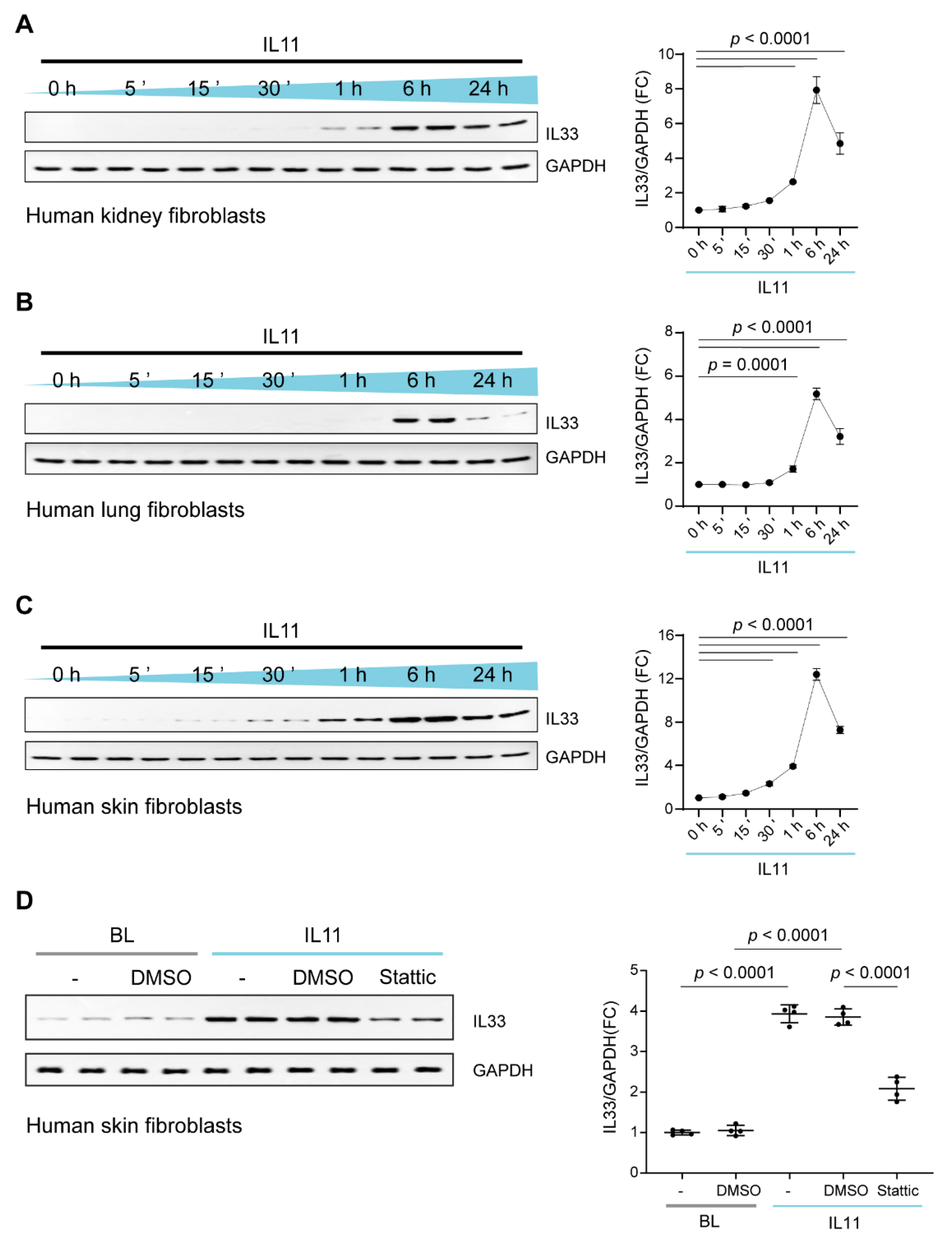
2.4. IL11 Stimulates STAT3-Dependent IL33 Upregulation in Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Antibodies
4.2. Recombinant Proteins
4.3. Chemicals
4.4. Cell Culture
4.5. RNA Sequencing
4.6. Western Blot
4.7. Quantitative Polymerase Chain Reaction (qPCR)
4.8. Proteomic Analysis Using Olink Proximity Extension Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, S.R.; Bennett, F.; Calvetti, J.A.; Kelleher, K.; Wood, C.R.; O’Hara, R.M., Jr.; Leary, A.C.; Sibley, B.; Clark, S.C.; Williams, D.A.; et al. Molecular Cloning of a cDNA Encoding Interleukin 11, a Stromal Cell-Derived Lymphopoietic and Hematopoietic Cytokine. Proc. Natl. Acad. Sci. USA 1990, 87, 7512–7516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widjaja, A.A.; Chothani, S.P.; Cook, S.A. Different Roles of Interleukin 6 and Interleukin 11 in the Liver: Implications for Therapy. Hum. Vaccines Immunother. 2020, 16, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.A.; Schafer, S. Hiding in Plain Sight: Interleukin-11 Emerges as a Master Regulator of Fibrosis, Tissue Integrity, and Stromal Inflammation. Annu. Rev. Med. 2020, 71, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Lin, B.; Huang, S.; Meng, J.; Zhang, F.; Zhou, M.; Hei, X.; Ke, Y.; Yang, H.; Huang, D. IL-11 Is Elevated and Drives the Profibrotic Phenotype Transition of Orbital Fibroblasts in Thyroid-Associated Ophthalmopathy. Front. Endocrinol. 2022, 13, 846106. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gu, S.; Liu, C.; Zhang, L.; Zhang, Z.; Zhao, Y.; Khoong, Y.; Li, H.; Gao, Y.; Liu, Y.; et al. CD39+ Fibroblasts Enhance Myofibroblast Activation by Promoting IL-11 Secretion in Hypertrophic Scars. J. Investig. Dermatol. 2022, 142, 1065–1076.e19. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Moreno-Moral, A.; DeLaughter, D.M.; Ng, B.; Patone, G.; Chow, K.; Khin, E.; et al. IL-11 Is a Crucial Determinant of Cardiovascular Fibrosis. Nature 2017, 552, 110–115. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Viswanathan, S.; Jinrui, D.; Singh, B.K.; Tan, J.; Wei Ting, J.G.; Lamb, D.; Shekeran, S.G.; George, B.L.; Schafer, S.; et al. Molecular Dissection of Pro-Fibrotic IL11 Signaling in Cardiac and Pulmonary Fibroblasts. Front. Mol. Biosci. 2021, 8, 926. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Dong, J.; Adami, E.; Viswanathan, S.; Ng, B.; Pakkiri, L.S.; Chothani, S.P.; Singh, B.K.; Lim, W.W.; Zhou, J.; et al. Redefining IL11 as a Regeneration-Limiting Hepatotoxin and Therapeutic Target in Acetaminophen-Induced Liver Injury. Sci. Transl. Med. 2021, 13, eaba8146. [Google Scholar] [CrossRef]
- Bai, X.; Zhao, G.; Chen, Q.; Li, Z.; Gao, M.; Ho, W.; Xu, X.; Zhang, X.-Q. Inhaled siRNA Nanoparticles Targeting IL11 Inhibit Lung Fibrosis and Improve Pulmonary Function Post-Bleomycin Challenge. Sci. Adv. 2022, 8, eabn7162. [Google Scholar] [CrossRef]
- Widjaja, A.; Shekeran, S.; Adami, E.; Goh, J.; Tan, J.; Viswanathan, S.; Lim, S.Y.; Tan, P.H.; Hubner, N.; Coffman, T.; et al. A Neutralizing IL-11 Antibody Improves Renal Function and Increases Lifespan in a Mouse Model of Alport Syndrome. J. Am. Soc. Nephrol. 2022, 33, 718–730. [Google Scholar] [CrossRef]
- Jasso, G.J.; Jaiswal, A.; Varma, M.; Laszewski, T.; Grauel, A.; Omar, A.; Silva, N.; Dranoff, G.; Porter, J.A.; Mansfield, K.; et al. Colon Stroma Mediates an Inflammation-Driven Fibroblastic Response Controlling Matrix Remodeling and Healing. PLoS Biol. 2022, 20, e3001532. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Louis, C.; Metcalfe, R.D.; Kosasih, C.C.; Wicks, I.P.; Griffin, M.D.W.; Putoczki, T.L. Emerging Roles for IL-11 in Inflammatory Diseases. Cytokine 2022, 149, 155750. [Google Scholar] [CrossRef] [PubMed]
- Bozza, M.; Bliss, J.L.; Maylor, R.; Erickson, J.; Donnelly, L.; Bouchard, P.; Dorner, A.J.; Trepicchio, W.L. Interleukin-11 Reduces T-Cell-Dependent Experimental Liver Injury in Mice. Hepatology 1999, 30, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Trepicchio, W.L.; Bozza, M.; Pedneault, G.; Dorner, A.J. Recombinant Human IL-11 Attenuates the Inflammatory Response through down-Regulation of Proinflammatory Cytokine Release and Nitric Oxide Production. J. Immunol. 1996, 157, 3627–3634. [Google Scholar] [PubMed]
- Shimizu, T.; Shiratori, K.; Sawada, T.; Kobayashi, M.; Hayashi, N.; Saotome, H.; Keith, J.C. Recombinant Human Interleukin-11 Decreases Severity of Acute Necrotizing Pancreatitis in Mice. Pancreas 2000, 21, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, M.; Butler, D.M.; Marinova-Mutafchieva, L.; Feldmann, M. An Anti-Inflammatory Role for Interleukin-11 in Established Murine Collagen-Induced Arthritis. Immunology 1998, 95, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kuo, D.; Ding, J.; Cohn, I.S.; Zhang, F.; Wei, K.; Rao, D.A.; Rozo, C.; Sokhi, U.K.; Shanaj, S.; Oliver, D.J.; et al. HBEGF Macrophages in Rheumatoid Arthritis Induce Fibroblast Invasiveness. Sci. Transl. Med. 2019, 11, eaau8587. [Google Scholar] [CrossRef]
- Ernst, M.; Najdovska, M.; Grail, D.; Lundgren-May, T.; Buchert, M.; Tye, H.; Matthews, V.B.; Armes, J.; Bhathal, P.S.; Hughes, N.R.; et al. STAT3 and STAT1 Mediate IL-11-Dependent and Inflammation-Associated Gastric Tumorigenesis in gp130 Receptor Mutant Mice. J. Clin. Investig. 2008, 118, 1727–1738. [Google Scholar] [CrossRef]
- Smillie, C.S.; Biton, M.; Ordovas-Montanes, J.; Sullivan, K.M.; Burgin, G.; Graham, D.B.; Herbst, R.H.; Rogel, N.; Slyper, M.; Waldman, J.; et al. Intra- and Inter-Cellular Rewiring of the Human Colon during Ulcerative Colitis. Cell 2019, 178, 714–730.e22. [Google Scholar] [CrossRef]
- Lim, W.-W.; Corden, B.; Ng, B.; Vanezis, K.; D’Agostino, G.; Widjaja, A.A.; Song, W.-H.; Xie, C.; Su, L.; Kwek, X.-Y.; et al. Interleukin-11 Is Important for Vascular Smooth Muscle Phenotypic Switching and Aortic Inflammation, Fibrosis and Remodeling in Mouse Models. Sci. Rep. 2020, 10, 17853. [Google Scholar] [CrossRef]
- Lim, W.-W.; Dong, J.; Ng, B.; Widjaja, A.A.; Xie, C.; Su, L.; Kwek, X.-Y.; Tee, N.G.Z.; Jian Pua, C.; Schafer, S.; et al. Inhibition of IL11 Signaling Reduces Aortic Pathology in Murine Marfan Syndrome. Circ. Res. 2022, 130, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Viswanathan, S.; Adami, E.; Singh, B.K.; Chothani, S.P.; Ng, B.; Lim, W.W.; Zhou, J.; Tripathi, M.; Ko, N.S.J.; et al. Hepatocyte-Specific IL11 Cis-Signaling Drives Lipotoxicity and Underlies the Transition from NAFLD to NASH. Nat. Commun. 2021, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.A.; Singh, B.K.; Adami, E.; Viswanathan, S.; Dong, J.; D’Agostino, G.A.; Ng, B.; Lim, W.W.; Tan, J.; Paleja, B.S.; et al. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Non-Alcoholic Steatohepatitis. Gastroenterology 2019, 157, 777–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishina, T.; Deguchi, Y.; Ohshima, D.; Takeda, W.; Ohtsuka, M.; Shichino, S.; Ueha, S.; Yamazaki, S.; Kawauchi, M.; Nakamura, E.; et al. Interleukin-11-Expressing Fibroblasts Have a Unique Gene Signature Correlated with Poor Prognosis of Colorectal Cancer. Nat. Commun. 2021, 12, 2281. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as Immune Regulators in Infection, Inflammation and Cancer. Nat. Rev. Immunol. 2021, 21, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Koliaraki, V.; Prados, A.; Armaka, M.; Kollias, G. The Mesenchymal Context in Inflammation, Immunity and Cancer. Nat. Immunol. 2020, 21, 974–982. [Google Scholar] [CrossRef]
- Zhu, M.; Lu, B.; Cao, Q.; Wu, Z.; Xu, Z.; Li, W.; Yao, X.; Liu, F. IL-11 Attenuates Liver Ischemia/Reperfusion Injury (IRI) through STAT3 Signaling Pathway in Mice. PLoS ONE 2015, 10, e0126296. [Google Scholar] [CrossRef]
- Mühl, H. STAT3, a Key Parameter of Cytokine-Driven Tissue Protection during Sterile Inflammation—The Case of Experimental Acetaminophen (Paracetamol)-Induced Liver Damage. Front. Immunol. 2016, 7, 163. [Google Scholar] [CrossRef] [Green Version]
- Schust, J.; Sperl, B.; Hollis, A.; Mayer, T.U.; Berg, T. Stattic: A Small-Molecule Inhibitor of STAT3 Activation and Dimerization. Chem. Biol. 2006, 13, 1235–1242. [Google Scholar] [CrossRef] [Green Version]
- Cayrol, C.; Girard, J.-P. Interleukin-33 (IL-33): A Critical Review of Its Biology and the Mechanisms Involved in Its Release as a Potent Extracellular Cytokine. Cytokine 2022, 156, 155891. [Google Scholar] [CrossRef]
- Trepicchio, W.L.; Wang, L.; Bozza, M.; Dorner, A.J. IL-11 Regulates Macrophage Effector Function through the Inhibition of Nuclear Factor-kappaB. J. Immunol. 1997, 159, 5661–5670. [Google Scholar] [PubMed]
- Maheshwari, A.; Janssens, K.; Bogie, J.; Van Den Haute, C.; Struys, T.; Lambrichts, I.; Baekelandt, V.; Stinissen, P.; Hendriks, J.J.A.; Slaets, H.; et al. Local Overexpression of Interleukin-11 in the Central Nervous System Limits Demyelination and Enhances Remyelination. Mediat. Inflamm. 2013, 2013, 685317. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.S.; Pfeiffer, C.J.; Keith, J.C., Jr. Protection by Recombinant Human Interleukin-11 against Experimental TNB-Induced Colitis in Rats. Dig. Dis. Sci. 1996, 41, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Bozza, M.; Bliss, J.L.; Dorner, A.J.; Trepicchio, W.L. Interleukin-11 Modulates Th1/Th2 Cytokine Production from Activated CD4+ T Cells. J. Interferon Cytokine Res. 2001, 21, 21–30. [Google Scholar] [CrossRef]
- Herrlinger, K.R.; Witthoeft, T.; Raedler, A.; Bokemeyer, B.; Krummenerl, T.; Schulzke, J.-D.; Boerner, N.; Kueppers, B.; Emmrich, J.; Mescheder, A.; et al. Randomized, Double Blind Controlled Trial of Subcutaneous Recombinant Human Interleukin-11 versus Prednisolone in Active Crohn’s Disease. Am. J. Gastroenterol. 2006, 101, 793–797. [Google Scholar] [CrossRef]
- Sands, B.E.; Winston, B.D.; Salzberg, B.; Safdi, M.; Barish, C.; Wruble, L.; Wilkins, R.; Shapiro, M.; Schwertschlag, U.S. RHIL-11 Crohn’s Study group Randomized, Controlled Trial of Recombinant Human Interleukin-11 in Patients with Active Crohn’s Disease. Aliment. Pharmacol. Ther. 2002, 16, 399–406. [Google Scholar] [CrossRef]
- Lawitz, E.J.; Hepburn, M.J.; Casey, T.J. A Pilot Study of Interleukin-11 in Subjects with Chronic Hepatitis C and Advanced Liver Disease Nonresponsive to Antiviral Therapy. Am. J. Gastroenterol. 2004, 99, 2359–2364. [Google Scholar] [CrossRef]
- Moreland, L.; Gugliotti, R.; King, K.; Chase, W.; Weisman, M.; Greco, T.; Fife, R.; Korn, J.; Simms, R.; Tesser, J.; et al. Results of a Phase-I/II Randomized, Masked, Placebo-Controlled Trial of Recombinant Human Interleukin-11 (rhIL-11) in the Treatment of Subjects with Active Rheumatoid Arthritis. Arthritis Res. 2001, 3, 247–252. [Google Scholar] [CrossRef]
- Trepicchio, W.L.; Ozawa, M.; Walters, I.B.; Kikuchi, T.; Gilleaudeau, P.; Bliss, J.L.; Schwertschlag, U.; Dorner, A.J.; Krueger, J.G. Interleukin-11 Therapy Selectively Downregulates Type I Cytokine Proinflammatory Pathways in Psoriasis Lesions. J. Clin. Investig. 1999, 104, 1527–1537. [Google Scholar] [CrossRef] [Green Version]
- Obana, M.; Miyamoto, K.; Murasawa, S.; Iwakura, T.; Hayama, A.; Yamashita, T.; Shiragaki, M.; Kumagai, S.; Miyawaki, A.; Takewaki, K.; et al. Therapeutic Administration of IL-11 Exhibits the Postconditioning Effects against Ischemia-Reperfusion Injury via STAT3 in the Heart. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H569–H577. [Google Scholar] [CrossRef] [Green Version]
- Obana, M.; Maeda, M.; Takeda, K.; Hayama, A.; Mohri, T.; Yamashita, T.; Nakaoka, Y.; Komuro, I.; Takeda, K.; Matsumiya, G.; et al. Therapeutic Activation of Signal Transducer and Activator of Transcription 3 by Interleukin-11 Ameliorates Cardiac Fibrosis after Myocardial Infarction. Circulation 2010, 121, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, R.; Maeda, M.; Arita, A.; Oshima, Y.; Obana, M.; Ito, T.; Yamamoto, Y.; Mohri, T.; Kishimoto, T.; Kawase, I.; et al. Identification of Cardiac Myocytes as the Target of Interleukin 11, a Cardioprotective Cytokine. Cytokine 2007, 38, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Eissmann, M.F.; Dijkstra, C.; Jarnicki, A.; Phesse, T.; Brunnberg, J.; Poh, A.R.; Etemadi, N.; Tsantikos, E.; Thiem, S.; Huntington, N.D.; et al. IL-33-Mediated Mast Cell Activation Promotes Gastric Cancer through Macrophage Mobilization. Nat. Commun. 2019, 10, 2735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maywald, R.L.; Doerner, S.K.; Pastorelli, L.; De Salvo, C.; Benton, S.M.; Dawson, E.P.; Lanza, D.G.; Berger, N.A.; Markowitz, S.D.; Lenz, H.-J.; et al. IL-33 Activates Tumor Stroma to Promote Intestinal Polyposis. Proc. Natl. Acad. Sci. USA 2015, 112, E2487–E2496. [Google Scholar] [CrossRef] [Green Version]
- Hatzioannou, A.; Banos, A.; Sakelaropoulos, T.; Fedonidis, C.; Vidali, M.-S.; Köhne, M.; Händler, K.; Boon, L.; Henriques, A.; Koliaraki, V.; et al. An Intrinsic Role of IL-33 in Treg Cell-Mediated Tumor Immunoevasion. Nat. Immunol. 2020, 21, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gallage, S.; Mellone, M.; Wuestefeld, T.; Klotz, S.; Hanley, C.J.; Raguz, S.; Acosta, J.C.; Innes, A.J.; Banito, A.; et al. mTOR Regulates MAPKAPK2 Translation to Control the Senescence-Associated Secretory Phenotype. Nat. Cell Biol. 2015, 17, 1205–1217. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Holland, J.W.; Bols, N.; Secombes, C.J. Cloning and Expression of the First Nonmammalian Interleukin-11 Gene in Rainbow Trout Oncorhynchus Mykiss. FEBS J. 2005, 272, 1136–1147. [Google Scholar] [CrossRef]
- Xu, L.; Podok, P.; Xie, J.; Lu, L. Comparative Analysis of Differential Gene Expression in Kidney Tissues of Moribund and Surviving Crucian Carp (Carassius auratus Gibelio) in Response to Cyprinid Herpesvirus 2 Infection. Arch. Virol. 2014, 159, 1961–1974. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Cao, Z.; Sun, Y.; Chen, Y.; Xiang, Y.; Wang, L.; Zhang, S.; Guo, W. Comparative Analysis of the Expression Patterns of IL-1β, IL-11, and IL-34 in Golden Pompano (Trachinotus ovatus) Following Different Pathogens Challenge. Fish Shellfish Immunol. 2019, 93, 863–870. [Google Scholar] [CrossRef]
- Wangkahart, E.; Secombes, C.J.; Wang, T. Studies on the Use of Flagellin as an Immunostimulant and Vaccine Adjuvant in Fish Aquaculture. Front. Immunol. 2018, 9, 3054. [Google Scholar] [CrossRef]
- Dawson, H.D.; Chen, C.; Li, R.W.; Bell, L.N.; Shea-Donohue, T.; Kringel, H.; Beshah, E.; Hill, D.E.; Urban, J.F. Molecular and Metabolomic Changes in the Proximal Colon of Pigs Infected with Trichuris Suis. Sci. Rep. 2020, 10, 12853. [Google Scholar] [CrossRef] [PubMed]
- Chothani, S.; Schäfer, S.; Adami, E.; Viswanathan, S.; Widjaja, A.A.; Langley, S.R.; Tan, J.; Wang, M.; Quaife, N.M.; Jian Pua, C.; et al. Widespread Translational Control of Fibrosis in the Human Heart by RNA-Binding Proteins. Circulation 2019, 140, 937–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 19 March 2022).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize Analysis Results for Multiple Tools and Samples in a Single Report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Assarsson, E.; Lundberg, M.; Holmquist, G.; Björkesten, J.; Thorsen, S.B.; Ekman, D.; Eriksson, A.; Rennel Dickens, E.; Ohlsson, S.; Edfeldt, G.; et al. Homogenous 96-Plex PEA Immunoassay Exhibiting High Sensitivity, Specificity, and Excellent Scalability. PLoS ONE 2014, 9, e95192. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widjaja, A.A.; Chothani, S.; Viswanathan, S.; Goh, J.W.T.; Lim, W.-W.; Cook, S.A. IL11 Stimulates IL33 Expression and Proinflammatory Fibroblast Activation across Tissues. Int. J. Mol. Sci. 2022, 23, 8900. https://doi.org/10.3390/ijms23168900
Widjaja AA, Chothani S, Viswanathan S, Goh JWT, Lim W-W, Cook SA. IL11 Stimulates IL33 Expression and Proinflammatory Fibroblast Activation across Tissues. International Journal of Molecular Sciences. 2022; 23(16):8900. https://doi.org/10.3390/ijms23168900
Chicago/Turabian StyleWidjaja, Anissa A., Sonia Chothani, Sivakumar Viswanathan, Joyce Wei Ting Goh, Wei-Wen Lim, and Stuart A. Cook. 2022. "IL11 Stimulates IL33 Expression and Proinflammatory Fibroblast Activation across Tissues" International Journal of Molecular Sciences 23, no. 16: 8900. https://doi.org/10.3390/ijms23168900
APA StyleWidjaja, A. A., Chothani, S., Viswanathan, S., Goh, J. W. T., Lim, W.-W., & Cook, S. A. (2022). IL11 Stimulates IL33 Expression and Proinflammatory Fibroblast Activation across Tissues. International Journal of Molecular Sciences, 23(16), 8900. https://doi.org/10.3390/ijms23168900






