Striatal Neurons Partially Expressing a Dopaminergic Phenotype: Functional Significance and Regulation
Abstract
:1. Introduction
2. Results
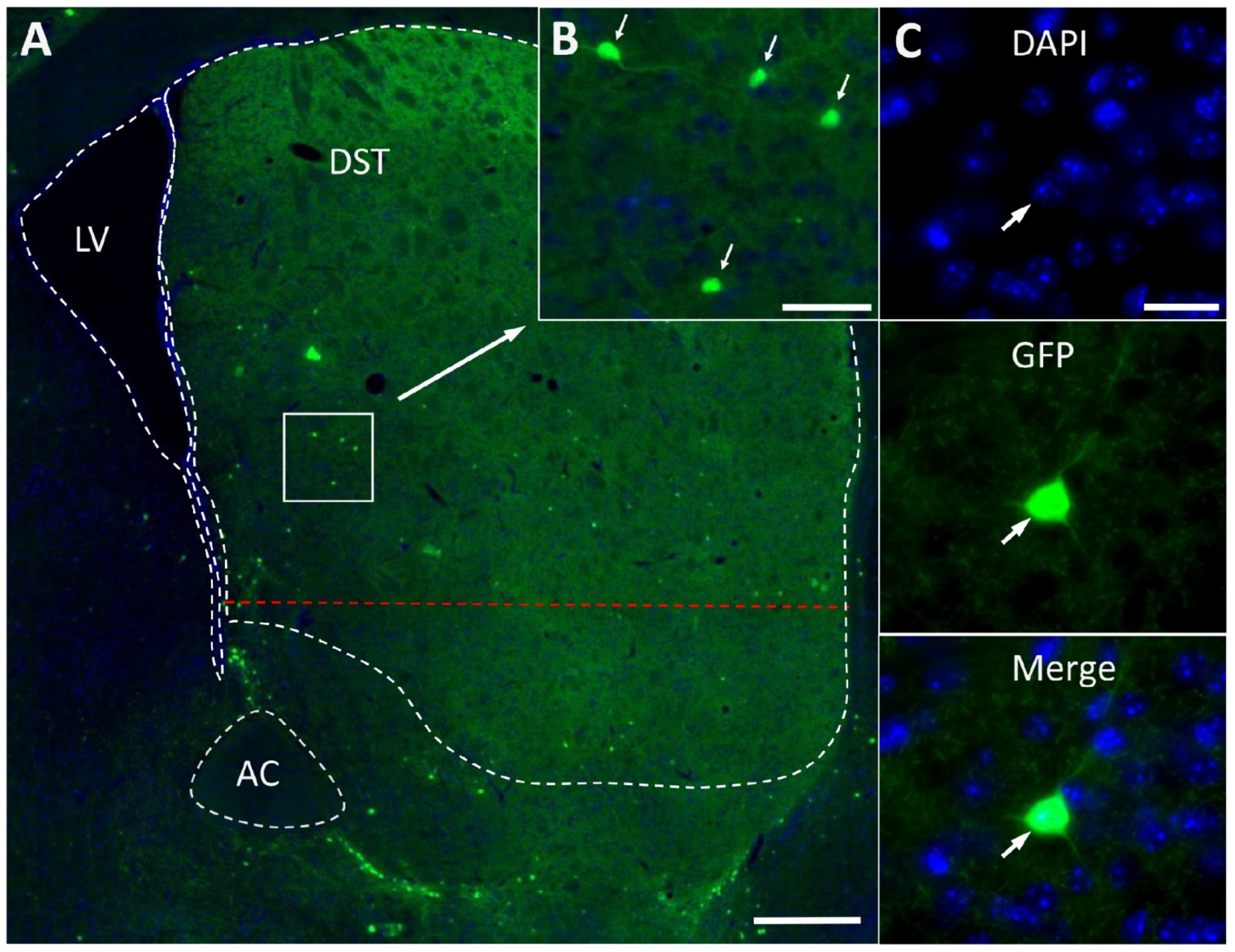
2.1. Fluorescence Microscopy of Striatal Neurons Expressing Green Fluorescence Protein
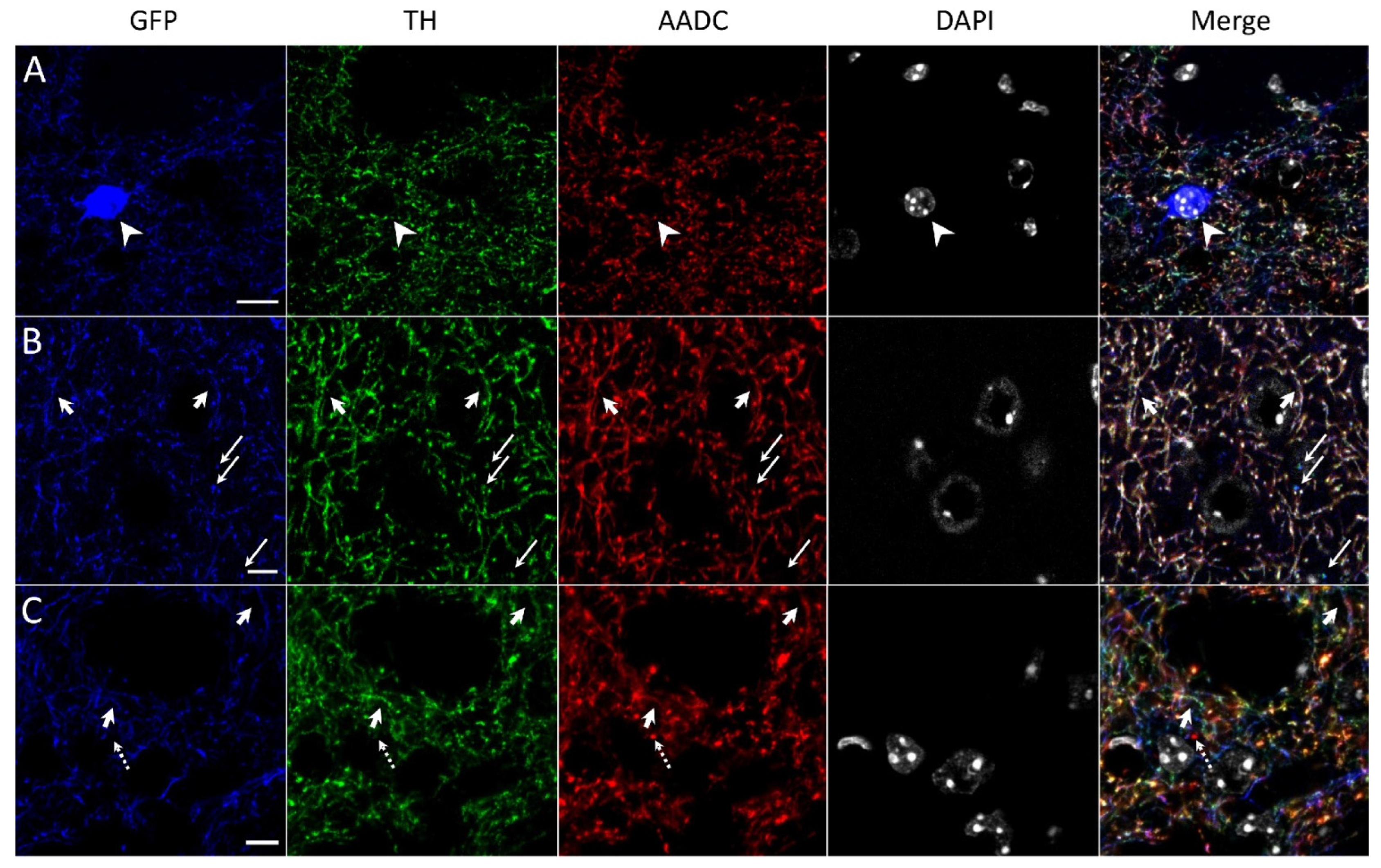
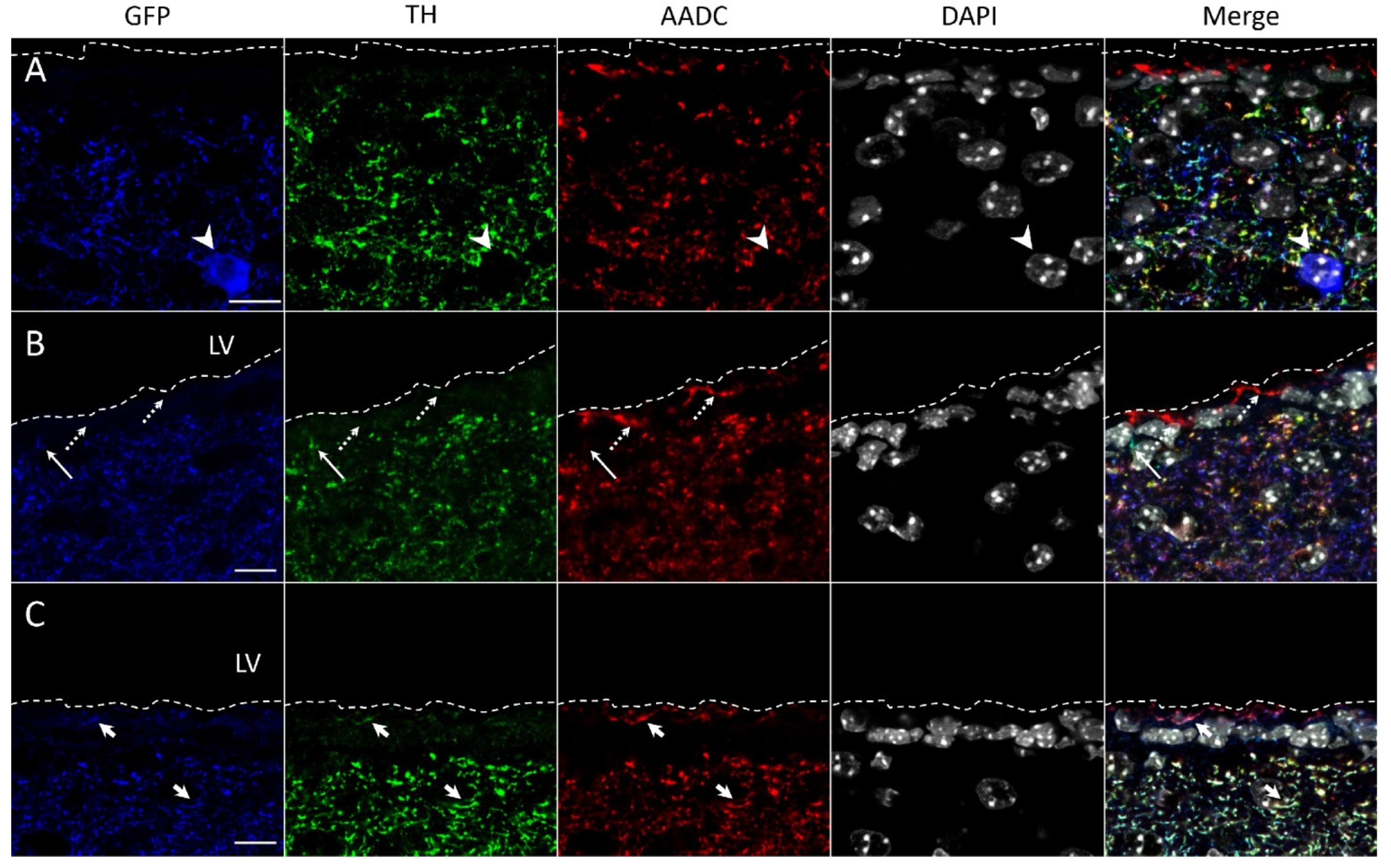
2.2. Confocal Microscopy of the Striatum of Transgenic Mice after Immunostaining for Tyrosine Hydroxylase and Aromatic L-Amino Acids Decarboxylase
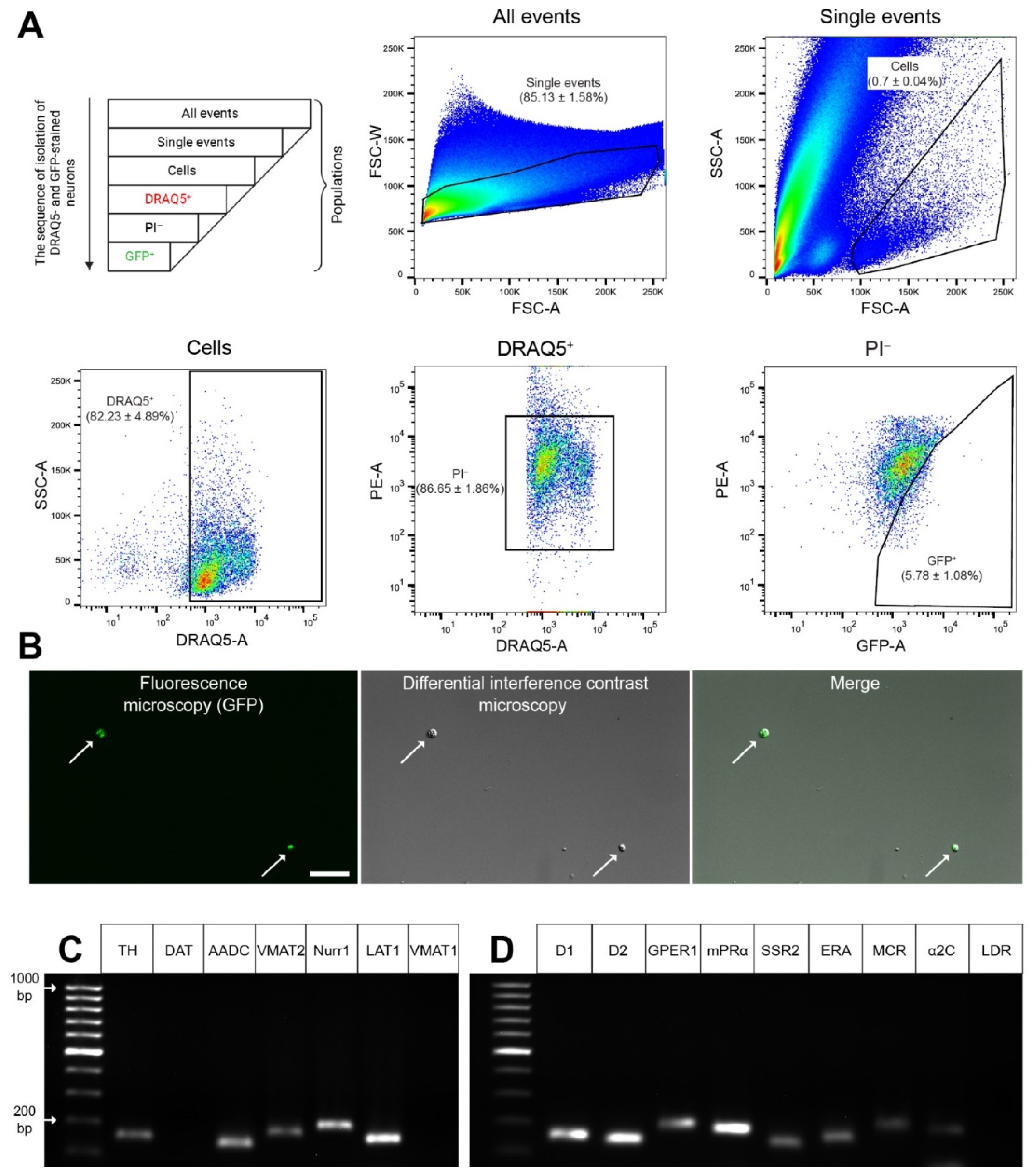
2.3. Obtaining a Fraction of GFP Neurons from the Dissociated Dorsal Striatum
2.4. Gene Expression Profile in GFP-Containing Neurons of the Dorsal Striatum
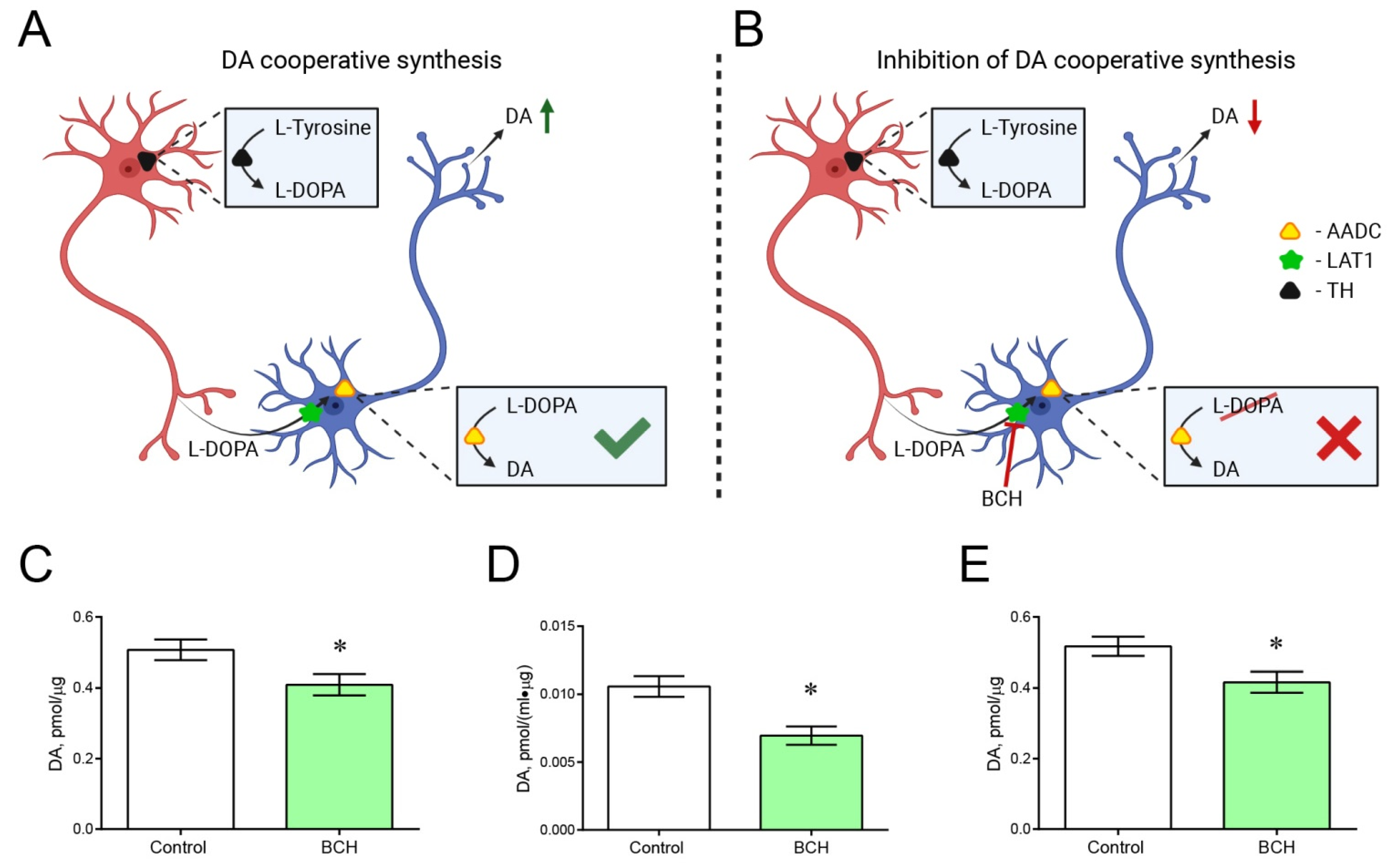
2.5. Incubation of Striatal Sections with and without 2-Amino-2-Norbornanecarboxylic Acid
3. Discussion
4. Materials and Methods
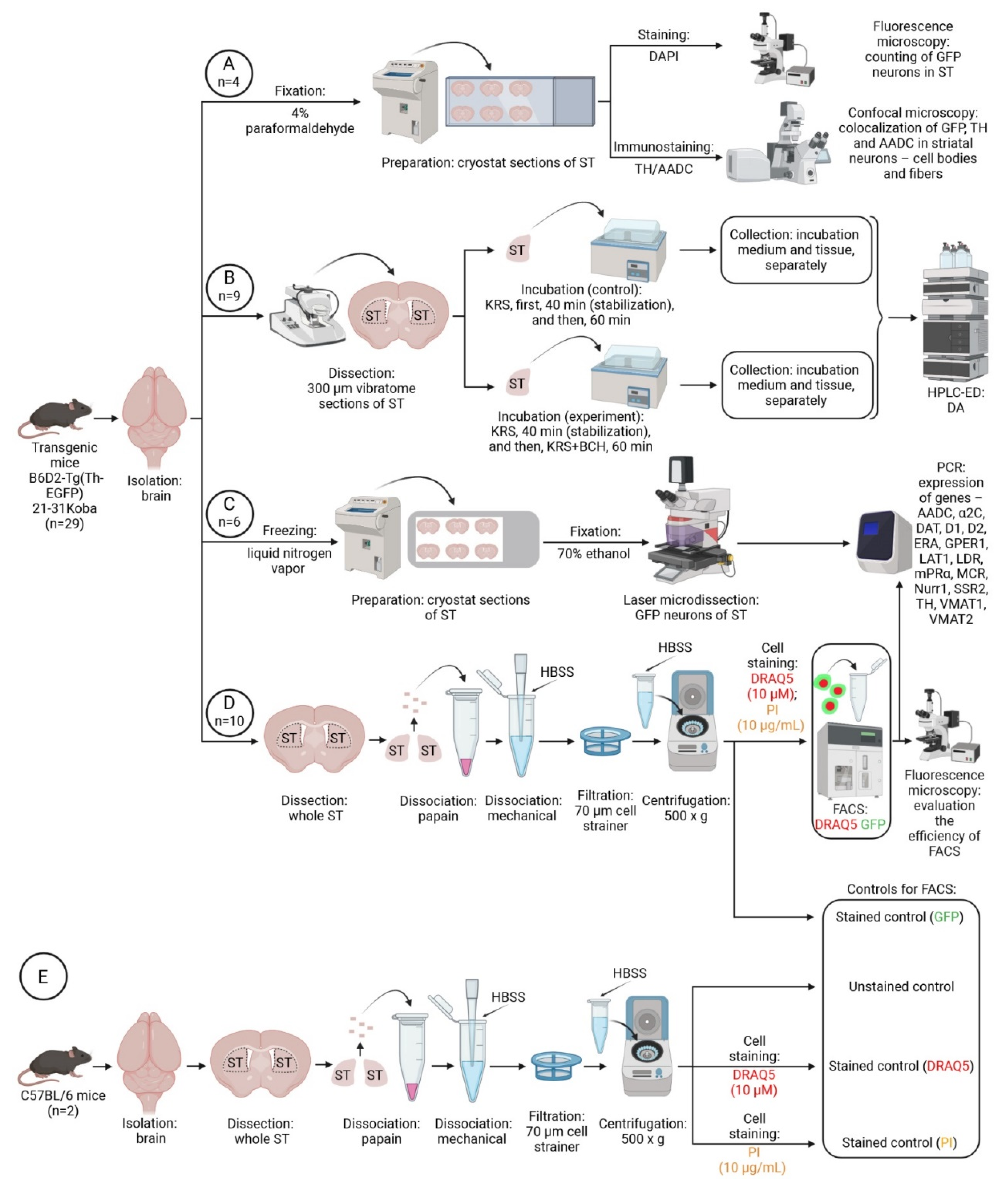
4.1. Animals and Experimental Procedures
4.2. Morphological Study
4.3. Incubation of Striatal Sections of Transgenic Mice
4.4. High-Performance Liquid Chromatography with Electrochemical Detection
4.5. Laser Microdissection
4.6. Preparation of a Cell Suspension from the Dorsal Striatum of Transgenic Mice and C57BL/6 Mice
4.7. Cell Sorting
4.8. RNA Isolation and PCR
4.9. Fluorescence Microscopy
4.10. Counting Cell Bodies of GFP Neurons in the Dorsal Striatum
4.11. Confocal Microscopy
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AADC | aromatic L-amino acid decarboxylase |
| BCH | 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid |
| DA | dopamine |
| DAT | dopamine transporter |
| GABA | gamma amino butyric acid |
| GFP | green fluorescent protein |
| LAT1 | L-amino acid transporter 1 |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| PCR | polymerase chain reaction |
| PI | propidium iodide |
| TH | tyrosine hydroxylase |
| VMAT | vesicular monoamine transporter |
References
- Kaminer, J.; Espinoza, D.; Bhimani, S.; Tepper, J.M.; Koos, T.; Shiflett, M.W. Loss of Striatal Tyrosine-hydroxylase Interneurons Impairs Instrumental Goal-directed Behavior. Eur. J. Neurosci. 2019, 50, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Agid, Y. Parkinson’s Disease: Pathophysiology. Lancet 1991, 337, 1321–1324. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Jenner, P.; Przedborski, S. Pathogenesis of Parkinson’s Disease. Mov. Disord. 2013, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M. Development of Early Diagnosis of Parkinson’s Disease: Illusion or Reality? CNS Neurosci. Ther. 2020, 26, 997–1009. [Google Scholar] [CrossRef]
- Cossette, M.; Lévesque, D.; Parent, A. Neurochemical Characterization of Dopaminergic Neurons in Human Striatum. Parkinsonism Relat. Disord. 2005, 11, 277–286. [Google Scholar] [CrossRef]
- Cossette, M.; Lecomte, F.; Parent, A. Morphology and Distribution of Dopaminergic Neurons Intrinsic to the Human Striatum. J. Chem. Neuroanat. 2005, 29, 1–11. [Google Scholar] [CrossRef]
- Dubach, M.; Schmidt, R.; Kunkel, D.; Bowden, D.M.; Martin, R.; German, D.C. Primate Neostriatal Neurons Containing Tyrosine Hydroxylase: Immunohistochemical Evidence. Neurosci. Lett. 1987, 75, 205–210. [Google Scholar] [CrossRef]
- Lopez-Real, A.; Rodriguez-Pallares, J.; Guerra, M.J.; Labandeira-Garcia, J.L. Localization and Functional Significance of Striatal Neurons Immunoreactive to Aromatic L-Amino Acid Decarboxylase or Tyrosine Hydroxylase in Rat Parkinsonian Models. Brain Res. 2003, 969, 135–146. [Google Scholar] [CrossRef]
- Tandé, D.; Höglinger, G.; Debeir, T.; Freundlieb, N.; Hirsch, E.C.; François, C. New Striatal Dopamine Neurons in MPTP-Treated Macaques Result from a Phenotypic Shift and Not Neurogenesis. Brain 2006, 129, 1194–1200. [Google Scholar] [CrossRef] [Green Version]
- Ugrumov, M.V. Non-Dopaminergic Neurons Partly Expressing Dopaminergic Phenotype: Distribution in the Brain, Development and Functional Significance. J. Chem. Neuroanat. 2009, 38, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M.V. Brain Neurons Partly Expressing Dopaminergic Phenotype. Location, Development, Functional Significance, and Regulation. Adv. Pharmacol. 2013, 68, 37–91. [Google Scholar] [CrossRef] [PubMed]
- Weihe, E.; Depboylu, C.; Schütz, B.; Schäfer, M.K.-H.; Eiden, L.E. Three Types of Tyrosine Hydroxylase-Positive CNS Neurons Distinguished by Dopa Decarboxylase and VMAT2 Co-Expression. Cell. Mol. Neurobiol. 2006, 26, 657–676. [Google Scholar] [CrossRef] [PubMed]
- Betarbet, R.; Turner, R.; Chockkan, V.; DeLong, M.R.; Allers, K.A.; Walters, J.; Levey, A.I.; Greenamyre, J.T. Dopaminergic Neurons Intrinsic to the Primate Striatum. J. Neurosci. 1997, 17, 6761–6768. [Google Scholar] [CrossRef]
- Depboylu, C.; Klietz, M.; Maurer, L.; Oertel, W.H.; Kobayashi, K.; Weihe, E.; Höglinger, G.U.; Schäfer, M.K.-H. Transcriptional and Structural Plasticity of Tyrosine Hydroxylase Expressing Neurons in Both Striatum and Nucleus Accumbens Following Dopaminergic Denervation. J. Chem. Neuroanat. 2014, 61–62, 169–175. [Google Scholar] [CrossRef]
- Huot, P.; Levesque, M.; Parent, A. The Fate of Striatal Dopaminergic Neurons in Parkinson’s Disease and Huntington’s Chorea. Brain 2006, 130, 222–232. [Google Scholar] [CrossRef]
- Masuda, M.; Miura, M.; Inoue, R.; Imanishi, M.; Saino-Saito, S.; Takada, M.; Kobayashi, K.; Aosaki, T. Postnatal Development of Tyrosine Hydroxylase MRNA-Expressing Neurons in Mouse Neostriatum. Eur. J. Neurosci. 2011, 34, 1355–1367. [Google Scholar] [CrossRef]
- Porritt, M.J.; Batchelor, P.E.; Hughes, A.J.; Kalnins, R.; Donnan, G.A.; Howells, D.W. New Dopaminergic Neurons in Parkinson’s Disease Striatum. Lancet 2000, 356, 44–45. [Google Scholar] [CrossRef]
- Ünal, B.; Shah, F.; Kothari, J.; Tepper, J.M. Anatomical and Electrophysiological Changes in Striatal TH Interneurons after Loss of the Nigrostriatal Dopaminergic Pathway. Brain Struct. Funct. 2015, 220, 331–349. [Google Scholar] [CrossRef]
- Darmopil, S.; Muñetón-Gómez, V.C.; de Ceballos, M.L.; Bernson, M.; Moratalla, R. Tyrosine Hydroxylase Cells Appearing in the Mouse Striatum after Dopamine Denervation Are Likely to Be Projection Neurones Regulated by L-DOPA. Eur. J. Neurosci. 2008, 27, 580–592. [Google Scholar] [CrossRef]
- Xenias, H.S.; Ibanez-Sandoval, O.; Koos, T.; Tepper, J.M. Are Striatal Tyrosine Hydroxylase Interneurons Dopaminergic? J. Neurosci. 2015, 35, 6584–6599. [Google Scholar] [CrossRef] [PubMed]
- Ibanez-Sandoval, O.; Tecuapetla, F.; Unal, B.; Shah, F.; Koos, T.; Tepper, J.M. Electrophysiological and Morphological Characteristics and Synaptic Connectivity of Tyrosine Hydroxylase-Expressing Neurons in Adult Mouse Striatum. J. Neurosci. 2010, 30, 6999–7016. [Google Scholar] [CrossRef]
- Basile, G.A.; Bertino, S.; Bramanti, A.; Ciurleo, R.; Anastasi, G.P.; Milardi, D.; Cacciola, A. Striatal Topographical Organization: Bridging the Gap between Molecules, Connectivity and Behavior. Eur. J. Histochem. 2021, 65 (Suppl. 1), 3284. [Google Scholar] [CrossRef]
- Tepper, J.M.; Koós, T.; Ibanez-Sandoval, O.; Tecuapetla, F.; Faust, T.W.; Assous, M. Heterogeneity and Diversity of Striatal GABAergic Interneurons: Update 2018. Front. Neuroanat. 2018, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.; Kobayashi, K.; Okano, H.; Saino-Saito, S. Cortical and Striatal Expression of Tyrosine Hydroxylase MRNA in Neonatal and Adult Mice. Cell. Mol. Neurobiol. 2003, 23, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Biezonski, D.K.; Trifilieff, P.; Meszaros, J.; Javitch, J.A.; Kellendonk, C. Evidence for Limited D1 and D2 Receptor Coexpression and Colocalization within the Dorsal Striatum of the Neonatal Mouse. J. Comp. Neurol. 2015, 523, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M.; Taxi, J.; Pronina, T.; Kurina, A.; Sorokin, A.; Sapronova, A.; Calas, A. Neurons Expressing Individual Enzymes of Dopamine Synthesis in the Mediobasal Hypothalamus of Adult Rats: Functional Significance and Topographic Interrelations. Neuroscience 2014, 277, 45–54. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef]
- Jin, X.; Costa, R.M. Start/Stop Signals Emerge in Nigrostriatal Circuits during Sequence Learning. Nature 2010, 466, 457–462. [Google Scholar] [CrossRef]
- Hidalgo-Balbuena, A.E.; Luma, A.Y.; Pimentel-Farfan, A.K.; Peña-Rangel, T.; Rueda-Orozco, P.E. Sensory Representations in the Striatum Provide a Temporal Reference for Learning and Executing Motor Habits. Nat. Commun. 2019, 10, 4074. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, Y.; Sugimoto, T.; Hattori, T.; Uemura, Y.; Nagatsu, I.; Kikuchi, H.; Mizuno, N. Tyrosine Hydroxylase-like Immunoreactive Neurons in the Striatum of the Rat. Neurosci. Lett. 1989, 97, 6–10. [Google Scholar] [CrossRef]
- Ünal, B.; Ibáñez-Sandoval, O.; Shah, F.; Abercrombie, E.D.; Tepper, J.M. Distribution of Tyrosine Hydroxylase-Expressing Interneurons with Respect to Anatomical Organization of the Neostriatum. Front. Syst. Neurosci. 2011, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Farrell, T.; Kellaghan, P.; Tan, Y.; Zahm, D.S.; Totterdell, S. Immunocytochemical Characterization of Catecholaminergic Neurons in the Rat Striatum Following Dopamine-Depleting Lesions. Eur. J. Neurosci. 1999, 11, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Bubak, A.N.; Redmond, D.E.; Elsworth, J.D.; Roth, R.H.; Collier, T.J.; Bjugstad, K.B.; Blanchard, B.C.; Sladek, J.R. A Potential Compensatory Role for Endogenous Striatal Tyrosine Hydroxylase-Positive Neurons in a Nonhuman Primate Model of Parkinson’s Disease. Cell Transplant. 2015, 24, 673–680. [Google Scholar] [CrossRef]
- Huot, P.; Parent, A. Dopaminergic Neurons Intrinsic to the Striatum. J. Neurochem. 2007, 101, 1441–1447. [Google Scholar] [CrossRef]
- Kozina, E.A.; Kim, A.R.; Kurina, A.Y.; Ugrumov, M.V. Cooperative Synthesis of Dopamine by Non-Dopaminergic Neurons as a Compensatory Mechanism in the Striatum of Mice with MPTP-Induced Parkinsonism. Neurobiol. Dis. 2017, 98, 108–121. [Google Scholar] [CrossRef]
- Iwawaki, T.; Kohno, K.; Kobayashi, K. Identification of a Potential Nurr1 Response Element That Activates the Tyrosine Hydroxylase Gene Promoter in Cultured Cells. Biochem. Biophys. Res. Commun. 2000, 274, 590–595. [Google Scholar] [CrossRef]
- Sawamoto, K.; Nakao, N.; Kobayashi, K.; Matsushita, N.; Takahashi, H.; Kakishita, K.; Yamamoto, A.; Yoshizaki, T.; Terashima, T.; Murakami, F.; et al. Visualization, Direct Isolation, and Transplantation of Midbrain Dopaminergic Neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 6423–6428. [Google Scholar] [CrossRef]
- Goshima, Y.; Misu, Y.; Arai, N.; Misugi, K. Nanomolar L-DOPA Facilitates Release of Dopamine via Presynaptic. BETA.-Adrenoceptors: Comparative Studies on the Actions in Striatal Slices from Control and 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine(MPTP)-Treated C57 Black Mice, an Animal Model for Parkinson’s Disease. Jpn. J. Pharmacol. 1991, 55, 93–100. [Google Scholar] [CrossRef]
- Kitahama, K.; Geffard, M.; Okamura, H.; Nagatsu, I.; Mons, N.; Jouvet, M. Dopamine- and DOPA-Immunoreactive Neurons in the Cat Forebrain with Reference to Tyrosine Hydroxylase-Immunohistochemistry. Brain Res. 1990, 518, 83–94. [Google Scholar] [CrossRef]
- Ugrumov, M.V.; Pavlova, E.N.; Kolacheva, A.A.; Dil’mukhametova, L.K.; Bogdanov, V.V.; Blokhin, V.; Pronina, T.S. The Periventricular Nucleus as a Brain Center Containing Dopaminergic Neurons and Neurons Expressing Individual Enzymes of Dopamine Synthesis. Int. J. Mol. Sci. 2022, 23, 6739. [Google Scholar] [CrossRef] [PubMed]
- Murtazina, A.R.; Bondarenko, N.S.; Pronina, T.S.; Chandran, K.I.; Bogdanov, V.V.; Dilmukhametova, L.K.; Ugrumov, M.V. A Comparative Analysis of CSF and the Blood Levels of Monoamines As Neurohormones in Rats during Ontogenesis. Acta Naturae 2021, 13, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.I.; Stout, K.A.; Miller, G.W. The Vesicular Monoamine Transporter 2: An Underexplored Pharmacological Target. Neurochem. Int. 2014, 73, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M.V.; Melnikova, V.I.; Lavrentyeva, A.V.; Kudrin, V.S.; Rayevsky, K.S. Dopamine Synthesis by Non-Dopaminergic Neurons Expressing Individual Complementary Enzymes of the Dopamine Synthetic Pathway in the Arcuate Nucleus of Fetal Rats. Neuroscience 2004, 124, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ecker, G. Insights into the Structure, Function, and Ligand Discovery of the Large Neutral Amino Acid Transporter 1, LAT1. Int. J. Mol. Sci. 2018, 19, 1278. [Google Scholar] [CrossRef] [PubMed]
- Kontostavlaki, D.P.; Panayotacopoulou, M.T.; Sluijs, J.A.; Unmehopa, U.A.; Huitinga, I.; Hol, E.M.; Swaab, D.F. Co-Expression of Tyrosine Hydroxylase and GTP Cyclohydrolase I in Arginine Vasopressin-Synthesizing Neurons of the Human Supraoptic Nucleus Demonstrated by Laser Microdissection and Real-Time PCR. Neuroendocrinology 2006, 84, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, C.B.; Albert, V.R.; Joh, T.H.; Reis, D.J. Aromatic L-Amino Acid Decarboxylase in the Rat Brain: Coexistence with Vasopressin in Small Neurons of the Suprachiasmatic Nucleus. Brain Res. 1983, 276, 362–366. [Google Scholar] [CrossRef]
- Busceti, C.L.; Biagioni, F.; Mastroiacovo, F.; Bucci, D.; Lenzi, P.; Pasquali, L.; Trabucco, A.; Nicoletti, F.; Fornai, F. High Number of Striatal Dopaminergic Neurons during Early Postnatal Development: Correlation Analysis with Dopaminergic Fibers. J. Neural Transm. 2008, 115, 1375–1383. [Google Scholar] [CrossRef]
- Stephens, S.B.Z.; Rouse, M.L.; Tolson, K.P.; Liaw, R.B.; Parra, R.A.; Chahal, N.; Kauffman, A.S. Effects of Selective Deletion of Tyrosine Hydroxylase from Kisspeptin Cells on Puberty and Reproduction in Male and Female Mice. eNeuro 2017, 4, ENEURO.0150-17.2017. [Google Scholar] [CrossRef]
- Ibáñez-Sandoval, O.; Xenias, H.S.; Tepper, J.M.; Koós, T. Dopaminergic and Cholinergic Modulation of Striatal Tyrosine Hydroxylase Interneurons. Neuropharmacology 2015, 95, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Luo, R.; Janssen, M.J.; Partridge, J.G.; Vicini, S. Direct and GABA-Mediated Indirect Effects of Nicotinic ACh Receptor Agonists on Striatal Neurones. J. Physiol. 2013, 591, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Espadas, I.; Darmopil, S.; Vergaño-Vera, E.; Ortiz, O.; Oliva, I.; Vicario-Abejón, C.; Martín, E.D.; Moratalla, R. L-DOPA-Induced Increase in TH-Immunoreactive Striatal Neurons in Parkinsonian Mice: Insights into Regulation and Function. Neurobiol. Dis. 2012, 48, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Viaro, R.; Longo, F.; Vincenzi, F.; Varani, K.; Morari, M. L-DOPA Promotes Striatal Dopamine Release through D1 Receptors and Reversal of Dopamine Transporter. Brain Res. 2021, 1768, 147583. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Guo, B.; Dai, C.; Yao, H.; Sun, T.; Liu, X.; Bai, Z.; Wang, W.; Wu, S. Striatal Distribution and Cytoarchitecture of Dopamine Receptor Subtype 1 and 2: Evidence from Double-Labeling Transgenic Mice. Front. Neural Circuits 2017, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, V.; Rokka, J.; Nyman, L.; Sallinen, J.; Tiihonen, J.; Tupala, E.; Haaparanta, M.; Hietala, J. Autoradiographic Characterization of A2C-Adrenoceptors in the Human Striatum. Synapse 2008, 62, 508–515. [Google Scholar] [CrossRef]
- Ohta, H.; Kohno, Y.; Arake, M.; Tamura, R.; Yukawa, S.; Sato, Y.; Morimoto, Y.; Nishida, Y.; Yawo, H. Adrenergic Receptor-Mediated Modulation of Striatal Firing Patterns. Neurosci. Res. 2016, 112, 47–56. [Google Scholar] [CrossRef]
- Sánchez-Soto, M.; Casadó-Anguera, V.; Yano, H.; Bender, B.J.; Cai, N.-S.; Moreno, E.; Canela, E.I.; Cortés, A.; Meiler, J.; Casadó, V.; et al. A2A- and A2C-Adrenoceptors as Potential Targets for Dopamine and Dopamine Receptor Ligands. Mol. Neurobiol. 2018, 55, 8438–8454. [Google Scholar] [CrossRef]
- Holmberg, M.; Scheinin, M.; Kurose, H.; Miettinen, R. Adrenergic A2C-Receptors Reside in Rat Striatal GABAergic Projection Neurons: Comparison of Radioligand Binding and Immunohistochemistry. Neuroscience 1999, 93, 1323–1333. [Google Scholar] [CrossRef]
- Hathway, G.J.; Humphrey, P.P.A.; Kendrick, K.M. Evidence That Somatostatin Sst2 Receptors Mediate Striatal Dopamine Release. Br. J. Pharmacol. 1999, 128, 1346–1352. [Google Scholar] [CrossRef]
- Hathway, G.J.; Humphrey, P.P.A.; Kendrick, K.M. Somatostatin Induces Striatal Dopamine Release and Contralateral Turning Behaviour in the Mouse. Neurosci. Lett. 2004, 358, 127–131. [Google Scholar] [CrossRef]
- Vanetti, M.; Ziólkowska, B.; Wang, X.; Horn, G.; Höllt, V. MRNA Distribution of Two Isoforms of Somatostatin Receptor 2 (MSSTR2A and MSSTR2B) in Mouse Brain. Mol. Brain Res. 1994, 27, 45–50. [Google Scholar] [CrossRef]
- Balthazart, J.; Choleris, E.; Remage-Healey, L. Steroids and the Brain: 50 Years of Research, Conceptual Shifts and the Ascent of Non-Classical and Membrane-Initiated Actions. Horm. Behav. 2018, 99, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M. Neurosteroids and Potential Therapeutics: Focus on Pregnenolone. J. Steroid Biochem. Mol. Biol. 2016, 160, 78–87. [Google Scholar] [CrossRef]
- Krentzel, A.A.; Willett, J.A.; Johnson, A.G.; Meitzen, J. Estrogen Receptor Alpha, G-protein Coupled Estrogen Receptor 1, and Aromatase: Developmental, Sex, and Region-specific Differences across the Rat Caudate–Putamen, Nucleus Accumbens Core and Shell. J. Comp. Neurol. 2021, 529, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Willett, J.A.; Dorris, D.M.; Meitzen, J. Sex Differences in Medium Spiny Neuron Excitability and Glutamatergic Synaptic Input: Heterogeneity Across Striatal Regions and Evidence for Estradiol-Dependent Sexual Differentiation. Front. Endocrinol. 2018, 9, 173. [Google Scholar] [CrossRef]
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen Receptor α and G-Protein Coupled Estrogen Receptor 1 Are Localized to GABAergic Neurons in the Dorsal Striatum. Neurosci. Lett. 2016, 622, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Yoest, K.E.; Quigley, J.A.; Becker, J.B. Rapid Effects of Ovarian Hormones in Dorsal Striatum and Nucleus Accumbens. Horm. Behav. 2018, 104, 119–129. [Google Scholar] [CrossRef]
- Lemoine, S.; Leroy, D.; Warembourg, M. Progesterone Receptor and Dopamine Synthesizing Enzymes in Hypothalamic Neurons of the Guinea Pig: An Immunohistochemical Triple-Label Analysis. J. Chem. Neuroanat. 2005, 29, 13–20. [Google Scholar] [CrossRef]
- Vogel, S.; Klumpers, F.; Krugers, H.J.; Fang, Z.; Oplaat, K.T.; Oitzl, M.S.; Joëls, M.; Fernández, G. Blocking the Mineralocorticoid Receptor in Humans Prevents the Stress-Induced Enhancement of Centromedial Amygdala Connectivity with the Dorsal Striatum. Neuropsychopharmacology 2015, 40, 947–956. [Google Scholar] [CrossRef]
- Admon, R.; Holsen, L.M.; Aizley, H.; Remington, A.; Whitfield-Gabrieli, S.; Goldstein, J.M.; Pizzagalli, D.A. Striatal Hypersensitivity During Stress in Remitted Individuals with Recurrent Depression. Biol Psychiatry 2015, 78, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Oswald, L.M.; Zandi, P.; Nestadt, G.; Potash, J.B.; Kalaydjian, A.E.; Wand, G.S. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology 2006, 31, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.K.; Mirshahi, F.; Mirshahi, M.; Rostene, W. Immunochemical Detection of the Mineralocorticoid Receptor in Rat Brain. Neuroendocrinology 1993, 58, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, N.; Okada, H.; Yasoshima, Y.; Takahashi, K.; Kiuchi, K.; Kobayashi, K. Dynamics of Tyrosine Hydroxylase Promoter Activity during Midbrain Dopaminergic Neuron Development. J. Neurochem. 2002, 82, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates, Compact, 2nd ed.; Academic Press: Cambridge, UK, 2001. [Google Scholar]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.; Fukasawa, Y.; et al. Human L-Type Amino Acid Transporter 1 (LAT1): Characterization of Function and Expression in Tumor Cell Lines. Biochim. Biophys. Acta-Biomembr. 2001, 1514, 291–302. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kanagawa, H. Effect of Equilibration Period on the Viability of Frozen-Thawed Mouse Morulae after Rapid Freezing. Mol. Reprod. Dev. 1990, 26, 105–110. [Google Scholar] [CrossRef]
- Buckanovich, R.J.; Sasaroli, D.; O’Brien-Jenkins, A.; Botbyl, J.; Conejo-Garcia, J.R.; Benencia, F.; Liotta, L.A.; Gimotty, P.A.; Coukos, G. Use of Immuno-LCM to Identify the in Situ Expression Profile of Cellular Constituents of the Tumor Microenvironment. Cancer Biol. Ther. 2006, 5, 635–642. [Google Scholar] [CrossRef]
- Eickhoff, A.; Tjaden, J.; Stahlke, S.; Vorgerd, M.; Theis, V.; Matschke, V.; Theiss, C. Effects of Progesterone on T-Type-Ca2+-Channel Expression in Purkinje Cells. Neural Regen. Res. 2022, 17, 2465. [Google Scholar] [CrossRef]
- Liu, Q.-R.; Rubio, F.J.; Bossert, J.M.; Marchant, N.J.; Fanous, S.; Hou, X.; Shaham, Y.; Hope, B.T. Detection of Molecular Alterations in Methamphetamine-Activated Fos-Expressing Neurons from a Single Rat Dorsal Striatum Using Fluorescence-Activated Cell Sorting (FACS). J. Neurochem. 2014, 128, 173–185. [Google Scholar] [CrossRef]
- Eriksen, J.; Rasmussen, S.G.F.; Rasmussen, T.N.; Vaegter, C.B.; Cha, J.H.; Zou, M.-F.; Newman, A.H.; Gether, U. Visualization of Dopamine Transporter Trafficking in Live Neurons by Use of Fluorescent Cocaine Analogs. J. Neurosci. 2009, 29, 6794–6808. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Wu, Y.-M.; Ji, Z.; Gao, X.-Y.; Pan, S.-Y. A Modified Technique for Culturing Primary Fetal Rat Cortical Neurons. J. Biomed. Biotechnol. 2012, 2012, 803930. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.J.; Wiltshire, M.; Errington, R.J. DRAQ5 Labeling of Nuclear DNA in Live and Fixed Cells. Curr. Protoc. Cytom. 2004, 28, 7–25. [Google Scholar] [CrossRef]
- Berteotti, C.; Lo Martire, V.; Alvente, S.; Bastianini, S.; Bombardi, C.; Matteoli, G.; Ohtsu, H.; Lin, J.-S.; Silvani, A.; Zoccoli, G. Orexin/Hypocretin and Histamine Cross-Talk on Hypothalamic Neuron Counts in Mice. Front. Neurosci. 2021, 15, 660518. [Google Scholar] [CrossRef] [PubMed]
- Skrapits, K.; Sárvári, M.; Farkas, I.; Göcz, B.; Takács, S.; Rumpler, É.; Váczi, V.; Vastagh, C.; Rácz, G.; Matolcsy, A.; et al. The Cryptic Gonadotropin-Releasing Hormone Neuronal System of Human Basal Ganglia. eLife 2021, 10, e67714. [Google Scholar] [CrossRef] [PubMed]
- Abercrombie, M. Estimation of Nuclear Population from Microtome Sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troshev, D.; Bannikova, A.; Blokhin, V.; Kolacheva, A.; Pronina, T.; Ugrumov, M. Striatal Neurons Partially Expressing a Dopaminergic Phenotype: Functional Significance and Regulation. Int. J. Mol. Sci. 2022, 23, 11054. https://doi.org/10.3390/ijms231911054
Troshev D, Bannikova A, Blokhin V, Kolacheva A, Pronina T, Ugrumov M. Striatal Neurons Partially Expressing a Dopaminergic Phenotype: Functional Significance and Regulation. International Journal of Molecular Sciences. 2022; 23(19):11054. https://doi.org/10.3390/ijms231911054
Chicago/Turabian StyleTroshev, Dmitry, Alyona Bannikova, Victor Blokhin, Anna Kolacheva, Tatiana Pronina, and Michael Ugrumov. 2022. "Striatal Neurons Partially Expressing a Dopaminergic Phenotype: Functional Significance and Regulation" International Journal of Molecular Sciences 23, no. 19: 11054. https://doi.org/10.3390/ijms231911054
APA StyleTroshev, D., Bannikova, A., Blokhin, V., Kolacheva, A., Pronina, T., & Ugrumov, M. (2022). Striatal Neurons Partially Expressing a Dopaminergic Phenotype: Functional Significance and Regulation. International Journal of Molecular Sciences, 23(19), 11054. https://doi.org/10.3390/ijms231911054






