Dendrimers with Tetraphenylmethane Moiety as a Central Core: Synthesis, a Pore Study and the Adsorption of Volatile Organic Compounds
Abstract
:1. Introduction
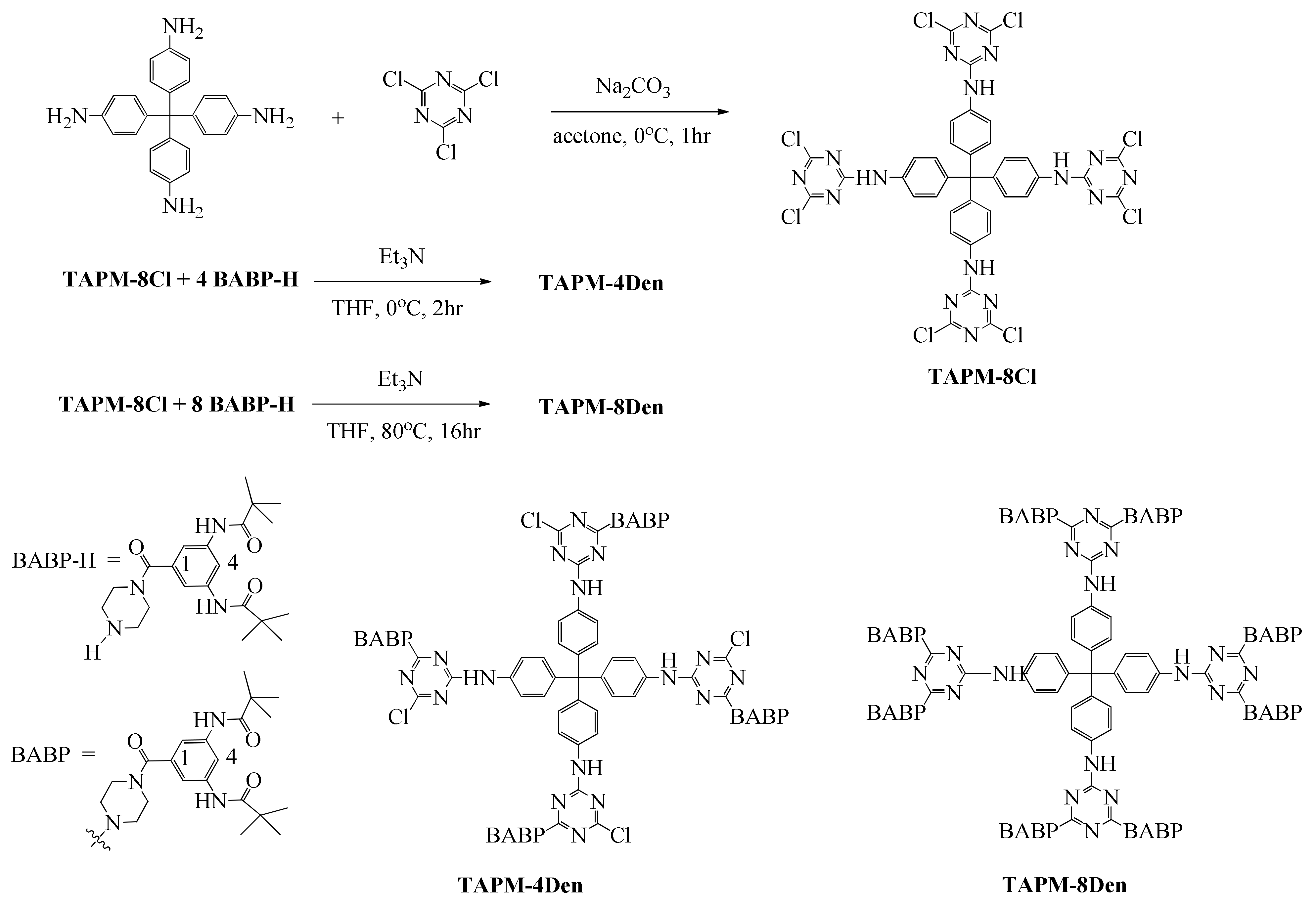
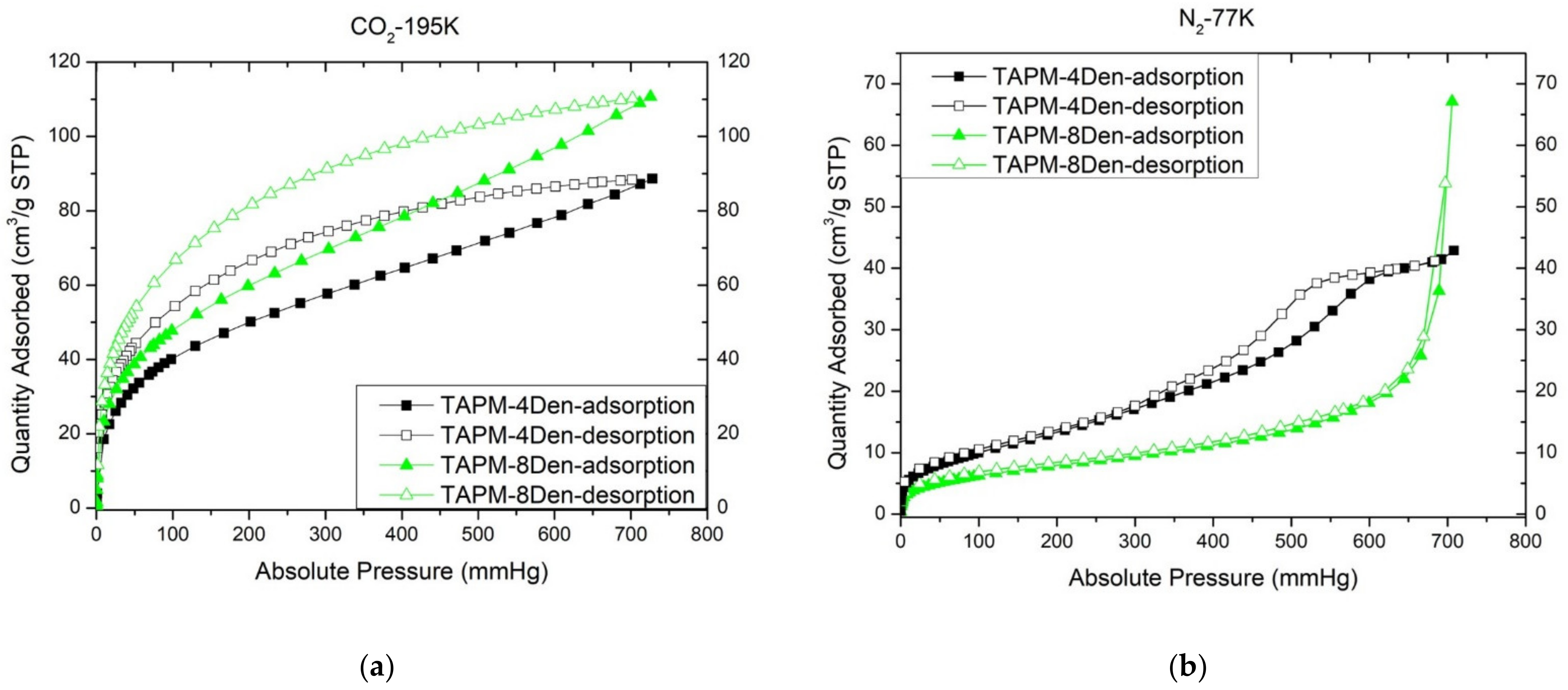
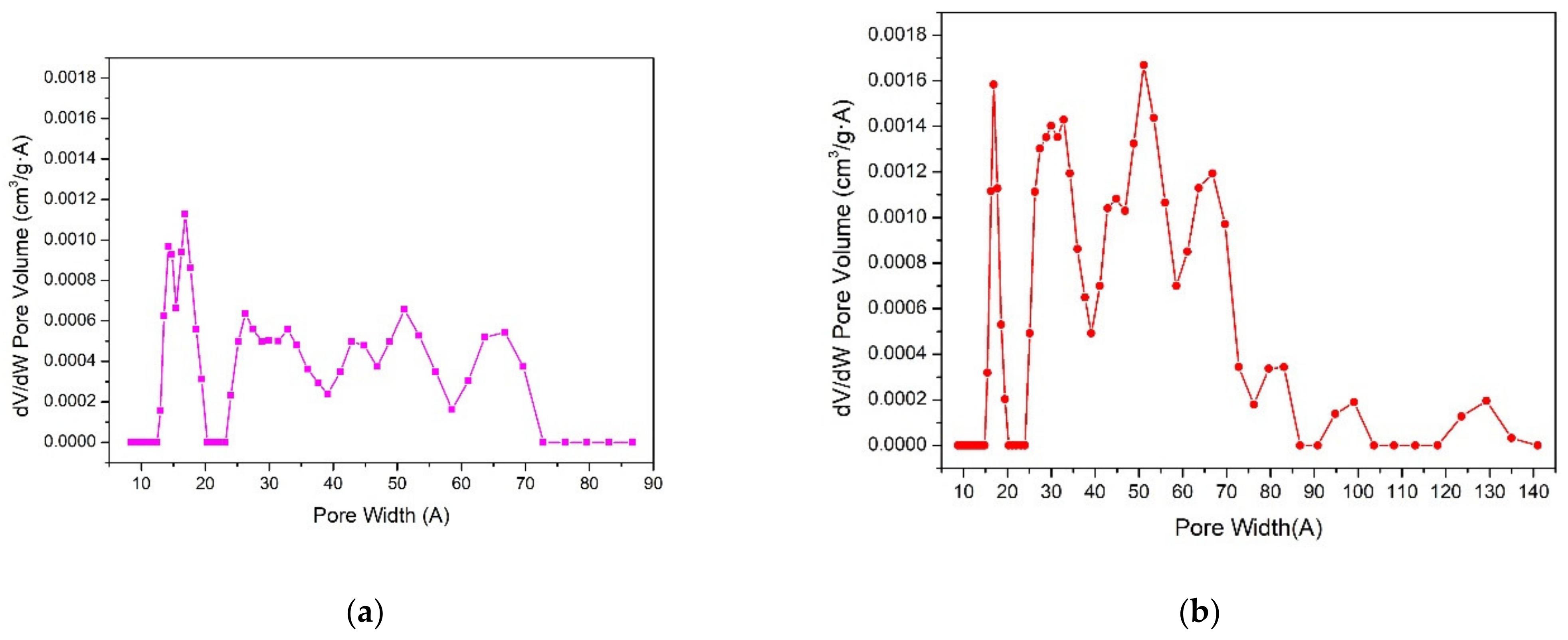
2. Results and Discussion
3. Methods and Materials
3.1. General
3.2. Preparation of TAPM-8Cl
3.3. Preparation of TAPM-8Den
3.4. Preparation of TAPM-4Den
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caminade, A.-M.; Turrin, C.-O.; Laurent, R.; Ouali, A.; Delavaux-Nicot, B. Dendrimers: Towards Catalytic, Material and Biomedical Uses; Wiley: Chichester, west Susses, UK, 2011. [Google Scholar]
- Lu, Y.-C.; Hsu, H.-F.; Lai, L.-L. Unconventional Approaches to Prepare Triazine-Based Liquid Crystal Dendrimers. Nanomaterials 2021, 11, 2112. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Huang, C.-C.; Li, C.-Y.; Lai, L.-L.; Lee, J.-J.; Hsu, H.-F. Both increasing the Iso-to-Col transition and lowering the solidifying temperatures of a triazine-based dendrimer by introducing CN polar groups in the dendritic core. J. Mater. Chem. C 2019, 7, 14232–14238. [Google Scholar] [CrossRef]
- Tsai, M.-J.; Hsieh, J.-W.; Lai, L.-L.; Cheng, K.-L.; Liu, S.-H.; Lee, J.-J.; Hsu, H.-F. Converting Nonliquid Crystals into Liquid Crystals by N-Methylation in the Central Linker of Triazine-Based Dendrimers. J. Org. Chem. 2016, 81, 5007–5013. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.-L.; Hsieh, J.-W.; Cheng, K.-L.; Liu, S.-H.; Lee, J.-J.; Hsu, H.-F. A Small Change in Central Linker Has a Profound Effect in Inducing Columnar Phases of Triazine-Based Unconventional Dendrimers. Chem. Eur. J. 2014, 20, 5160–5166. [Google Scholar] [CrossRef]
- Lai, L.-L.; Wang, S.-W.; Cheng, K.-L.; Lee, J.-J.; Wang, T.-H.; Hsu, H.-F. Induction of the Columnar Phase of Unconventional Dendrimers by Breaking the C2 Symmetry of Molecules. Chem. Eur. J. 2012, 18, 15361–15367. [Google Scholar] [CrossRef]
- Lai, L.-L.; Hsu, S.-J.; Hsu, H.-C.; Wang, S.-W.; Cheng, K.-L.; Chen, C.-J.; Wang, T.-H.; Hsu, H.-F. Formation of Columnar Liquid Crystals on the Basis of Unconventional Triazine-Based Dendrimers by the C3-Symmetric Approach. Chem. Eur. J. 2012, 18, 6542–6547. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef]
- Liu, Y.; Lopes, R.P.; Lüdtke, T.; Di Silvio, D.; Moya, S.; Hamon, J.-R.; Astruc, D. “Click” dendrimer-Pd nanoparticle assemblies as enzyme mimics: Catalytic o-phenylenediamine oxidation and application in colorimetric H2O2 detection. Inorg. Chem. Front. 2021, 8, 3301–3307. [Google Scholar] [CrossRef]
- Yamamoto, K.; Imaoka, T.; Tanabe, M.; Kambe, T. New Horizon of Nanoparticle and Cluster Catalysis with Dendrimers. Chem. Rev. 2020, 120, 1397–1437. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-H.; Cangiotti, M.; Kao, C.-L.; Ottaviani, M.F. EPR Characterization of Copper(II) Complexes of PAMAM-Py Dendrimers for Biocatalysis in the Absence and Presence of Reducing Agents and a Spin Trap. J. Phys. Chem. B 2017, 121, 10498–10507. [Google Scholar] [CrossRef] [PubMed]
- Pirzadeh, K.; Ghoreyshi, A.A.; Rohani, S.; Rahimnejad, M. Strong Influence of Amine Grafting on MIL-101 (Cr) Metal–Organic Framework with Exceptional CO2/N2 Selectivity. Ind. Eng. Chem. Res. 2020, 59, 366–378. [Google Scholar] [CrossRef]
- Sudan, S.; Gładysiak, A.; Valizadeh, B.; Lee, J.-H.; Stylianou, K.C. Sustainable Capture of Aromatic Volatile Organic Compounds by a Pyrene-Based Metal–Organic Framework under Humid Conditions. Inorg. Chem. 2020, 59, 9029–9036. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.M.H.; Jhung, S.H. Oxidative modification of metal-organic framework-derived carbon: An effective strategy for adsorptive elimination of carbazole and benzonitrile. Fuel 2022, 307, 121764. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Lu, Q. Polyamidoamine dendrimer functionalized cellulose nanocrystals for CO2 capture. Cellulose 2021, 28, 4241–4251. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Chien, C.-Y.; Hsu, H.-F.; Lai, L.-L. Adsorbing Volatile Organic Chemicals by Soluble Triazine-Based Dendrimers under Ambient Conditions with the Adsorption Capacity of Pyridine up to 946.2 mg/g. Molecules 2021, 26, 4862. [Google Scholar] [CrossRef]
- Lee, C.-H.; Soldatov, D.V.; Tzeng, C.-H.; Lai, L.-L.; Lu, K.-L. Design of a Peripheral Building Block for H-Bonded Dendritic Frameworks and Analysis of the Void Space in the Bulk Dendrimers. Sci. Rep. 2017, 7, 3649. [Google Scholar] [CrossRef]
- Lee, C.-H.; Tsai, M.-R.; Chang, Y.-T.; Lai, L.-L.; Lu, K.-L.; Cheng, K.-L. Preparation of Unconventional Dendrimers that Contain Rigid NH—Triazine Linkages and Peripheral tert-Butyl Moieties for CO2-Selective Adsorption. Chem. Eur. J. 2013, 19, 10573–10579. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Simon, S.C.; Wagner, M.; Druzhinin, S.I.; Zachariasse, K.A.; Müllen, K. Polypyrene Dendrimers. Angew. Chem. Int. Ed. 2008, 47, 10175–10178. [Google Scholar] [CrossRef]
- Hammer, B.A.G.; Moritz, R.; Stangenberg, R.; Baumgarten, M.; Müllen, K. The polar side of polyphenylene dendrimers. Chem. Soc. Rev. 2015, 44, 4072–4090. [Google Scholar] [CrossRef] [PubMed]
- Balzani, V.; Campagna, S.; Denti, G.; Juris, A.; Serroni, S.; Venturi, M. Designing Dendrimers Based on Transition-Metal Complexes. Light-Harvesting Properties and Predetermined Redox Patterns. Acc. Chem. Res. 1998, 31, 26–34. [Google Scholar] [CrossRef]
- Balzani, V.; Bergamini, G.; Ceroni, P.; Marchi, E. Designing light harvesting antennas by luminescent dendrimers. New J. Chem. 2011, 35, 1944–1954. [Google Scholar] [CrossRef]
- Li, F.; Srivatsa, S.C.; Bhattacharya, S. A review on catalytic pyrolysis of microalgae to high-quality bio-oil with low oxygeneous and nitrogenous compounds. Renew. Sustain. Energy Rev. 2019, 108, 481–497. [Google Scholar] [CrossRef]
- Vellingiri, K.; Kumar, P.; Deep, A.; Kim, K.-H. Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem. Eng. J. 2017, 307, 1116–1126. [Google Scholar] [CrossRef]
- Shafiei, M.; Alivand, M.S.; Rashidi, A.; Samimi, A.; Mohebbi-Kalhori, D. Synthesis and adsorption performance of a modified micro-mesoporous MIL-101(Cr) for VOCs removal at ambient conditions. Chem. Eng. J. 2018, 341, 164–174. [Google Scholar] [CrossRef]
- Kecili, R.; Hussain, C.M. Chapter 4—Mechanism of Adsorption on Nanomaterials. In Nanomaterials in Chromatography; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–115. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Pan, H.; Ritter, J.A.; Balbuena, P.B.J.L. Examination of the approximations used in determining the isosteric heat of adsorption from the Clausius-Clapeyron equation. Langmuir 1998, 14, 6323–6327. [Google Scholar] [CrossRef]
- Zukal, A.; Pawlesa, J.; Čejka, J. Isosteric heats of adsorption of carbon dioxide on zeolite MCM-22 modified by alkali metal cations. Adsorption 2009, 15, 264–270. [Google Scholar] [CrossRef]
- Pribylov, A.A.; Murdmaa, K.O.; Solovtsova, O.V.; Knyazeva, M.K. Methane adsorption on various metal-organic frameworks and determination of the average adsorption heats at supercritical temperatures and pressures. Russ. Chem. Bull. 2018, 67, 1807–1813. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Mofarahi, M. A New Approach in Predicting Gas Adsorption Isotherms and Isosteric Heats Based on Two-Dimensional Equations of State. Arab. J. Sci. Eng. 2019, 44, 5513–5526. [Google Scholar] [CrossRef]
- Skrovanek, D.J.; Howe, S.E.; Painter, P.C.; Coleman, M.M. Hydrogen bonding in polymers: Infrared temperature studies of an amorphous polyamide. Macromolecules 1985, 18, 1676–1683. [Google Scholar] [CrossRef]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Bárcia, P.S.; Nicolau, M.P.M.; Gallegos, J.M.; Chen, B.; Rodrigues, A.E.; Silva, J.A.C. Modeling adsorption equilibria of xylene isomers in a microporous metal–organic framework. Microporous Mesoporous Mater. 2012, 155, 220–226. [Google Scholar] [CrossRef]
- Woods, P.M.; Millar, T.J.; Zijlstra, A.A.; Herbst, E. The Synthesis of Benzene in the Proto–planetary Nebula CRL 618. Astrophys. J. 2002, 574, L167–L170. [Google Scholar] [CrossRef]
- U.S. Department of Commerce, Washington, DC, USA. NIST Chemistry WebBook. 2017. Available online: https://webbook.nist.gov/chemistry/ (accessed on 30 June 2022).
- Solvent Physical Properties. Available online: https://people.chem.umass.edu/xray/solvent.html (accessed on 30 June 2022).
- Bhadra, B.N.; Jhung, S.H. Adsorptive removal of nitrogenous compounds from microalgae-derived bio-oil using metal-organic frameworks with an amino group. Chem. Eng. J. 2020, 388, 124195. [Google Scholar] [CrossRef]
- Mondol, M.M.H.; Bhadra, B.N.; Jhung, S.H. Removal of nitrogen-containing compounds from microalgae derived biofuel by adsorption over functionalized metal organic frameworks. Fuel 2020, 280, 118622. [Google Scholar] [CrossRef]
- Li, F.; Katz, L.; Qiu, S. Adsorptive Selectivity and Mechanism of Three Different Adsorbents for Nitrogenous Compounds Removal from Microalgae Bio-Oil. Ind. Eng. Chem. Res. 2019, 58, 3959–3968. [Google Scholar] [CrossRef]
- Li, F.; Katz, L.; Hu, Z. Adsorption of Major Nitrogen-Containing Components in Microalgal Bio-Oil by Activated Carbon: Equilibrium, Kinetics, and Ideal Adsorbed Solution Theory (IAST) Model. ACS Sustain. Chem. Eng. 2019, 7, 16529–16538. [Google Scholar] [CrossRef]




| Host | hexane | cyano-benzene | nitro-benzene | toluene | o-xylene | m-xylene | p-xylene | |
|---|---|---|---|---|---|---|---|---|
| Guest | ||||||||
| TAPM-4Den | 0.54 | 4.24 | 3.61 | 1.54 | 0.38 | 0.43 | 0.38 | |
| TAPM-8Den | 0.34 | 16.02 | 9.73 | 1.80 | 1.92 | 1.57 | 1.61 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.-T.; Tzeng, C.-H.; Chien, H.-J.; Chen, C.-C.; Lai, L.-L. Dendrimers with Tetraphenylmethane Moiety as a Central Core: Synthesis, a Pore Study and the Adsorption of Volatile Organic Compounds. Int. J. Mol. Sci. 2022, 23, 11155. https://doi.org/10.3390/ijms231911155
Gu Z-T, Tzeng C-H, Chien H-J, Chen C-C, Lai L-L. Dendrimers with Tetraphenylmethane Moiety as a Central Core: Synthesis, a Pore Study and the Adsorption of Volatile Organic Compounds. International Journal of Molecular Sciences. 2022; 23(19):11155. https://doi.org/10.3390/ijms231911155
Chicago/Turabian StyleGu, Zi-Ting, Chung-Hao Tzeng, Hung-Jui Chien, Chun-Chi Chen, and Long-Li Lai. 2022. "Dendrimers with Tetraphenylmethane Moiety as a Central Core: Synthesis, a Pore Study and the Adsorption of Volatile Organic Compounds" International Journal of Molecular Sciences 23, no. 19: 11155. https://doi.org/10.3390/ijms231911155
APA StyleGu, Z.-T., Tzeng, C.-H., Chien, H.-J., Chen, C.-C., & Lai, L.-L. (2022). Dendrimers with Tetraphenylmethane Moiety as a Central Core: Synthesis, a Pore Study and the Adsorption of Volatile Organic Compounds. International Journal of Molecular Sciences, 23(19), 11155. https://doi.org/10.3390/ijms231911155





