GDNF Increases Inhibitory Synaptic Drive on Principal Neurons in the Hippocampus via Activation of the Ret Pathway
Abstract
:1. Introduction
1.1. GDNF
1.2. Epilepsy
1.3. GDNF and Epilepsy
2. Results
2.1. GDNF Enhances Inhibitory Inputs to CA1 Pyramidal Neurons
2.2. Inhibitory Synapse Density Is Increased in the Pyramidal Layer
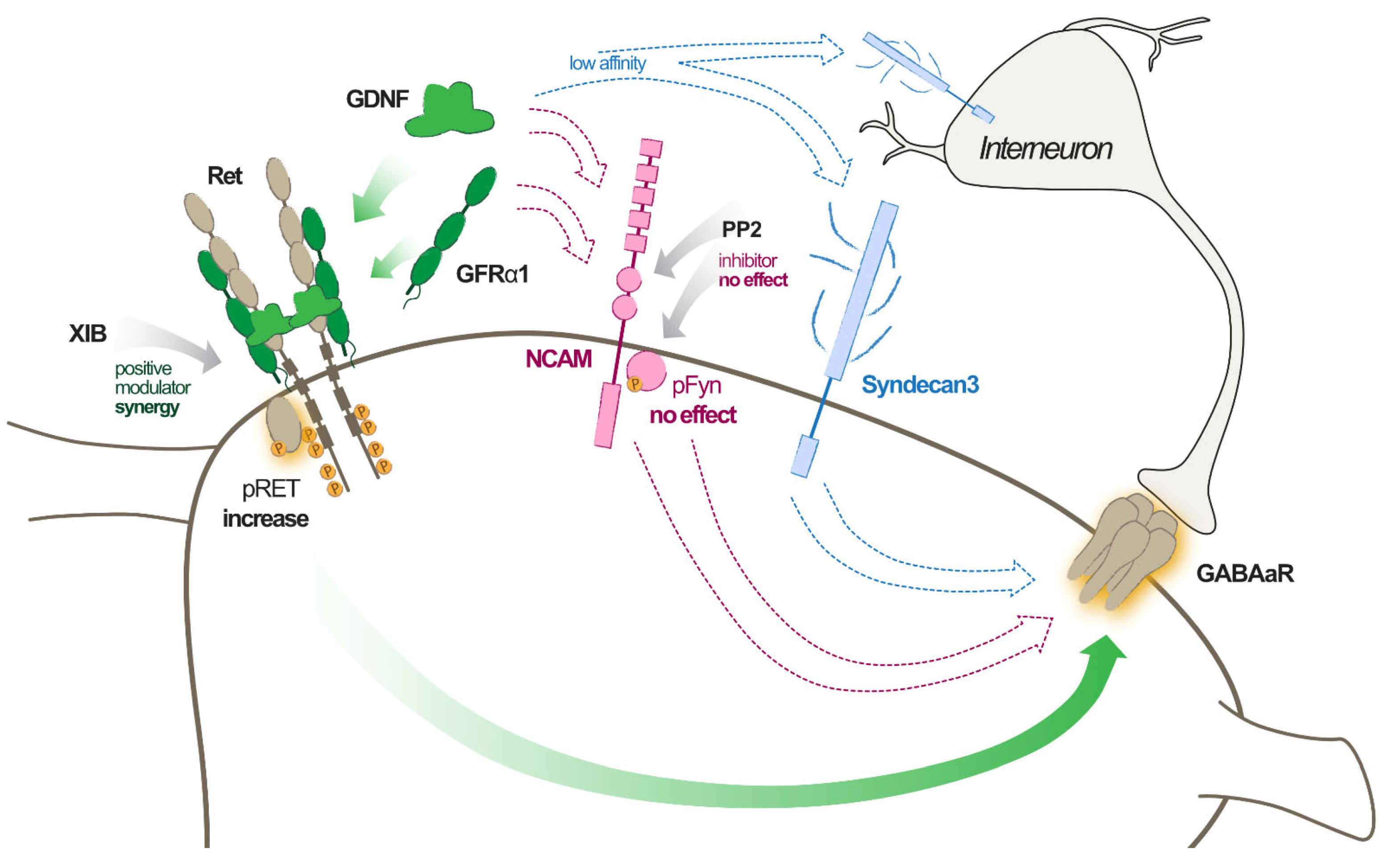
2.3. GDNF Effect Is Mediated by Ret Pathway Activation
2.4. Potential Involvement of Syndecan3 Pathway in GDNF Effect
2.5. Potential Involvement of NCAM Pathway in GDNF Effect
2.6. Validation in Human Brain Tissue
3. Discussion
3.1. Enhanced Synaptic Inhibition of Principal Neurons by Increased Extracellular Levels of GDNF
3.2. GDNF Signalling Pathways
4. Materials and Methods
4.1. Code Accessibility
4.2. Animals and Ethical Information
4.3. Slice Preparation
4.4. Slice Incubation
4.5. Patch-Clamp Recordings
4.6. Immunohistochemistry–Imaging
4.7. Array Tomography
4.8. Western Blot
4.9. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, L.F.H.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A Glial Cell Line-Derived Neurotrophic Factor for Midbrain Dopaminergic Neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Treanor, J.J.S.; Goodman, L.; Sauvage, F.; Stone, D.M.; Poulsen, K.T.; Beck, C.D.; Gray, C.; Armanini, M.P.; Pollock, R.A.; Hefti, F.; et al. Characterization of a Multicomponent Receptor for GDNF. Nature 1996, 382, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Paratcha, G.; Ledda, F.; Ibáñez, C.F. The Neural Cell Adhesion Molecule NCAM Is an Alternative Signaling Receptor for GDNF Family Ligands. Cell 2003, 113, 867–879. [Google Scholar] [CrossRef] [Green Version]
- Ledda, F.; Paratcha, G.; Sandoval-Guzmán, T.; Ibáñez, C.F. GDNF and GFRalpha1 Promote Formation of Neuronal Synapses by Ligand-Induced Cell Adhesion. Nat. Neurosci. 2007, 10, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bespalov, M.M.; Sidorova, Y.A.; Tumova, S.; Ahonen-Bishopp, A.; Magalhães, A.C.; Kulesskiy, E.; Paveliev, M.; Rivera, C.; Rauvala, H.; Saarma, M. Heparan Sulfate Proteoglycan Syndecan-3 Is a Novel Receptor for GDNF, Neurturin, and Artemin. J. Cell Biol. 2011, 192, 153–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springer, J.E.; Mu, X.; Bergmann, L.W.; Trojanowski, J.Q. Expression of GDNF MRNA in Rat and Human Nervous Tissue. Exp. Neurol. 1994, 127, 167–170. [Google Scholar] [CrossRef]
- Trupp, M.; Belluardo, N.; Funakoshi, H.; Ibáñez, C.F. Complementary and Overlapping Expression of Glial Cell Line-Derived Neurotrophic Factor (GDNF), c-Ret Proto-Oncogene, and GDNF Receptor-α Indicates Multiple Mechanisms of Trophic Actions in the Adult Rat CNS. J. Neurosci. 1997, 17, 3554–3567. [Google Scholar] [CrossRef] [Green Version]
- Pochon, N.A.M.; Menoud, A.; Tseng, J.L.; Zurn, A.D.; Aebischer, P. Neuronal GDNF Expression in the Adult Rat Nervous System Identified By In Situ Hybridization. Eur. J. Neurosci. 1997, 9, 463–471. [Google Scholar] [CrossRef]
- Burazin, T.C.; Gundlach, A.L. Localization of GDNF/Neurturin Receptor (c-Ret, GFRalpha-1 and Alpha-2) MRNAs in Postnatal Rat Brain: Differential Regional and Temporal Expression in Hippocampus, Cortex and Cerebellum. Brain Res. Mol. Brain Res. 1999, 73, 151–171. [Google Scholar] [CrossRef]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients with Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. Jama Neurol. 2017, 75, 279. [Google Scholar] [CrossRef]
- Schmidt, D. Drug Treatment of Epilepsy: Options and Limitations. Epilepsy Behav. 2009, 15, 56–65. [Google Scholar] [CrossRef]
- Engel, J. Excitation and Inhibition in Epilepsy. Can. J. Neurol. Sci. 1996, 23, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Shorvon, S.D. The Etiologic Classification of Epilepsy: Etiologic Classification of Epilepsy. Epilepsia 2011, 52, 1052–1057. [Google Scholar] [CrossRef]
- Kanter-Schlifke, I.; Georgievska, B.; Kirik, D.; Kokaia, M. Seizure Suppression by GDNF Gene Therapy in Animal Models of Epilepsy. Mol. Ther. 2007, 15, 1106–1113. [Google Scholar] [CrossRef]
- Kanter-Schlifke, I.; Fjord-Larsen, L.; Kusk, P.; Ängehagen, M.; Wahlberg, L.; Kokaia, M. GDNF Released from Encapsulated Cells Suppresses Seizure Activity in the Epileptic Hippocampus. Exp. Neurol. 2009, 216, 413–419. [Google Scholar] [CrossRef]
- Nanobashvili, A.; Melin, E.; Emerich, D.; Tornøe, J.; Simonato, M.; Wahlberg, L.; Kokaia, M. Unilateral Ex Vivo Gene Therapy by GDNF in Epileptic Rats. Gene Ther. 2018, 37, 2887. [Google Scholar] [CrossRef]
- Paolone, G.; Falcicchia, C.; Lovisari, F.; Kokaia, M.; Bell, W.J.; Fradet, T.; Barbieri, M.; Wahlberg, L.U.; Emerich, D.F.; Simonato, M. Long-Term, Targeted Delivery of GDNF from Encapsulated Cells Is Neuroprotective and Reduces Seizures in the Pilocarpine Model of Epilepsy. J. Neurosci. 2019, 39, 2144–2156. [Google Scholar] [CrossRef] [Green Version]
- Kirik, D.; Georgievska, B.; Björklund, A. Localized Striatal Delivery of GDNF as a Treatment for Parkinson Disease. Nat. Neurosci. 2004, 7, 105–110. [Google Scholar] [CrossRef]
- Sergaki, M.C.; López-Ramos, J.C.; Stagkourakis, S.; Gruart, A.; Broberger, C.; Delgado-García, J.M.; Ibáñez, C.F. Compromised Survival of Cerebellar Molecular Layer Interneurons Lacking GDNF Receptors GFRα1 or RET Impairs Normal Cerebellar Motor Learning. Cell Rep. 2017, 19, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Takaku, S.; Yanagisawa, H.; Watabe, K.; Horie, H.; Kadoya, T.; Sakumi, K.; Nakabeppu, Y.; Poirier, F.; Sango, K. GDNF Promotes Neurite Outgrowth and Upregulates Galectin-1 through the RET/PI3K Signaling in Cultured Adult Rat Dorsal Root Ganglion Neurons. Neurochem Int. 2013, 62, 330–339. [Google Scholar] [CrossRef]
- Rocha, S.M.; Cristovão, A.C.; Campos, F.L.; Fonseca, C.P.; Baltazar, G. Astrocyte-Derived GDNF Is a Potent Inhibitor of Microglial Activation. Neurobiol. Dis. 2012, 47, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bédard, C.; Destexhe, A. Generalized Cable Theory for Neurons in Complex and Heterogeneous Media. Phys. Rev. E 2013, 88, 022709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choii, G.; Ko, J. Gephyrin: A Central GABAergic Synapse Organizer. Exp. Mol. Med. 2015, 47, e158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanus, C.; Vannier, C.; Triller, A. Intracellular Association of Glycine Receptor with Gephyrin Increases Its Plasma Membrane Accumulation Rate. J. Neurosci. 2004, 24, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedstrom, K.L.; Murtie, J.C.; Albers, K.; Calcutt, N.A.; Corfas, G. Treating Small Fiber Neuropathy by Topical Application of a Small Molecule Modulator of Ligand-Induced GFRα/RET Receptor Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 2325–2330. [Google Scholar] [CrossRef] [Green Version]
- Markram, H.; Tsodyks, M. Redistribution of Synaptic Efficacy between Neocortical Pyramidal Neurons. Nature 1996, 382, 807–810. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Sheng, M. Regulated Expression and Subcellular Localization of Syndecan Heparan Sulfate Proteoglycans and the Syndecan-Binding Protein CASK/LIN-2 during Rat Brain Development. J. Neurosci. 1999, 19, 7415–7425. [Google Scholar] [CrossRef]
- Nielsen, J.; Gotfryd, K.; Li, S.; Kulahin, N.; Soroka, V.; Rasmussen, K.K.; Bock, E.; Berezin, V. Role of Glial Cell Line-Derived Neurotrophic Factor (GDNF)-Neural Cell Adhesion Molecule (NCAM) Interactions in Induction of Neurite Outgrowth and Identification of a Binding Site for NCAM in the Heel Region of GDNF. J. Neurosci. 2009, 29, 11360–11376. [Google Scholar] [CrossRef] [Green Version]
- Paratcha, G.; Ibáñez, C.F.; Ledda, F. GDNF Is a Chemoattractant Factor for Neuronal Precursor Cells in the Rostral Migratory Stream. Mol. Cell. Neurosci. 2005, 31, 505–514. [Google Scholar] [CrossRef]
- Pozas, E.; Ibáñez, C.F. GDNF and GFRalpha1 Promote Differentiation and Tangential Migration of Cortical GABAergic Neurons. Neuron 2005, 45, 701–713. [Google Scholar] [CrossRef]
- Canty, A.J.; Dietze, J.; Harvey, M.; Enomoto, H.; Milbrandt, J.; Ibáñez, C.F. Regionalized Loss of Parvalbumin Interneurons in the Cerebral Cortex of Mice with Deficits in GFRalpha1 Signaling. J. Neurosci. 2009, 29, 10695–10705. [Google Scholar] [CrossRef] [Green Version]
- Wierenga, C.J.; Wadman, W.J. Miniature Inhibitory Postsynaptic Currents in CA1 Pyramidal Neurons After Kindling Epileptogenesis. J. Neurophysiol. 1999, 82, 1352–1362. [Google Scholar] [CrossRef] [Green Version]
- Kaplinovsky, T.; Cunningham, A.M. Differential Expression of RET Receptor Isoforms in the Olfactory System. Neuroscience 2010, 175, 49–65. [Google Scholar] [CrossRef]
- Richardson, D.S.; Rodrigues, D.M.; Hyndman, B.D.; Crupi, M.J.F.; Nicolescu, A.C.; Mulligan, L.M. Alternative Splicing Results in RET Isoforms with Distinct Trafficking Properties. Mol. Biol. Cell 2012, 23, 3838–3850. [Google Scholar] [CrossRef]
- Olander, E.R.; Janzen, D.; Villmann, C.; Jensen, A.A. Comparison of Biophysical Properties of A1β2 and A3β2 GABAA Receptors in Whole-Cell Patch-Clamp Electrophysiological Recordings. PLoS ONE 2020, 15, e0234080. [Google Scholar] [CrossRef]
- . Sun, C.; Sieghart, W.; Kapur, J. Distribution of A1, A4, Γ2, and δ Subunits of GABAA Receptors in Hippocampal Granule Cells. Brain Res. 2004, 1029, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Ledri, M.; Sørensen, A.T.; Madsen, M.G.; Christiansen, S.H.; Ledri, L.N.; Cifra, A.; Bengzon, J.; Lindberg, E.; Pinborg, L.H.; Jespersen, B.; et al. Differential Effect of Neuropeptides on Excitatory Synaptic Transmission in Human Epileptic Hippocampus. J. Neurosci. 2015, 35, 9622–9631. [Google Scholar] [CrossRef] [Green Version]
- Cik, M.; Masure, S.; Lesage, A.S.J.; Linden, I.V.; Gompel, P.V.; Pangalos, M.N.; Gordon, R.D.; Leysen, J.E. Binding of GDNF and Neurturin to Human GDNF Family Receptor α 1 and 2. J. Biol. Chem. 2000, 275, 27505–27512. [Google Scholar] [CrossRef] [Green Version]
- Weissberg, I.; Wood, L.; Kamintsky, L.; Vazquez, O.; Milikovsky, D.Z.; Alexander, A.; Oppenheim, H.; Ardizzone, C.; Becker, A.; Frigerio, F.; et al. Albumin Induces Excitatory Synaptogenesis through Astrocytic TGF-β/ALK5 Signaling in a Model of Acquired Epilepsy Following Blood–Brain Barrier Dysfunction. Neurobiol. Dis. 2015, 78, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, F.; Sano, Y.; Saito, K.; Abe, M.; Maeda, T.; Haruki, H.; Kanda, T. Pericyte-Derived Glial Cell Line-Derived Neurotrophic Factor Increase the Expression of Claudin-5 in the Blood–Brain Barrier and the Blood-Nerve Barrier. Neurochem. Res. 2011, 37, 401–409. [Google Scholar] [CrossRef]
- Vezzani, A.; Viviani, B. Neuromodulatory Properties of Inflammatory Cytokines and Their Impact on Neuronal Excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Wickham, J.; Ledri, M.; Bengzon, J.; Jespersen, B.; Pinborg, L.H.; Englund, E.; Woldbye, D.P.D.; Andersson, M.; Kokaia, M. Inhibition of Epileptiform Activity by Neuropeptide Y in Brain Tissue from Drug-Resistant Temporal Lobe Epilepsy Patients. Sci. Rep. 2019, 9, 19393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, J.; Brödjegård, N.G.; Vighagen, R.; Pinborg, L.H.; Bengzon, J.; Woldbye, D.P.D.; Kokaia, M.; Andersson, M. Prolonged Life of Human Acute Hippocampal Slices from Temporal Lobe Epilepsy Surgery. Sci. Rep. 2018, 8, 4158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, K.R.; Smith, C.; Wright, A.K.; Serrano-Pozo, A.; Pooler, A.M.; Koffie, R.; Bastin, M.E.; Bak, T.H.; Abrahams, S.; Kopeikina, K.J.; et al. Studying Synapses in Human Brain with Array Tomography and Electron Microscopy. Nat. Protoc. 2013, 8, 1366–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henstridge, C.M.; Jackson, R.J.; Kim, J.M.; Herrmann, A.G.; Wright, A.K.; Harris, S.E.; Bastin, M.E.; Starr, J.M.; Wardlaw, J.; Gillingwater, T.H.; et al. Post-Mortem Brain Analyses of the Lothian Birth Cohort 1936: Extending Lifetime Cognitive and Brain Phenotyping to the Level of the Synapse. Acta Neuropathol. Commun 2015, 3, 53. [Google Scholar] [CrossRef] [Green Version]
- Thevenaz, P.; Ruttimann, U.E.; Unser, M. A Pyramid Approach to Subpixel Registration Based on Intensity. IEEE Trans. Image Process. 1998, 7, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Micheva, K.D.; Busse, B.; Weiler, N.C.; O’Rourke, N.; Smith, S.J. Single-Synapse Analysis of a Diverse Synapse Population: Proteomic Imaging Methods and Markers. Neuron 2010, 68, 639–653. [Google Scholar] [CrossRef] [Green Version]
- Jonas, P.; Major, G.; Sakmann, B. Quantal Components of Unitary EPSCs at the Mossy Fibre Synapse on CA3 Pyramidal Cells of Rat Hippocampus. J. Physiology 1993, 472, 615–663. [Google Scholar] [CrossRef] [Green Version]
- Mikroulis, A. XPSC-Detection—Template Correlation-Based Detection of Postsynaptic Currents.
- Mikroulis, A.; Ledri, M.; Ruffolo, G.; Palma, E.; Sperk, G.; Dalli, J.; Vezzani, A.; Kokaia, M. Lipid Mediator N-3 Docosapentaenoic Acid-derived Protectin D1 Enhances Synaptic Inhibition of Hippocampal Principal Neurons by Interaction with a G-protein-coupled Receptor. FASEB J. 2022, 36, e22203. [Google Scholar] [CrossRef]
- Schneggenburger, R.; Meyer, A.C.; Neher, E. Released Fraction and Total Size of a Pool of Immediately Available Transmitter Quanta at a Calyx Synapse. Neuron 1999, 23, 399–409. [Google Scholar] [CrossRef]
- Bird, A.D.; Wall, M.J.; Richardson, M.J.E. Bayesian Inference of Synaptic Quantal Parameters from Correlated Vesicle Release. Front. Comput. Neurosci. 2016, 10, 116. [Google Scholar] [CrossRef]









| Frequency (Hz) | Amplitude (pA) | Rise Time (ms) | ||||
|---|---|---|---|---|---|---|
| sIPSCs | mIPSCs | sIPSCs | mIPSCs | sIPSCs | mIPSCs | |
| Ctrl | 4.1 ± 0.1 (4.1), n = 7 | 3.5 ± 0.1 (3.4), n = 7 | 34.0 ± 0.3 (34.2), n = 7 | 30.3 ± 0.5 (29.7), n = 7 | 1.62 ± 0.01 (1.62), n = 7 | 1.31 ± 0.01 (1.34), n = 7 |
| GDNF 2nM | 4.8 ± 0.1 (4.8), n = 6 | 3.8 ± 0.1 (3.8), n = 8 | 35.4 ± 0.4 (35.3), n = 6 | 30.2 ± 0.4 (30.4), n = 8 | 1.21 ± 0.01 (1.20), n = 6 | 1.08 ± 0.01 (1.10), n = 8 |
| Mann–Whitney p | 0.002 | 0.036 | 0.013 | 0.477 | 0.002 | 0.001 |
| Frequency (Hz) | Amplitude (pA) | Rise Time (ms) | ||||
|---|---|---|---|---|---|---|
| sIPSCs | mIPSCs | sIPSCs | mIPSCs | sIPSCs | mIPSCs | |
| GDNF 2nM (DMSO) | 3.6 ± 0.4 (4.1), n = 11 | 3.5 ± 0.4 (3.5), n = 9 | 18.5 ± 3.8 (18.5), n = 11 | 16.8 ± 2.6 (14.0), n = 9 | 1.60 ± 0.09 (1.52), n = 11 | 1.56 ± 0.06 (1.52), n = 9 |
| GDNF 2nM + XIB4035 | 4.1 ± 0.4 (4.4), n = 11 | 4.3 ± 0.4 (4.5), n = 8 | 21.9 ± 2.8 (22.1), n = 11 | 33.8 ± 11.0 (22.3), n = 8 | 1.56 ± 0.06 (1.54), n = 11 | 1.50 ± 0.063 (1.47), n = 8 |
| Mann–Whitney p | 0.277 | 0.180 | 0.119 | 0.056 | 0.396 | 0.235 |
| Patient | Age (Years) | Sex |
|---|---|---|
| 1 | 18 | Male |
| 2 | 4 | Female |
| 3 | 43 | Female |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikroulis, A.; Waloschková, E.; Bengzon, J.; Woldbye, D.; Pinborg, L.H.; Jespersen, B.; Avila, A.S.; Laszlo, Z.I.; Henstridge, C.; Ledri, M.; et al. GDNF Increases Inhibitory Synaptic Drive on Principal Neurons in the Hippocampus via Activation of the Ret Pathway. Int. J. Mol. Sci. 2022, 23, 13190. https://doi.org/10.3390/ijms232113190
Mikroulis A, Waloschková E, Bengzon J, Woldbye D, Pinborg LH, Jespersen B, Avila AS, Laszlo ZI, Henstridge C, Ledri M, et al. GDNF Increases Inhibitory Synaptic Drive on Principal Neurons in the Hippocampus via Activation of the Ret Pathway. International Journal of Molecular Sciences. 2022; 23(21):13190. https://doi.org/10.3390/ijms232113190
Chicago/Turabian StyleMikroulis, Apostolos, Eliška Waloschková, Johan Bengzon, David Woldbye, Lars H. Pinborg, Bo Jespersen, Anna Sanchez Avila, Zsofia I. Laszlo, Christopher Henstridge, Marco Ledri, and et al. 2022. "GDNF Increases Inhibitory Synaptic Drive on Principal Neurons in the Hippocampus via Activation of the Ret Pathway" International Journal of Molecular Sciences 23, no. 21: 13190. https://doi.org/10.3390/ijms232113190
APA StyleMikroulis, A., Waloschková, E., Bengzon, J., Woldbye, D., Pinborg, L. H., Jespersen, B., Avila, A. S., Laszlo, Z. I., Henstridge, C., Ledri, M., & Kokaia, M. (2022). GDNF Increases Inhibitory Synaptic Drive on Principal Neurons in the Hippocampus via Activation of the Ret Pathway. International Journal of Molecular Sciences, 23(21), 13190. https://doi.org/10.3390/ijms232113190







