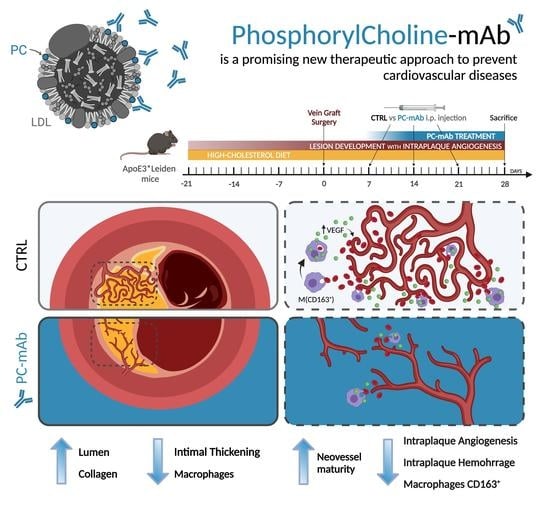
Phosphorylcholine Monoclonal Antibody Therapy Decreases Intraplaque Angiogenesis and Intraplaque Hemorrhage in Murine Vein Grafts
Abstract
1. Introduction
2. Results
2.1. PC-mAb Decreases Vein Graft Thickening and Increases Lumen Area
2.2. PC-mAb Increases Collagen Content
2.3. PC-mAb Decreases ICAM-1 and VCAM-1 Expression
2.4. PC-mAb Decreases Intraplaque Angiogenesis and Intraplaque Hemorrhage
2.5. PC-mAb Decreases EC Metabolic Activity and Migration and Sprouts Formation
2.6. PC-mAb Targets M(CD163) Macrophages In Vivo and In Vitro by Decreasing CD163 Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Vein Graft Surgery
4.3. Treatment
4.4. Histology and Immunostainings in Vein Grafts
4.5. Cell Culture
4.6. MTT Assay
4.7. Migration Assay
4.8. Aortic Ring Sprouting Assay
4.9. Protein Expression Analysis
4.10. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IPA | Intraplaque angiogenesis |
| IPH | Intraplaque hemorrhage |
| PC | Phosphorylcholine |
| Hb | Hemoglobin |
| VEGF | Vascular endothelial growth factor |
| EC | Endothelial cell |
| VSMC | Vascular smooth muscle cell |
| ACTA2 | Actin alpha 2 |
| VG | Vein graft |
| DAMPs | Damage associated molecular patterns |
References
- De Vries, M.R.; Simons, K.H.; Jukema, J.W.; Braun, J.; Quax, P.H. Vein graft failure: From pathophysiology to clinical outcomes. Nat. Rev. Cardiol. 2016, 13, 451–470. [Google Scholar] [CrossRef] [PubMed]
- Parma, L.; Baganha, F.; Quax, P.H.A.; de Vries, M.R. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur. J. Pharmacol. 2017, 816, 107–115. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Finn, A.V.; Gold, H.K.; Tulenko, T.N.; Wrenn, S.P.; Narula, J. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression.n of coronary atheroma. N. Engl J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.B.; Virmani, R.; Arbustini, E.; Pasterkamp, G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur. Heart J. 2011, 32, 1977–1985, 1985a, 1985b, 1985c. [Google Scholar] [CrossRef]
- Habib, A.; Finn, A.V. The role of iron metabolism as a mediator of macrophage inflammation and lipid handling in atherosclerosis. Front. Pharmacol. 2014, 5, 195. [Google Scholar] [CrossRef]
- de Vries, M.R.; Quax, P.H. Plaque angiogenesis and its relation to inflammation and atherosclerotic plaque destabilization. Curr. Opin. Lipidol. 2016, 27, 499–506. [Google Scholar] [CrossRef]
- Guo, L.; Akahori, H.; Harari, E.; Smith, S.L.; Polavarapu, R.; Karmali, V.; Otsuka, F.; Gannon, R.L.; Braumann, R.E.; Dickinson, M.H.; et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Invest. 2018, 128, 1106–1124. [Google Scholar] [CrossRef]
- Camejo, G.; Halberg, C.; Manschik-Lundin, A.; Hurt-Camejo, E.; Rosengren, B.; Olsson, H.; Hansson, G.I.; Forsberg, G.B.; Ylhen, B. Hemin binding and oxidation of lipoproteins in serum: Mechanisms and effect on the interaction of LDL with human macrophages. J. Lipid Res. 1998, 39, 755–766. [Google Scholar] [CrossRef]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef]
- Tokumura, A.; Toujima, M.; Yoshioka, Y.; Fukuzawa, K. Lipid peroxidation in low density lipoproteins from human plasma and egg yolk promotes accumulation of 1-acyl analogues of platelet-activating factor-like lipids. Lipids 1996, 31, 1251–1258. [Google Scholar] [CrossRef]
- Yeagle, P.L. The Membranes of Cells, 3rd ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 57–71. [Google Scholar]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef]
- Afonyushkin, T.; Oskolkova, O.V.; Philippova, M.; Resink, T.J.; Erne, P.; Binder, B.R.; Bochkov, V.N. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: Novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1007–1013. [Google Scholar] [CrossRef]
- Yan, F.X.; Li, H.M.; Li, S.X.; He, S.H.; Dai, W.P.; Li, Y.; Wang, T.T.; Shi, M.M.; Yuan, H.X.; Xu, Z.; et al. The oxidized phospholipid POVPC impairs endothelial function and vasodilation via uncoupling endothelial nitric oxide synthase. J. Mol. Cell Cardiol. 2017, 112, 40–48. [Google Scholar] [CrossRef]
- Yahagi, K.; Kolodgie, F.D.; Otsuka, F.; Finn, A.V.; Davis, H.R.; Joner, M.; Virmani, R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 79–98. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Nikiforov, N.G.; Sukhorukov, V.N.; Kubekina, M.V.; Sobenin, I.A.; Wu, W.K.; Foxx, K.K.; Pintus, S.; Stegmaier, P.; Stelmashenko, D.; et al. Role of Phagocytosis in the Pro-Inflammatory Response in LDL-Induced Foam Cell Formation; a Transcriptome Analysis. Int. J. Mol. Sci. 2020, 21, 817. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Bezsonov, E.E.; Orekhova, V.A.; Popkova, T.V.; Starodubova, A.V.; Orekhov, A.N. Recognition of Oxidized Lipids by Macrophages and Its Role in Atherosclerosis Development. Biomedicines 2021, 9, 915. [Google Scholar] [CrossRef]
- Que, X.; Hung, M.Y.; Yeang, C.; Gonen, A.; Prohaska, T.A.; Sun, X.; Diehl, C.; Määttä, A.; Gaddis, D.E.; Bowden, K.; et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature 2018, 558, 301–306. [Google Scholar] [CrossRef]
- Binder, C.J.; Shaw, P.X.; Chang, M.K.; Boullier, A.; Hartvigsen, K.; Hörkkö, S.; Miller, Y.I.; Woelkers, D.A.; Corr, M.; Witztum, J.L. The role of natural antibodies in atherogenesis. J. Lipid Res. 2005, 46, 1353–1363. [Google Scholar] [CrossRef]
- Bird, D.A.; Gillotte, K.L.; Hörkkö, S.; Friedman, P.; Dennis, E.A.; Witztum, J.L.; Steinberg, D. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: Implications with respect to macrophage recognition of apoptotic cells. Proc. Natl. Acad. Sci. USA 1999, 96, 6347–6352. [Google Scholar] [CrossRef]
- Hörkkö, S.; Bird, D.A.; Miller, E.; Itabe, H.; Leitinger, N.; Subbanagounder, G.; Berliner, J.A.; Friedman, P.; Dennis, E.A.; Curtiss, L.K.; et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 1999, 103, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Bergmark, C.; Laurila, A.; Hörkkö, S.; Han, K.H.; Friedman, P.; Dennis, E.A.; Witztum, J.L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: Evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. USA 1999, 96, 6353–6358. [Google Scholar] [CrossRef] [PubMed]
- Faria-Neto, J.R.; Chyu, K.Y.; Li, X.; Dimayuga, P.C.; Ferreira, C.; Yano, J.; Cercek, B.; Shah, P.K. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis 2006, 189, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Sobel, M.; Moreno, K.I.; Yagi, M.; Kohler, T.R.; Tang, G.L.; Clowes, A.W.; Zhou, X.H.; Eugenio, E. Low levels of a natural IgM antibody are associated with vein graft stenosis and failure. J. Vasc. Surg. 2013, 58, e1001–e1002. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.R.; Ewing, M.M.; de Jong, R.C.M.; MacArthur, M.R.; Karper, J.C.; Peters, E.A.B.; Nordzell, M.; Karabina, S.A.P.; Sexton, D.; Dahlbom, I.; et al. Identification of IgG1 isotype phosphorylcholine antibodies for the treatment of inflammatory cardiovascular diseases. J. Intern. Med. 2021, 290, 141–156. [Google Scholar] [CrossRef]
- Ståhle, M.; Silvola, J.M.U.; Hellberg, S.; de Vries, M.; Quax, P.H.A.; Kroon, J.; Rinne, P.; de Jong, A.; Liljenbäck, H.; Savisto, N.; et al. Therapeutic Antibody Against Phosphorylcholine Preserves Coronary Function and Attenuates Vascular (18)F-FDG Uptake in Atherosclerotic Mice. JACC Basic Transl. Sci. 2020, 5, 360–373. [Google Scholar] [CrossRef]
- de Vries, M.R.; Niessen, H.W.; Löwik, C.W.; Hamming, J.F.; Jukema, J.W.; Quax, P.H. Plaque rupture complications in murine atherosclerotic vein grafts can be prevented by TIMP-1 overexpression. PLoS ONE 2012, 7, e47134. [Google Scholar] [CrossRef]
- de Vries, M.R.; Parma, L.; Peters, H.A.B.; Schepers, A.; Hamming, J.F.; Jukema, J.W.; Goumans, M.; Guo, L.; Finn, A.V.; Virmani, R.; et al. Blockade of vascular endothelial growth factor receptor 2 inhibits intraplaque haemorrhage by normalization of plaque neovessels. J. Intern. Med. 2019, 285, 59–74. [Google Scholar] [CrossRef]
- Baganha, F.; de Jong, R.C.M.; Peters, E.A.; Voorham, W.; Jukema, J.W.; Delibegovic, M.; de Vries, M.R.; Quax, P.H.A. Atorvastatin pleiotropically decreases intraplaque angiogenesis and intraplaque haemorrhage by inhibiting ANGPT2 release and VE-Cadherin internalization. Angiogenesis 2021, 24, 567–581. [Google Scholar] [CrossRef]
- Lardenoye, J.H.; de Vries, M.R.; Löwik, C.W.; Xu, Q.; Dhore, C.R.; Cleutjens, J.P.; van Hinsbergh, V.W.; van Bockel, J.H.; Quax, P.H. Accelerated atherosclerosis and calcification in vein grafts: A study in APOE*3 Leiden transgenic mice. Circ. Res. 2002, 91, 577–584. [Google Scholar] [CrossRef]
- Frostegård, A.G.; Su, J.; Hua, X.; Vikström, M.; de Faire, U.; Frostegård, J. Antibodies against native and oxidized cardiolipin and phosphatidylserine and phosphorylcholine in atherosclerosis development. PLoS ONE 2014, 9, e111764. [Google Scholar] [CrossRef]
- Jeurissen, M.L.J.; Walenbergh, S.M.A.; Houben, T.; Gijbels, M.J.J.; Li, J.; Hendrikx, T.; Oligschlaeger, Y.; van Gorp, P.J.; Binder, C.J.; Donners, M.; et al. Prevention of oxLDL uptake leads to decreased atherosclerosis in hematopoietic NPC1-deficient Ldlr(-/-) mice. Atherosclerosis 2016, 255, 59–65. [Google Scholar] [CrossRef][Green Version]
- Pluijmert, N.J.; de Jong, R.C.M.; de Vries, M.R.; Pettersson, K.; Atsma, D.E.; Jukema, J.W.; Quax, P.H.A. Phosphorylcholine Antibodies Preserve Cardiac Function and Reduce Infarct Size by Attenuating the Post-Ischemic Inflammatory Response. JACC Basic Transl. Sci. 2020, 5, 1228–1239. [Google Scholar] [CrossRef]
- Pluijmert, N.J.; de Jong, R.C.M.; de Vries, M.R.; Pettersson, K.; Atsma, D.E.; Jukema, J.W.; Quax, P.H.A. Phosphorylcholine antibodies restrict infarct size and left ventricular remodelling by attenuating the unreperfused post-ischaemic inflammatory response. J. Cell Mol. Med. 2021, 25, 7772–7782. [Google Scholar] [CrossRef]
- Fu, P.; Birukov, K.G. Oxidized phospholipids in control of inflammation and endothelial barrier. Transl. Res. 2009, 153, 166–176. [Google Scholar] [CrossRef]
- de Vries, M.R.; Quax, P.H.A. Inflammation in Vein Graft Disease. Front. Cardiovasc. Med. 2018, 5, 3. [Google Scholar] [CrossRef]
- Boullier, A.; Gillotte, K.L.; Hörkkö, S.; Green, S.R.; Friedman, P.; Dennis, E.A.; Witztum, J.L.; Steinberg, D.; Quehenberger, O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J. Biol. Chem. 2000, 275, 9163–9169. [Google Scholar] [CrossRef]
- Podrez, E.A.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.J.; Shan, L.; Febbraio, M.; Hajjar, D.P.; et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 2002, 277, 38517–38523. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Hoogeveen, R.M.; Ali, L.; Prange, K.H.M.; Waissi, F.; van Weeghel, M.; Bachmann, J.C.; Versloot, M.; Borrelli, M.J.; Yeang, C.; et al. Atherogenic Lipoprotein(a) Increases Vascular Glycolysis, Thereby Facilitating Inflammation and Leukocyte Extravasation. Circ. Res 2020, 126, 1346–1359. [Google Scholar] [CrossRef]
- Baganha, F.; Ritsma, L.; Quax, P.H.A.; de Vries, M.R. Assessment of Microvessel Permeability in Murine Atherosclerotic Vein Grafts Using Two-Photon Intravital Microscopy. Int. J. Mol. Sci. 2020, 21, 9244. [Google Scholar] [CrossRef]
- de Jong, A.; Sier, V.Q.; Peters, H.A.B.; Schilder, N.K.M.; Jukema, J.W.; Goumans, M.; Quax, P.H.A.; de Vries, M.R. Interfering in the ALK1 Pathway Results in Macrophage-Driven Outward Remodeling of Murine Vein Grafts. Front. Cardiovasc Med. 2021, 8, 784980. [Google Scholar] [CrossRef] [PubMed]
- Welten, S.M.; Bastiaansen, A.J.; de Jong, R.C.; de Vries, M.R.; Peters, E.A.; Boonstra, M.C.; Sheikh, S.P.; La Monica, N.; Kandimalla, E.R.; Quax, P.H.; et al. Inhibition of 14q32 MicroRNAs miR-329, miR-487b, miR-494, and miR-495 increases neovascularization and blood flow recovery after ischemia. Circ. Res. 2014, 115, 696–708. [Google Scholar] [CrossRef] [PubMed]
- Parma, L.; Peters, H.A.B.; Sluiter, T.J.; Simons, K.H.; Lazzari, P.; de Vries, M.R.; Quax, P.H.A. bFGF blockade reduces intraplaque angiogenesis and macrophage infiltration in atherosclerotic vein graft lesions in ApoE3*Leiden mice. Sci. Rep. 2020, 10, 15968. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baganha, F.; Sluiter, T.J.; de Jong, R.C.M.; van Alst, L.A.; Peters, H.A.B.; Jukema, J.W.; Delibegovic, M.; Pettersson, K.; Quax, P.H.A.; de Vries, M.R. Phosphorylcholine Monoclonal Antibody Therapy Decreases Intraplaque Angiogenesis and Intraplaque Hemorrhage in Murine Vein Grafts. Int. J. Mol. Sci. 2022, 23, 13662. https://doi.org/10.3390/ijms232113662
Baganha F, Sluiter TJ, de Jong RCM, van Alst LA, Peters HAB, Jukema JW, Delibegovic M, Pettersson K, Quax PHA, de Vries MR. Phosphorylcholine Monoclonal Antibody Therapy Decreases Intraplaque Angiogenesis and Intraplaque Hemorrhage in Murine Vein Grafts. International Journal of Molecular Sciences. 2022; 23(21):13662. https://doi.org/10.3390/ijms232113662
Chicago/Turabian StyleBaganha, Fabiana, Thijs J. Sluiter, Rob C. M. de Jong, Louise A. van Alst, Hendrika A. B. Peters, J. Wouter Jukema, Mirela Delibegovic, Knut Pettersson, Paul H. A. Quax, and Margreet R. de Vries. 2022. "Phosphorylcholine Monoclonal Antibody Therapy Decreases Intraplaque Angiogenesis and Intraplaque Hemorrhage in Murine Vein Grafts" International Journal of Molecular Sciences 23, no. 21: 13662. https://doi.org/10.3390/ijms232113662
APA StyleBaganha, F., Sluiter, T. J., de Jong, R. C. M., van Alst, L. A., Peters, H. A. B., Jukema, J. W., Delibegovic, M., Pettersson, K., Quax, P. H. A., & de Vries, M. R. (2022). Phosphorylcholine Monoclonal Antibody Therapy Decreases Intraplaque Angiogenesis and Intraplaque Hemorrhage in Murine Vein Grafts. International Journal of Molecular Sciences, 23(21), 13662. https://doi.org/10.3390/ijms232113662