Dietary Polyphenols and In Vitro Intestinal Fructose Uptake and Transport: A Systematic Literature Review
Abstract
:1. Introduction
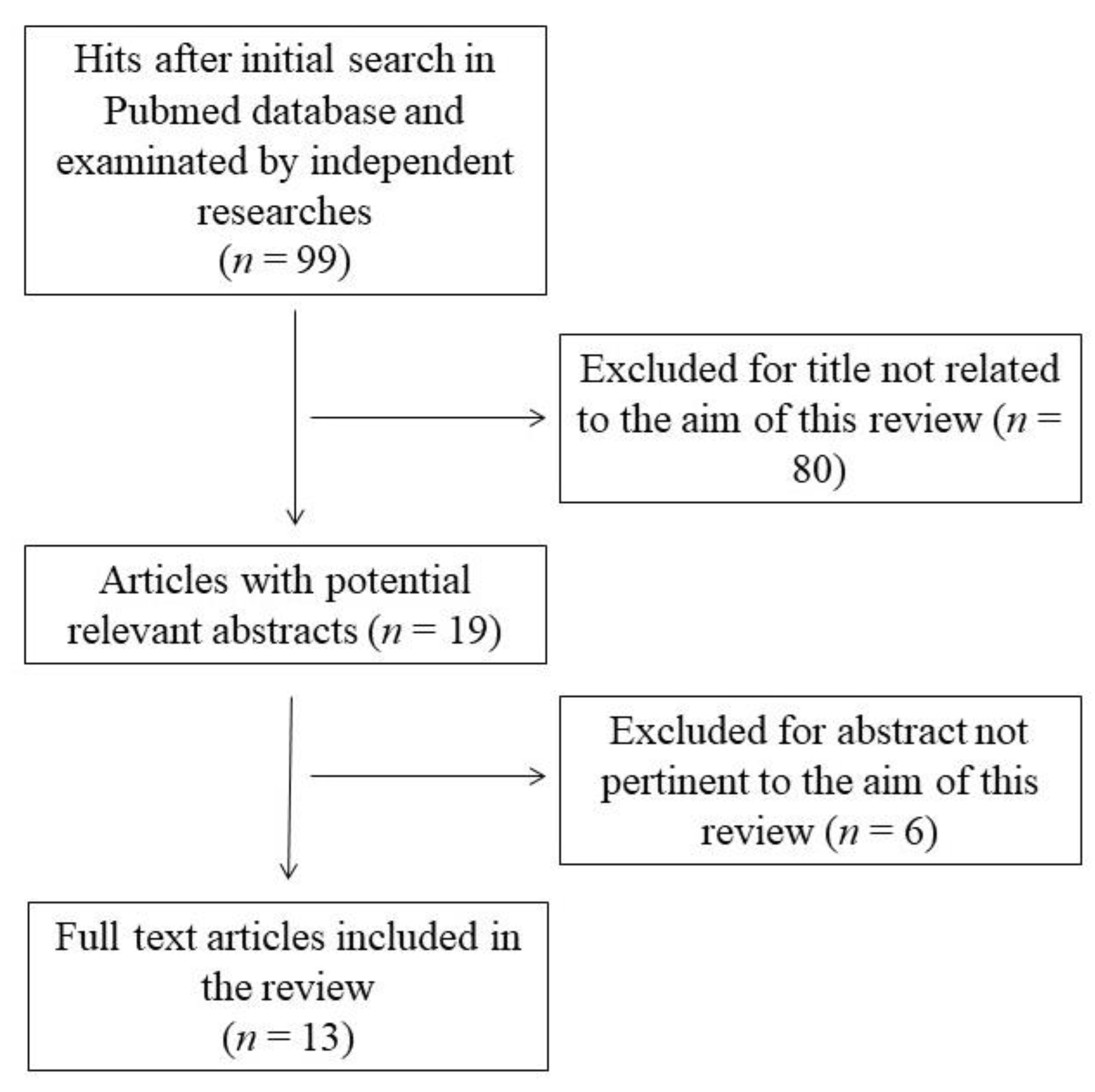
2. Methods
3. Results and Discussion
3.1. Effect of Single PP on FRU Uptake and Transport in Intestinal Cells
3.2. Effect of Polyphenol-Rich Products on FRU Uptake and Transport in Intestinal Cells
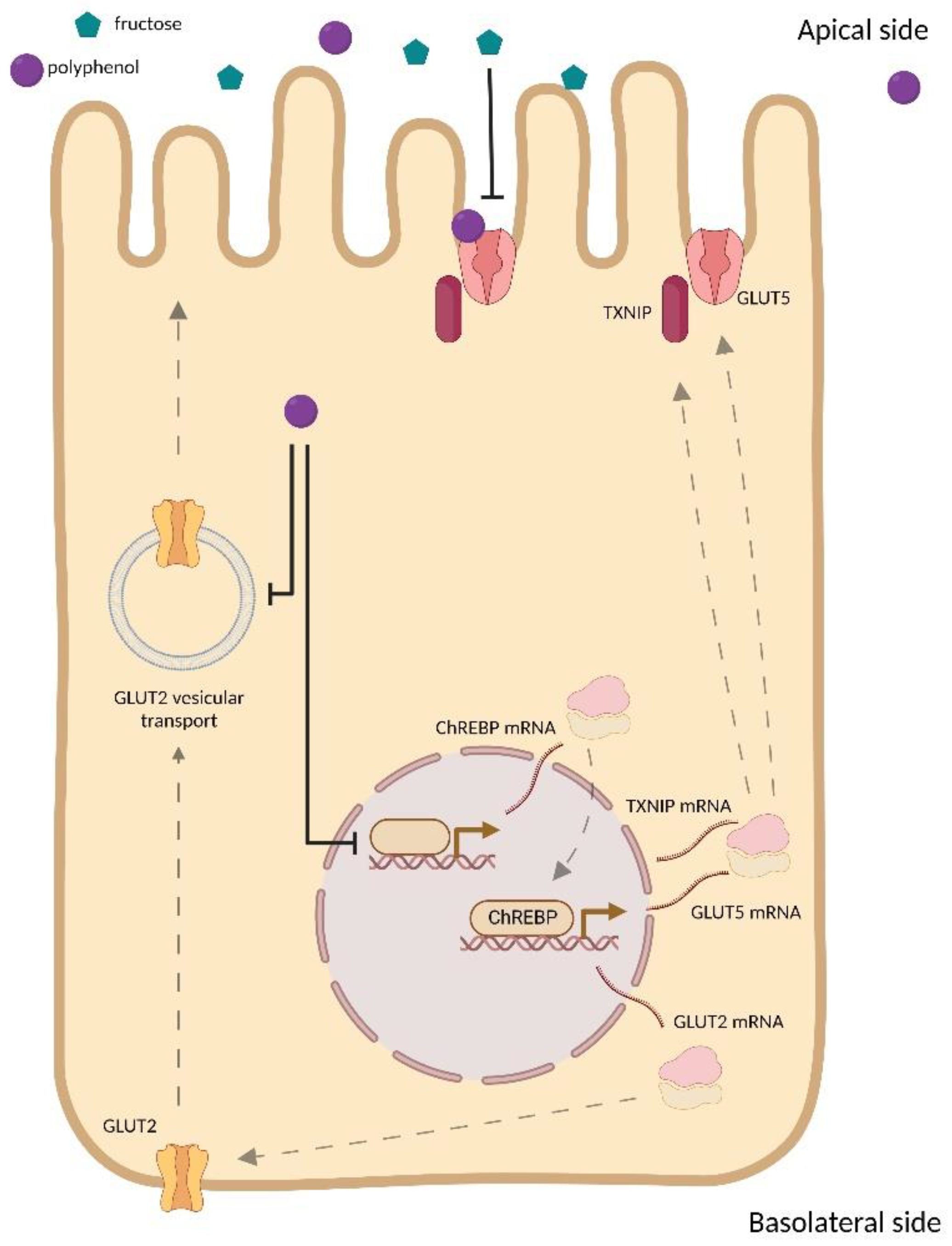
3.3. Effect of Polyphenols on GLUT Family Expression
3.4. Polyphenols and GLUT Family Interactions
3.5. Effect of Polyphenols on GLUT2 Translocation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferraris, R.P.; Choe, J.Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Iossa, S.; Cigliano, L. Sweet but Bitter: Focus on Fructose Impact on Brain Function in Rodent Models. Nutrients 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Rosset, R. Health outcomes of a high fructose intake: The importance of physical activity. J. Physiol. 2019, 597, 3561–3571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Busnatu, S.-S.; Salmen, T.; Pana, M.-A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Risk Factor: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.; Yu, G. The Role of Fructose in Type 2 Diabetes and Other Metabolic Diseases. Nutr. Food Sci. 2017, 8, 1–4. [Google Scholar] [CrossRef]
- Federico, A.; Rosato, V.; Masarone, M.; Torre, P.; Dallio, M.; Romeo, M.; Persico, M. The Role of Fructose in Non-Alcoholic Steatohepatitis: Old Relationship and New Insights. Nutrients 2021, 13, 1314. [Google Scholar] [CrossRef]
- Yuan, C.; Joh, H.K.; Wang, Q.L.; Zhang, Y.; Smith-Warner, S.A.; Wang, M.; Song, M.; Cao, Y.; Zhang, X.; Zoltick, E.S.; et al. Sugar-sweetened beverage and sugar consumption and colorectal cancer incidence and mortality according to anatomic subsite. Am. J. Clin. Nutr. 2022, 115, 1481–1489. [Google Scholar] [CrossRef]
- Zwarts, I.; van Zutphen, T.; Kruit, J.K.; Liu, W.; Oosterveer, M.H.; Verkade, H.J.; Uhlenhaut, N.H.; Jonker, J.W. Identification of the fructose transporter GLUT5 (SLC2A5) as a novel target of nuclear receptor LXR. Sci. Rep. 2019, 9, 9299. [Google Scholar] [CrossRef] [Green Version]
- Schmidl, S.; Ursu, O.; Iancu, C.V.; Oreb, M.; Oprea, T.I.; Choe, J.Y. Identification of new GLUT2-selective inhibitors through in silico ligand screening and validation in eukaryotic expression systems. Sci. Rep. 2021, 11, 13751. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Hengist, A.; Koumanov, F.; Gonzalez, J. Fructose and metabolic health: Governed by hepatic glycogen status? J. Physiol. 2019, 597, 3573–3585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, L.U.; Yoon, J.H.; Jenkins, D.J.; Wolever, T.M.; Jenkins, A.L. Relationship between polyphenol intake and blood glucose response of normal and diabetic individuals. Am. J. Clin. Nutr. 1984, 39, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals from Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Alarcón, K.; Victoriano, M.; Mardones, L.; Villagran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Cruz-Martins, N.; Sharifi-Rad, J.; Martorell, M. Phytochemicals as Potential Epidrugs in Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 656978. [Google Scholar] [CrossRef] [PubMed]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care 2013, 36, 1454–1461. [Google Scholar] [CrossRef] [Green Version]
- Lecoultre, V.; Carrel, G.; Egli, L.; Binnert, C.; Boss, A.; MacMillan, E.L.; Kreis, R.; Boesch, C.; Darimont, C.; Tappy, L. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am. J. Clin. Nutr. 2014, 99, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Miralles, B.; Amigo, L.; Recio, I. Intestinal Signaling of Proteins and Digestion-Derived Products Relevant to Satiety. J. Agric. Food Chem. 2018, 66, 10123–10131. [Google Scholar] [CrossRef] [Green Version]
- Alzaid, F.; Cheung, H.-M.; Preedy, V.R.; Sharp, P.A. Regulation of Glucose Transporter Expression in Human Intestinal Caco-2 Cells following Exposure to an Anthocyanin-Rich Berry Extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, T.L.; Ellam, S.L.; Forrelli, T.; Williamson, G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with SGLT1 and GLUT2 transporters. BioFactors 2013, 39, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Horita, M.; Nagai, H.; Nagatomo, A.; Nishida, N.; Matsuura, Y.; Nagaoka, S. Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract. Mol. Nutr. Food Res. 2012, 56, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ikumi, Y.; Kida, T.; Sakuma, S.; Yamashita, S.; Akashi, M. Polymer–phloridzin conjugates as an anti-diabetic drug that Inhibits glucose absorption through the Na+/glucose cotransporter (SGLT1) in the small intestine. J. Control. Release 2008, 125, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Terazono, Y.; Hirasaki, N.; Tatemichi, Y.; Kinoshita, E.; Obata, A.; Matsui, T. Inhibition of Glucose Transport by Tomatoside A, a Tomato Seed Steroidal Saponin, through the Suppression of GLUT2 Expression in Caco-2 Cells. J. Agric. Food Chem. 2018, 66, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Martínez, M.M. Unraveling the Inhibition of Intestinal Glucose Transport by Dietary Phenolics: A Review. Curr. Pharm. Des. 2019, 25, 3418–3433. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Gauer, J.S.; Tumova, S.; Lippiat, J.D.; Kerimi, A.; Williamson, G. Differential patterns of inhibition of the sugar transporters GLUT2, GLUT5 and GLUT7 by flavonoids. Biochem. Pharmacol. 2018, 152, 11–20. [Google Scholar] [CrossRef]
- Sugimoto, K.; Amako, M.; Takeuchi, H.; Nakagawa, K.; Yoshimura, M.; Amakura, Y.; Fujita, T.; Takenaka, S.; Inui, H. Oenothein B in Eucalyptus Leaf Extract Suppresses Fructose Absorption in Caco-2 Cells. Molecules 2022, 27, 122. [Google Scholar] [CrossRef]
- Kerimi, A.; Gauer, J.S.; Crabbe, S.; Cheah, J.W.; Lau, J.; Walsh, R.; Cancalon, P.F.; Williamson, G. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Br. J. Nutr. 2019, 121, 782–792. [Google Scholar] [CrossRef]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.I.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Extract. J. Am. Coll. Nutr. 2019, 38, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, J.A.; Kerimi, A.; Abranko, L.; Tumova, S.; Ford, L.; Blackburn, R.S.; Rayner, C.; Williamson, G. Acute metabolic actions of the major polyphenols in chamomile: An in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Sci. Rep. 2018, 8, 5471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satsu, H.; Awara, S.; Unno, T.; Shimizu, M. Suppressive effect of nobiletin and epicatechin gallate on fructose uptake in human intestinal epithelial Caco-2 cells. Biosci. Biotechnol. Biochem. 2018, 82, 636–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, N.; Araújo, J.R.; Correia-Branco, A.; Carletti, J.V.; Martel, F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct. Foods 2017, 36, 429–439. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, Y.; Kwon, O. Selected Phytochemicals and Culinary Plant Extracts Inhibit Fructose Uptake in Caco-2 Cells. Molecules 2015, 20, 17393–17404. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Andrade, N.; Andrade, S.; Silva, C.; Guimarães, J.T.; Keating, E.; Calhau, C.; Martel, F.; Rodrigues, I.; Marques, C. Effect of chrysin on changes in intestinal environment and microbiome induced by fructose-feeding in rats. Food Funct. 2019, 10, 4566–4576. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.; Das, R.; Emran, T.; Nainu, F.; Chakraborty, A.; Ahmad, I.; Tallei, T.; Idris, A.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2022, 2022, 1–23. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pietrzyk, N.; Kowalska-Baron, A.; Nowak, A.; Chałaśkiewicz, K.; Ratajewski, M.; Budryn, G.; Koziołkiewicz, M. Phenolics-Rich Extracts of Dietary Plants as Regulators of Fructose Uptake in Caco-2 Cells via GLUT5 Involvement. Molecules 2021, 26, 4745. [Google Scholar] [CrossRef]
- Schreck, K.; Melzig, M.F. Traditionally Used Plants in the Treatment of Diabetes Mellitus: Screening for Uptake Inhibition of Glucose and Fructose in the Caco2-Cell Model. Front. Pharm. 2021, 12, 692566. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Aydin, E.; Gauer, J.S.; Pyner, A.; Williamson, G.; Kerimi, A. Green and Chamomile Teas, but not Acarbose, Attenuate Glucose and Fructose Transport via Inhibition of GLUT2 and GLUT5. Mol. Nutr. Food Res. 2017, 61, 1700566. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.; Lim, J.; Chegeni, M.; Wightman, J.D.; Hamaker, B.R.; Ferruzzi, M.G. Concord and Niagara Grape Juice and Their Phenolics Modify Intestinal Glucose Transport in a Coupled in Vitro Digestion/Caco-2 Human Intestinal Model. Nutrients 2016, 8, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Betoret, E.; Taccari, A.; Dalla Rosa, M.; Bordoni, A. Impact of processing on the nutritional and functional value of mandarin juice. J. Sci. Food Agric. 2020, 100, 4558–4564. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef] [Green Version]
- Di Nunzio, M.; Toselli, M.; Verardo, V.; Caboni, M.F.; Bordoni, A. Counteraction of oxidative damage by pomegranate juice: Influence of the cultivar. J. Sci. Food Agric. 2013, 93, 3565–3573. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Chiarello, E.; Di Nunzio, M.; Bordoni, A.; Gianotti, A. Colonic In Vitro Model Assessment of the Prebiotic Potential of Bread Fortified with Polyphenols Rich Olive Fiber. Nutrients 2021, 13, 787. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Bernardi, S.; Del Bo’, C.; Marino, M.; Gargari, G.; Cherubini, A.; Andrés-Lacueva, C.; Hidalgo-Liberona, N.; Peron, G.; González-Dominguez, R.; Kroon, P.; et al. Polyphenols and Intestinal Permeability: Rationale and Future Perspectives. J. Agric. Food Chem. 2020, 68, 1816–1829. [Google Scholar] [CrossRef]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Lizák, B.; Szarka, A.; Kim, Y.; Choi, K.-s.; Németh, C.E.; Marcolongo, P.; Benedetti, A.; Bánhegyi, G.; Margittai, É. Glucose Transport and Transporters in the Endomembranes. Int. J. Mol. Sci. 2019, 20, 5898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzoli, A.; Gatto, C.; Crescenzo, R.; Spagnuolo, M.S.; Nazzaro, M.; Iossa, S.; Cigliano, L. Gut and liver metabolic responses to dietary fructose—Are they reversible or persistent after switching to a healthy diet? Food Funct. 2021, 12, 7557–7568. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tuo, B.; Dong, H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients 2016, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Hininger-Favier, I.; Kelly, M.A.; Benaraba, R.; Dawson, H.D.; Coves, S.; Roussel, A.M.; Anderson, R.A. Green Tea Polyphenol Extract Regulates the Expression of Genes Involved in Glucose Uptake and Insulin Signaling in Rats Fed a High Fructose Diet. J. Agric. Food Chem. 2007, 55, 6372–6378. [Google Scholar] [CrossRef] [PubMed]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotimas, J.R.; Lee, A.W.; Schmider, A.B.; Carroll, S.H.; Shah, A.; Bilen, J.; Elliott, K.R.; Myers, R.B.; Soberman, R.J.; Yoshioka, J.; et al. Diabetes regulates fructose absorption through thioredoxin-interacting protein. eLife 2016, 5, e18313. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Dagdeviren, S.; Lewandowski, J.P.; Schmider, A.B.; Ricci-Blair, E.M.; Natarajan, N.; Hundal, H.; Noh, H.L.; Friedline, R.H.; Vidoudez, C.; et al. Thioredoxin Interacting Protein Is Required for a Chronic Energy-Rich Diet to Promote Intestinal Fructose Absorption. iScience 2020, 23, 101521. [Google Scholar] [CrossRef]
- Iizuka, K.; Bruick, R.K.; Liang, G.; Horton, J.D.; Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA 2004, 101, 7281–7286. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, K. The Role of Carbohydrate Response Element Binding Protein in Intestinal and Hepatic Fructose Metabolism. Nutrients 2017, 9, 181. [Google Scholar] [CrossRef]
- Kim, M.; Astapova, I.I.; Flier, S.N.; Hannou, S.A.; Doridot, L.; Sargsyan, A.; Kou, H.H.; Fowler, A.J.; Liang, G.; Herman, M.A. Intestinal, but not hepatic, ChREBP is required for fructose tolerance. JCI Insight 2017, 2, e96703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.S.; Andrade, N.; Martel, F. Intestinal fructose absorption: Modulation and relation to human diseases. PharmaNutrition 2020, 14, 100235. [Google Scholar] [CrossRef]
- Sun, L.R.; Zhou, W.; Zhang, H.M.; Guo, Q.S.; Yang, W.; Li, B.J.; Sun, Z.H.; Gao, S.H.; Cui, R.J. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Ebert, K.; Ewers, M.; Geillinger-Kästle, K.; Schoberth, G.; Essenwanger, J.; Stolz, J.; Daniel, H.; Witt, H. Reassessment of GLUT7 and GLUT9 as Putative Fructose and Glucose Transporters. J. Membr. Biol. 2017, 250, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Kitsberg, D.; Tsytkin, S.; Shulman, M.; Aroeti, B.; Nahmias, Y. Live imaging of GLUT2 glucose-dependent trafficking and its inhibition in polarized epithelial cysts. Open Biol. 2014, 4, 140091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Othman, S.; Kaldmäe, H.; Rätsep, R.; Bleive, U.; Aluvee, A.; Rinken, T. Optimization of Ultrasound-Assisted Extraction of Phloretin and Other Phenolic Compounds from Apple Tree Leaves (Malus domestica Borkh.) and Comparison of Different Cultivars from Estonia. Antioxidants 2021, 10, 189. [Google Scholar] [CrossRef]
- Szarka, L.A.; Camilleri, M. Methods for the assessment of small-bowel and colonic transit. Semin. Nucl. Med. 2012, 42, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Chiarello, E.; Di Nunzio, M.; Picone, G.; Antonelli, G.; Capozzi, F.; Bordoni, A. Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu. Nutrients 2022, 14, 517. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Bordoni, A.; Di Nunzio, M.; Viadel, B.; Gallego, E.; Villalba, M.P.; Tomás-Cobos, L.; Taneyo Saa, D.L.; Gianotti, A. Integrated Evaluation of the Potential Health Benefits of Einkorn-Based Breads. Nutrients 2017, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
| Ref. | Cell Model | Substrate and Concentration | Combination and Time of Incubation | Phenolic Compounds | Phenolic Concentration | Results |
|---|---|---|---|---|---|---|
| Sugimoto et al. [30] | Caco-2 | 1 mM FRU | Co-incubation for 3 h | Oenothein B | 5 µg/mL (3.19 µM) | ↓ FRU transport by 63% |
| Gallic acid | 50 µg/mL (293 µM) | ↓ FRU transport by <20% | ||||
| Ellagic acid | 50 µg/mL (165 µM) | ↓ FRU transport by <30% | ||||
| Quercetin 3-O-b-D-glucoronide | 50 µg/mL (104 µM) | ↓ FRU transport by <30% | ||||
| Kaempferol-3-O-glucoronide | 50 µg/mL (108 µM) | ↓ FRU transport by <30% | ||||
| Kerimi et al. [31] | Caco-2/TC7 | 130 mM 14C-FRU | Co-incubation for 30 min | Hesperidin | 800 µM | ↓ FRU transport approximately by 25% |
| Ji et al. [32] | Caco-2 | 100 nM NBDF | Pretreatment with compounds for 20 min and subsequent incubation with FRU for 30 min | Quercetin | 0–100 µg/mL (0–331 µM) | ↓ NBDF uptake in a concentration-dependent manner with a IC50 = 3.604 µg/mL (11.9 µM). ↓ GLUT2 mRNA expression in a concentration-dependent manner with a IC50 = 2.645 µg/mL (8.75 µM). ↓ GLUT5 mRNA expression in a concentration dependent manner with a IC50 = 1.788 µg/mL (5.91 µM) |
| Villa-Rodriguez et al. [33] | Caco-2/TC7 | 5 mM 14C-FRU | Pretreatment with compounds for 25 min and subsequent incubation with FRU for 60 min | Apigenin-7-O-glucoside | 200 µM | ↓ FRU transport approximately by 25% |
| Apigenin | 50 µM | ↓ FRU transport approximately by 20% | ||||
| Apigenin-7-O-glucoside + apigenin | 148 µM + 12 µM | ↓ FRU transport approximately by 80% | ||||
| E-2-β-D-glucopyranosyloxy-4-methoxycinnamic acid, Z-2-β-D-glucopyranosyloxy-4-methoxycinnamic acid | Not reported | No effect | ||||
| Satsu et al. [34] | Caco-2 | 200 nM 3H-FRU | Co-incubation for 10 min | Apigenin | 10 µM | ↓ FRU uptake approximately by 15% |
| Kaempferol | 10 µM | ↓ FRU uptake approximately by 10% | ||||
| Tangeretin | 10 µM and 25 µM | ↓ FRU uptake approximately by 40% and 50% at 10 µM and 25 µM, respectively. | ||||
| Sinensetin | 25 µM | ↓ FRU uptake approximately by 55% | ||||
| Catechin gallate | 25 µM | ↓ FRU uptake approximately by 70% | ||||
| Nobiletin | 10 µM, 25 µM, and 0–150 µM | ↓ FRU uptake approximately by 40% and 65% at 10 µM and 25 µM, respectively. ↓ FRU uptake and transport in a concentration-dependent matter | ||||
| Epicatechin gallate | 10 µM, 25 µM, and 0–150 µM | ↓ FRU uptake approximately by 40% and 70% at 10 µM and 25 µM, respectively. ↓ FRU uptake and transport in a concentration-dependent matter | ||||
| Galangin, fisetin, myricetin, morin, puerarin, diosmin, flavonol, flavone, hesperetin, genistein, daidzein, naringin, naringenin, caffeine, catechin, epicatechin, epigallocatechin, epigallocatechin gallate, rutin, baicalein, flavanone, daidzin, glycitin, glycitein, quercitrin, quercetin, genistin, ginkgolides b, ginkgolides j, equol | 10 µM | No effect | ||||
| Hesperetin, catechin, epicatechin, epigallocatechin, epigallocatechin gallate, gallocatechin, gallocatechin gallate | 25 µM | No effect | ||||
| Andrade et al. [35] | Caco-2 | 100 nM 14C-FRU | Pretreatment with compound for 20 min or 24 h and subsequent incubation with FRU for 6 min | Sinapinic acid | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 10% at 1 µM for 20 min. |
| Ferulic acid | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 20% at 1 µM for 20 min. ↑ FRU uptake approximately by 15% at 1 µM and 10 µM for 24 h | ||||
| Caffeic acid | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 15% at 1 µM and 10 µM for 20 min. ↑ FRU uptake approximately by 15% at 100 µM for 24 h | ||||
| Coumaric acid | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 15% at 10 µM for 20 min. | ||||
| Proctocatenoic acid | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 15% at 10 µM for 20 min. ↑ FRU uptake approximately by 10% at 100 µM for 24 h | ||||
| Apigenin | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 20% and 25% at 10 µM and 100 µM for 20 min, respectively. ↓ GLUT2 mRNA and GLUT5 mRNA levels approximately by 90% and 75% at 100 µM for 24 h, respectively | ||||
| Chrysin | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 20% at all concentrations for 20 min. ↑ and ↓ FRU uptake approximately by 20% and 25% FRU at 1 µM and 100 µM for 24 h, respectively. ↓ GLUT2 mRNA and GLUT5 mRNA levels approximately by 90% and 75% at 100 µM for 24 h, respectively | ||||
| Hesperidin | 1 µM, 10 µM, and 100 µM | ↑ FRU uptake approximately by 15% and 20% at 1 µM and 10 µM for 24 h, respectively | ||||
| Naringenin | 1 µM, 10 µM, and 100 µM | ↑ FRU uptake approximately by 30% and 20% at 1 µM and 10 µM for 20 min, respectively. ↓ FRU uptake approximately by 15% at 100 µM for 24 h | ||||
| Rutin | 1 µM, 10 µM, and 100 µM | ↑ FRU uptake approximately by 10% at 1 µM for 20 min | ||||
| Quercetin | 1 µM, 10 µM, and 100 µM | ↑ and ↓ FRU uptake approximately by 20% and 25% at 1 µM and 100 µM for 24 h, respectively. ↓ GLUT2 mRNA and GLUT5 mRNA levels approximately by 90% and 75% at 100 µM for 24 h, respectively | ||||
| Kaempferol | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 15% at 100 µM for 24 h. | ||||
| Catechin | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 10% at 10 µM for 20 min. | ||||
| Epicatechin | 1 µM, 10 µM, and 100 µM | ↑ FRU uptake approximately by 25% at 10 µM for 20 min | ||||
| Epigallocatechin | 1 µM, 10 µM, and 100 µM | ↑ FRU uptake approximately by 25% at 10 µM for 20 min | ||||
| Delphinidin | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 15% at 1 µM and 10 µM for 20 min. | ||||
| Malvidin-3-glucoside | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 15% at 100 µM for 20 min, and at 10 µM and 100 µM for 24 h | ||||
| Cyanidin-3-glucoside | 1 µM, 10 µM, and 100 µM | ↓ FRU uptake approximately by 20% at 1 µM and 10 µM for 20 min. | ||||
| Gallic acid, ellagic acid, xantohumol, catechin, epigallocatechin gallate, delphinidin-3-glucoside, malvidin, resveratrol, sinapinic acid | 1 µM, 10 µM, and 100 µM | No effect | ||||
| Lee et al. [36] | Caco-2 | 10 mM 14C-FRU | Co-incubation for 10 min | Quercetin | 0–30 µg/mL (0–99 µM) | ↓ FRU uptake in a concentration-dependent manner with a IC50 = 29.64 µg/mL (98 µM) |
| Catechin | 0–30 µg/mL (0–103 µM) | ↓ FRU uptake in a concentration-dependent manner with an IC50 = 87.08 µg/mL (300 µM) | ||||
| Curcumin | 0–30 µg/mL (0–-81 µM) | ↓ FRU uptake in a concentration-dependent manner with an IC50 = 65.57 µg/mL (178 µM) | ||||
| Bisdemethoxycurcumin | 0–30 µg/mL (0–97 µM) | ↓ FRU uptake in a concentration-dependent manner with a IC50 = 83.6 µg/mL (271 µM) | ||||
| Demethoxycurcumin | 0–30 µg/mL (0–88 µM) | ↓ FRU uptake in a concentration-dependent manner with IC50 = 37.43 µg/mL (110 µM) | ||||
| Kwon et al. [37] | Caco-2 | 10 mM 14C-FRU | Co-incubation for 5 min | Quercetin | 0–100 µM | ↓ FRU uptake and transport in a concentration-dependent manner |
| 50 mM 14C-FRU | Co-incubation for various times (5–45 min) | Quercetin | 200 µM | ↓ FRU uptake and transport in a concentration-dependent manner | ||
| Andrade et al. [38] | Ex vivo rat jejunum in an Ussing chamber | 1 µM 14C-FRU | Rats were fed for 18 weeks with the compound. Incubation of rat jejunum with FRU for 90 min | Chrysentin | 100 mg/kg body weight | ↓ FRU permeability approximately by 80% |
| Ref. | Cell Model | Substrate and Concentration | Combination and Time of Incubation | Phenolic Compounds | PP-Rich Product | Phenolic/Extract Concentration | Results |
|---|---|---|---|---|---|---|---|
| Zakłos-Szyda et al. [40] | Caco-2 | 100 µM NBDF | Pretreatment with extract for 24 h and subsequent incubation with NBDF for 3 h | Gallic acid (3.96 mg/g), ferulic acid (7.25 mg/g), procyanidin C1 (70.43 mg/g), rutin (4.98 mg/g), quercetin-3-O-glucoside (0.45 mg/g), kaempferol-3-O-glucoside (10.4 mg/g), isorhamnetin-3-O-rutinoside (4.72 mg/g), isorhamnetin-3-O-glucoside (9.83 mg/g), kaempferol (30.97 mg/g), apigenin (165.81 mg/g) | Purified hydroalcoholic Brassica juncea (var. Green giant) leaf extract | 0.25 mg/mL | ↓ NBDF uptake approximately by 20%. ↓ GLUT5 mRNA and protein expression nearly by 20% |
| Caffeic acid (2.02 mg/g), salicylic acid (24.63 mg/g), 3,5-dicaffeoylquinic acid (12.85 mg/g), ferulic acid (17.78 mg/g), sinapic acid (6.4 mg/g), procyanidin C1 (2.32 mg/g), epigallocatechin-3-gallate (51.62 mg/g), rutin (25.28 mg/g), quercetin-3-O-glucoside (27.70 mg/g), quercetin-3-O-glucuronide (22.2 mg/g), kaempferol-3-O-glucoside (18.56 mg/g), isorhamnetin-3-O-glucoside (1.05 mg/g), apigenin-7-O-glucoside (43.66 mg/g), apigenin (199.12 mg/g) | Purified hydroalcoholic Brassica juncea (var. Red giant) leaf extract | 0.25 mg/mL | ↓ NBDF uptake approximately by 30%. ↓ GLUT5 mRNA and protein expression nearly by 40% and 45%, respectively | ||||
| Chlorogenic acid (0.96 mg/g), caffeic acid (0.81 mg/g), 3-coumaric acid (0.17 mg/g), salicylic acid (28 mg/g), ferulic acid (36.2 mg/g), epigallocatechin-3-gallate (4.94 mg/g), quercetin-3-O-glucoside (1.9 mg/g), kaempferol-3-O-glucoside (6.21 mg/g), isorhamnetin-3-O-glucoside (20.17 mg/g), luteolin (6.45 mg/g), apigenin (40.47 mg/g) | Purified hydroalcoholic Matricaria chamomilla flower extract | 0.25 mg/mL | ↓ NBDF uptake approximately by 30%. ↓ GLUT5 mRNA and protein expression nearly by 25%, respectively | ||||
| Gallic acid (12.68 mg/g), 3-coumaric acid (6.24 mg/g), salicylic acid (41.88 mg/g), 3,5-dicaffeoylquinic acid (6.23 mg/g), ferulic acid (5.19 mg/g), procyanidin B2 (7.11 mg/g), (−)-epicatechin (6.27 mg/g), procyanidin C1 (256.76 mg/g), epigallocatechin-3-gallate (6.3 mg/g), epicatechin-3-gallate (6.51 mg/g), apigenin-7-O-glucoside (302.01 mg/g) | Purified hydroalcoholic Apium graveolens L., (var. Rapaceum) root extract | 0.25 mg/mL | No effect | ||||
| Sugimoto et al. [30] | Caco-2 | 1 mM FRU | Co-incubation for 3 h | Oenothein B, gallic acid, ellagic acid, quercetin 3-O-b-D-glucoronide, kaempferol-3-O-glucoronide | Hydroalcoholic Eucalyptus globulus leaf extract | 1 mg/mL | ↓ FRU transport by 65% |
| Schreck et al. [41] | Caco-2 | 54 nM 14C-FRU | Co-incubation for 1 h | Not reported | Methanolic Juglans regia leaf extract | 1 mg/mL | ↓ FRU uptake by 30.2% |
| Methanolic Peumus boldus leaf extract | 1 mg/mL | ↓ FRU uptake by 32.6% | |||||
| Methanolic and aqueous Adenophora triphylla root, Allium sativum bulb, Aronia melanocarpa fruit, Artemisia dracunculus leaf, Brassica oleracea (var. Capitata alba) leaf, Camellia sinensis (var. Assam) leaf, Camellia sinensis (var. Darjeeling) leaf, Camellia sinensis (var. Gunpowder) leaf, Camellia sinensis (var. Sencha) leaf, Ceratonia siliqua fruit, Citrus limon fruit skin, Coffea arabica green seed, Cornus officinalis fruit, Crataegus pinnatifida fruit, Cynara cardunculus herb, Eucommia ulmoides bark, Hibiscus sabdariffa flower, Ilex paraguariensis leaf, Lycium chinense fruit, Melissa officinalis leaf, Mentha aquatica leaf, Momordica charantia fruit, Nigella sativa seed, Olea europaea leaf, Origanum creticum leaf, Panax ginseng root, Potentilla aurea herb, Pueraria lobata root, Rosa rugosa flower, Rosmarinus officinalis leaf, Salvia officinalis leaf, Sarcopoterium spinosum root, Syzygium aromaticum flower, Thymus vulgaris herb, Vaccinium myrtillus fruit, Vitis vinifera seed (pomace) extracts. Aqueous Juglans regia leaf, Peumus boldus leaf extracts | 0.01–1 mg/mL | No effect | |||||
| Ji et al. [32] | Caco-2 | 100 nM NBDF | Pretreatment with extract for 20 min and subsequent incubation with FRU for 30 min | Apigenin (1.89 µg/g), luteolin (5.3 µg/g), tricin (27.4 µg/g) | Hydroalcoholic sugarcane extract | 0.01–10 mg/mL | ↓ NBDF uptake in a concentration-dependent manner with a IC50 = 4.468 mg/mL. ↓ and ↑ of GLUT2 mRNA and GLUT5 mRNA expression in a concentration-dependent manner with a IC50 = 3.396 mg/mL and IC50 = 4.941 mg/mL, respectively |
| Villa-Rodriguez et al. [33] | Caco-2/TC7 | 5 mM 14C-FRU | Pretreatment with extract for 25 min incubation with FRU for 60 min | 3-Caffeoylquinic acid (0.01%), 5-caffeoylquinic acid (0.07%), luteolin-7-O-glucoside (0.13%), umbelliferone (0.09%), di-caffeoylquinic acid (0.13%), apigenin-7-O-glucoside (12.3%), luteolin (0.01%) apigenin (0.28%), Z-2-β-D-glucopyranosyloxy-4-methoxycinnamic acid, E-2-β-D-glucopyranosyloxy-4-methoxycinnamic acid | Hydroalcoholic Matricaria recutita extract | 1 mg/mL | ↓ FRU transport by 28% |
| Villa-Rodriguez et al. [42] | Caco-2/TC7 | 5 mM 14C-FRU | Co-incubation for 60 min and pretreatment with extract for 25 min or 16 h and subsequent incubation with FRU for 60 min | 3-Caffeoylquinic acid (0.01%), 5-caffeoylquinic acid (0.07%), luteolin-7-O-glucoside (0.13%), umbelliferone (0.09%), di-caffeoylquinic acid (0.13%), apigenin-7-O-glucoside (12.3%), luteolin (0.01%) apigenin (0.28%) | Hydroalcoholic Matricaria recutita extract | 0–2 mg/mL and 1 mg/mL | ↓ FRU uptake and transport in a concentration-dependent manner with a IC50 = 2 mg/mL and IC50 = 1 mg/mL, respectively. ↓ FRU uptake and transport approximately by 20% and 30% at 1 mg/mL for 25 min, respectively. |
| Co-incubation for 60 min | (−)-epigallocatechin gallate (240 mg/g), (−)-epigallocatechin (70 mg/g), (−)-epicatechin (40 mg/g), (+)-catechin (17 mg/g). | Aqueous Camellia sinensis leaf extract | 0–2 mg/mL | ↓ FRU uptake and transport in a concentration dependent manner with a IC50 = 0.7 mg/mL and IC50 = 0.8 mg/mL, respectively. | |||
| Lee et al. [36] | Caco-2 | 10 mM 14C-FRU | Co-incubation for 10 min | Bisdemethoxycurcumin, demethoxycurcumin, curcumin | Acetonic turmeric extract | 500 µg/mL | ↓ FRU uptake approximately by 50% |
| Catechin, quercetin | Aqueous guava leaf extract | 500 µg/mL | ↓ FRU uptake approximately by 50% | ||||
| Not reported | Hydroalcoholic rosemary extract | 500 µg/mL | ↓ FRU uptake approximately by 40% | ||||
| Not reported | Hydroalcoholic chrysanthemum, bayberry, Korea ginseng extracts. Aqueous onion, passionflower, touchi extracts | 500 µg/mL | No effect | ||||
| Kerimi et al. [31] | Caco-2/TC7 | 130 mM 14C-FRU | Co-supplementation for 30 min | Not reported | Orange juice | Regular strength | No effect |
| Moser et al. [43] | Caco-2 | 9 mM FRU + 3 mM d7-FRU | Co-incubation for 60 min | Gallic acid (5 mg/100 mL), caffeic acid (3.4 mg/100 mL), caftaric acid (4.2 mg/100 mL), quercetin-3,4-O-diglucoside (2.2 mg/100 mL), quercetin (16.8 mg/100 mL), isorhamnetin (4.9 mg/100 mL), piceid (0.8 mg/100 mL), resveratrol (7.1 mg/100 mL). | Grape juice (var. Niagara), harvested in 2013 | 10 µM, 50 µM, and 100 µM as GAE | ↓ FRU transport by 10.5%, 28%, and 26.1% at 10 µM, 50 µM, and 100 µM, respectively. |
| Gallic acid (1.9 mg/100 mL), caffeic acid (5.2 mg/100 mL), caftaric acid (9.6 mg/100 mL), epicatechin (1.5 mg/100 mL), quercetin-3,4-O-diglucoside (2 mg/100 mL), quercetin (19.8 mg/100 mL), isorhamnetin (6.2 mg/100 mL), piceid (5.3 mg/100 mL), resveratrol (13.3 mg/100 mL). | Grape juice with SO2 (var. Niagara), harvested in 2013 | 10 µM, 50 µM, and 100 µM as GAE | ↓ FRU transport by 11.7%, 31.7%, and 29.5% at 10 µM, 50 µM, and 100 µM, respectively. | ||||
| Gallic acid (8.1 mg/100 mL), caffeic acid (11.1 mg/100 mL), caftaric acid (20.8 mg/100 mL), epicatechin (7.9 mg/100 mL), quercetin-3-O-glucoside (5.9 mg/100 mL), quercetin-3,4-O-diglucoside (4.2 mg/100 mL), quercetin-3-O-glucuronide (4.9 mg/100 mL), quercetin (30 mg/100 mL), isorhamnetin (12.6 mg/100 mL), piceid (2.2 mg/100 mL), resveratrol (3.5 mg/100 mL), cyanidin-3,5-O-diglucoside (623.5 ng/100 mL), cyanidin-3-O-glucoside (74.8 ng/100 mL), cyanidin-3-O-acetyl-glucoside (87.5 ng/100 mL), peonidin-3,5-O-diglucoside (144.7 ng/100 mL), peonidin-3-O -glucoside (13.6 ng/100 mL), peonidin-3-O-acetyl-glucoside (10.7 ng/100 mL), delphinidin-3-O-glucoside (620.5 ng/100 mL), delphinidin-3-O-acetyl-glucoside (113.2 ng/100 mL), delphinidin-3-O-p-coumaroyl-5-O-diglucoside (122.3 ng/100 mL), delphinidin-3-O-p-coumaroyl glucoside (199.8 ng/100 mL), petunidin-3-O-glucoside (191.1 ng/100 mL), petunidin-3-O-acetyl-glucoside (35 ng/100 mL), petunidin-3-O-p-coumaroyl-5-O-diglucoside (35.3 ng/100 mL), malvidin-3-O-glucoside (133.4 ng/100 mL), malvidin-3-O-acetyl-glucoside (22.3 ng/100 mL). | Grape juice (var. Concord), harvested in 2013 | 10 µM, 50 µM, and 100 µM as GAE | ↓ FRU transport by 25.2%, 22.1%, and 30.9% at 10 µM, 50 µM, and 100 µM, respectively. | ||||
| Gallic acid (4 mg/100 mL), caffeic acid (4.2 mg/100 mL), caftaric acid (2.9 mg/100 mL), epicatechin (2.4 mg/100 mL), quercetin-3,4-O-diglucoside (2.5 mg/100 mL), quercetin (20.6 mg/100 mL), isorhamnetin (5 mg/100 mL), piceid (1.4 mg/100 mL), resveratrol (10.3 mg/100 mL). | Grape juice (var. Niagara), harvested in 2014 | 10 µM, 50 µM, and 100 µM as GAE | ↓ FRU transport by 14.5%, 21.1%, and 41.3% at 10 µM, 50 µM, and 100 µM, respectively. | ||||
| Gallic acid (2.9 mg/100 mL), caffeic acid (7.6 mg/100 mL), caftaric acid (16.9 mg/100 mL), epicatechin (11.6 mg/100 mL), quercetin-3,4-O-diglucoside (3.3 mg/100 mL), quercetin (31.1 mg/100 mL), isorhamnetin (10.3 mg/100 mL), piceid (4.9 mg/100 mL), resveratrol (11.7 mg/100 mL). | Grape juice with SO2 (var. Niagara), harvested in 2014 | 10 µM, 50 µM, and 100 µM as GAE | ↓ FRU transport by 12.9%, 23.3%, and 40.4% at 10 µM, 50 µM, and 100 µM, respectively. | ||||
| Gallic acid (8.9 mg/100 mL), caffeic acid (12.8 mg/100 mL), caftaric acid (25.1 mg/100 mL), epicatechin (12.6 mg/100 mL), quercetin-3-O-glucoside (7.8 mg/100 mL), quercetin-3,4-O-diglucoside (4.3 mg/100 mL), quercetin-3-O-glucuronide (3.8 mg/100 mL), quercetin (34.3 mg/100 mL), isorhamnetin (13.1 mg/100 mL), piceid (4.3 mg/100 mL), resveratrol (5.1 mg/100 mL), cyanidin-3,5-O-diglucoside (710 ng/100 mL), cyanidin-3-O -glucoside (106.9 ng/100 mL), cyanidin-3-O-acetyl-glucoside (40.8 ng/100 mL), peonidin-3,5-O-diglucoside (150.4 ng/100 mL), peonidin-3-O-glucoside (19.2 ng/100 mL), peonidin-3-O-acetyl glucoside (15.9 ng/100 mL), delphinidin-3-O-glucoside (877.4 ng/100 mL), delphinidin-3-O-acetyl-glucoside (210.3 ng/100 mL), delphinidin-3-O-p-coumaroyl-5-O-diglucoside (212.1 ng/100 mL), delphinidin-3-O-p-coumaroyl glucoside (211.7 ng/100 mL), petunidin-3-O-glucoside (210.1 ng/100 mL), petunidin-3-O-acetyl-glucoside (61.9 ng/100 mL), petunidin-3-O-p-coumaroyl-5-O-diglucoside (58.2 ng/100 mL), malvidin-3-O-glucoside (168.4 ng/100 mL), malvidin-3-O-acetyl-glucoside (38.8 ng/100 mL). | Grape juice (var. Concord), harvested in 2014 | 10 µM, 50 µM, and 100 µM as GAE | ↓ FRU transport by 10.9%, 18.3%, and 18.1% at 10 µM, 50 µM, and 100 µM, respectively. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iametti, S.; Bonomi, F.; Di Nunzio, M. Dietary Polyphenols and In Vitro Intestinal Fructose Uptake and Transport: A Systematic Literature Review. Int. J. Mol. Sci. 2022, 23, 14355. https://doi.org/10.3390/ijms232214355
Iametti S, Bonomi F, Di Nunzio M. Dietary Polyphenols and In Vitro Intestinal Fructose Uptake and Transport: A Systematic Literature Review. International Journal of Molecular Sciences. 2022; 23(22):14355. https://doi.org/10.3390/ijms232214355
Chicago/Turabian StyleIametti, Stefania, Francesco Bonomi, and Mattia Di Nunzio. 2022. "Dietary Polyphenols and In Vitro Intestinal Fructose Uptake and Transport: A Systematic Literature Review" International Journal of Molecular Sciences 23, no. 22: 14355. https://doi.org/10.3390/ijms232214355
APA StyleIametti, S., Bonomi, F., & Di Nunzio, M. (2022). Dietary Polyphenols and In Vitro Intestinal Fructose Uptake and Transport: A Systematic Literature Review. International Journal of Molecular Sciences, 23(22), 14355. https://doi.org/10.3390/ijms232214355






